Abstract
Purpose
The purposes of this study were to quantify the increase in tibial force imbalance (i.e. magnitude of difference between medial and lateral tibial forces) and changes in laxities caused by 2° and 4° of varus–valgus (V–V) malalignment of the femoral component in kinematically aligned total knee arthroplasty (TKA) and use the results to detemine sensitivities to errors in making the distal femoral resections. Because V–V malalignment would introduce the greatest changes in the alignment of the articular surfaces at 0° flexion, the hypotheses were that the greatest increases in tibial force imbalance would occur at 0° flexion, that primarily V–V laxity would significantly change at this flexion angle, and that the tibial force imbalance would increase and laxities would change in proportion to the degree of V–V malalignment.
Methods
Kinematically aligned TKA was performed on ten human cadaveric knee specimens using disposable manual instruments without soft tissue release. One 3D-printed reference femoral component, with unmodified geometry, was aligned to restore the native distal and posterior femoral joint lines. Four 3D-printed femoral components, with modified geometry, introduced V–V malalignments of 2° and 4° from the reference component. Medial and lateral tibial forces were measured during passive knee flexion–extension between 0° to 120° using a custom tibial force sensor. Eight laxities were measured from 0° to 120° flexion using a six degree-of-freedom load application system.
Results
With the tibial component kinematically aligned, the increase in the tibial force imbalance from that of the reference component at 0° of flexion was sensitive to the degree of V–V malalignment of the femoral component. Sensitivities were 54 N/deg (medial tibial force increasing > lateral tibial force) (p < 0.0024) and 44 N/deg (lateral tibial force increasing > medial tibial force) (p < 0.0077) for varus and valgus malalignments, respectively. Varus–valgus malalignment did not significantly change varus, internal–external rotation, anterior–posterior, and compression–distraction laxities from 0° to 120° flexion. At only 30° of flexion, 4° of varus malalignment increased valgus laxity 1° (p = 0.0014).
Conclusion
At 0° flexion, V–V malalignment of the femoral component caused the tibial force imbalance to increase significantly, whereas the laxities were relatively unaffected. Because tibial force imbalance has the potential to adversely affect patient-reported outcomes and satisfaction, surgeons should strive to limit errors in resecting the distal femoral condyles to within ± 0.5 mm which in turn limits the average increase in tibial force imbalance to 68 N. Because laxities were generally unaffected, instability resulting from large increases in laxity is not a clinical concern within the ± 4° range tested.
Level of evidence
Therapeutic, Level II.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
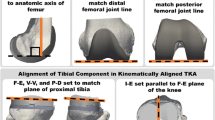
The goal of kinematically aligned total knee arthroplasty (TKA) is to restore native alignments of the limb, knee, and joint lines with the intent of restoring knee function closely to native without soft tissue release. Hence, to achieve kinematic alignment of the femoral component, the thicknesses of the distal and posterior resections of the femoral condyles must be equal to the thicknesses of the corresponding regions of the femoral component after compensating for cartilage wear and kerf of the saw blade [27]. To achieve kinematic alignment of the tibial component, the resection plane must be simultaneously parallel to the proximal tibial joint line viewed in the coronal plane and the medial tibial joint line viewed in the sagittal plane and the thickness of the resection must avoid damage to the insertion of the posterior cruciate ligament [27].
The use of manual cutting guides and oscillating saws can lead to errors in making these resections [6, 24, 31, 47]. Because the resections to achieve kinematic alignment of the femoral component are made without reference to the resection for the tibial component, errors in the femoral resections can be made independently from errors in the tibial resection. Errors in making either femoral or tibial resections may adversely affect tibiofemoral joint function by increasing the tibial force imbalance (i.e., magnitude of difference between medial and lateral tibial forces) and/or laxities. Increased tibial force imbalance has been associated with decreased patient-reported outcomes and satisfaction [22] and might lead to complications such as wear and aseptic loosening which often are causes of revision [14, 16, 54]. Increased laxities lead to instability which is a leading cause of revision [14, 16, 54].
Focusing attention on the femoral component, varus–valgus (V–V) alignment of the femoral component in the coronal plane in kinematic alignment is set by the resection thicknesses of the two distal femoral condyles. If the two resections are not equal in thickness to the corresponding regions of the femoral component after accounting for cartilage wear and kerf of the saw blade, then the femoral component will be malaligned in either varus or valgus rotation. Hence for surgeons to make informed decisions as to whether some adjustment is needed in the femoral cuts after calipered measurement of the thickness of the initial resections [27, 28], important information is the sensitivies to errors in making these resections as indicated by increased tibial force imbalance and changes in laxities during passive motion.
Various approaches have been taken to study the effects of V–V malalignments on tibiofemoral joint function and include laboratory experiments using cadaveric limbs [59, 62], finite element analysis [36], and multi-body dynamics simulation of gait [11, 55]. However, these studies did not assess tibiofemoral joint function passively and/or did not kinematically align the TKA components.
Accordingly, with the tibial component kinematically aligned, the objectives of this study were to determine the increase in tibial force imbalance caused by 2° and 4° of V–V malalignment of the femoral component in kinematically aligned TKA and changes in laxities and use the results to determine sensitivities to errors in making the distal femoral resections. The degrees of freedom of interest were V–V rotation, internal–external (I–E) rotation, anterior–posterior (A–P) translation, and compressive–distractive (C–D) translation. Because V–V malalignment would introduce the greatest changes in the alignment of the articular surfaces at 0° flexion, the hypotheses were that the greatest increases in tibial force imbalance would occur at 0° flexion, that primarily V–V laxity would significantly change at this flexion angle, and that the tibial force imbalance would increase and laxities would change in proportion to the degree of V–V malalignment. If the latter hypothesis was supported, then this would provide the framework for determining the sensitivities of increases in tibial force imbalance and changes in laxities to errors in making the distal femoral resections.
Materials and methods
Based on a power analysis to be described later in the “Statistical analysis” subsection, ten fresh-frozen human cadaveric knees (average age = 82 years, range = 65–98 years, 9 males, 1 female) were included. An anteroposterior radiograph was taken of 23 knee specimens. A trained orthopedic surgeon viewed each radiograph and excluded specimens when there were either signs of degenerative joint disease (i.e., marginal osteophytes, joint space narrowing, chondrocalcinosis, or subchondral sclerosis) or evidence of previous surgery to the knee. Five specimens were excluded due to signs of previous surgery to the knee. Eight specimens were excluded due to radiographic signs of degenerative joint disease. Ten specimens were included in the study. Each included specimen was stored frozen at − 20° C. Specimen preparation and testing spanned a total of four consecutive days. As described below, dissection and alignment in the load application system was performed on Day 1, kinematic alignment TKA was performed on Day 2, and testing was performed on Days 3 and 4. In between, the specimen was refrigerated overnight.
After thawing overnight, a native knee specimen was dissected and aligned in a six degree-of-freedom load application system (Fig. 1) in preparation for measuring tibial forces in the medial and lateral compartments and laxities using previously described protocols [51, 53]. The thigh was transected 20 cm proximal and the shank was transected 25 cm distal to the joint line of the knee. Soft tissues other than skin and fat were retained between 15 cm proximal and 12 cm distal of the joint line of the knee. To apply muscle forces, straps were sutured on to the semimembranosus/semitendinosus, quadriceps, and biceps femoris tendons. Intramedullary rods cemented into the medullary canals of the femur and tibia were attached to alignment fixtures connected to the load application system. Subsequent to a functional axis alignment procedure [4], the shafts of the femur and tibia were cemented within square aluminum tubes, which rigidly fixed the position and orientation of the knee and enabled removal and reinsertion of the native knee and the TKA knee during subsequent testing in the load application system [4]. The knee was subjected to a preconditioning protocol consisting of first cycling the knee five times between ± 2.5 N m in flexion–extension (F–E) and then extending the knee under 2.5 N m to define 0° flexion [37].
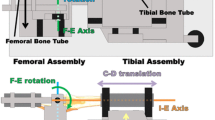
Functional diagram of the custom six degree-of-freedom load application system. The system consists of two independent assemblies, the femoral assembly and the tibial assembly. The system embodies the coordinate system of Grood and Suntay [20]. As such, the flexion–extension axis is fixed in the femur and the internal–external rotation axis is fixed in the tibia. The femoral assembly allows two degrees of freedom, flexion–extension (F–E) rotation and medial–lateral (M–L) translation. The tibial assembly allows internal–external (I–E) rotation, compressive–distractive (C–D) translation, varus–valgus (V–V) rotation and anterior–posterior (A–P) translation. The patella points down. The system operates under closed-loop load control with actuators for each degree of freedom except medial–lateral translation. Forces of major muscle groups crossing the knee also can be applied. Transducers include load cells for each actuator and highly accurate motion sensors (LVDTs and RVDTs) for each degree of freedom. Specimens are aligned following a functional axis approach whereby the flexion–extension axis of the tibiofemoral joint, which is fixed to the femur [25], is aligned with the flexion–extension axis of the load application system and the internal–external rotation axis of the tibiofemoral joint, which is fixed in the tibia [25], is aligned with the internal–external rotation axis of the load application system
Varus–valgus malalignment of the femoral component was simulated by modifying a commercially available femoral component (Persona CR, Zimmer Biomet, Inc.) using 3D modeling software (SolidWorks 2014, Dassault Systèmes) and 3D printing the malaligned femoral components and a reference femoral component with no malalignment using an acrylic-like plastic (VeroWhite, Objet Eden260VS, Stratasys, Ltd.). The Persona femoral component design was modified by rotating the exterior surfaces of the femoral component relative to the interior surfaces such that the malaligned femoral components with modified geometry and the reference femoral component with unmodified geometry could all be implanted using the same cement mantle on the same cadaveric knee specimen (Fig. 2). Five femoral components were 3D printed with malalignments of 2° varus, 4° varus, 2° valgus, and 4° valgus and a 0° reference femoral component with unmodified geometry. Values of 2° and 4° were selected based on previous literature which has examined femoral component malalignment in vitro and in vivo [1, 18, 42, 43, 49], a pilot test conducted in our laboratory, and the clinical experience of an experienced surgeon. All malalignments were made about the center of the distal joint line of the femoral component. The thickness of each condyle of each 3D-printed femoral component was within ± 0.1 mm of the designed thickness.
Rendering of 3D models showing the posterior view of the 3D-printed reference femoral component and the 3D-printed femoral component with a 4° varus malalignment. The orange line shows the orientation of the distal joint line of the femoral component, and the blue line shows the orientation of the inside surface of the femoral component. A 4° varus rotation of the exterior surfaces relative to the interior surfaces about the center of the reference femoral component (i.e., midpoint between the lugs) reduced the thickness of the medial distal femoral condyle, increased the thickness of the lateral distal femoral condyle, and malaligned the distal femoral joint line. Reducing the thickness of the medial distal condyle of the femoral component and increasing the thickness of the lateral distal condyle of the femoral component simulated an angular error in resecting the articular surfaces of the distal femoral condyles of the bone
A kinematically aligned TKA was performed using cruciate-retaining components (Persona CR, Zimmer Biomet, Inc, Warsaw, IN, USA) and disposable manual instruments without soft tissue release following a previously described technique [27, 28, 46]. In brief, a mid-sagittal osteotomy of the patella exposed the knee [41]. A distal femoral and a posterior femoral reference guide was used to resect the femur with the goal of maintaining the native the distal and posterior femoral joint lines, respectively. This goal was accomplished by matching the thicknesses of the distal medial, distal lateral, posterior medial, and posterior lateral femoral resections as measured with a caliper to the corresponding condylar regions of the femoral component after correcting for the kerf of the saw blade [28]. The I–E rotation of the tibial component was set parallel to the F–E plane of the knee. Because the reliability of using the tibial tubercle to identify the F–E plane of the native knee has been questioned [9, 26], the A–P axis of the tibial component was aligned parallel to the F–E plane of the native knee using templates which have been shown to align the A–P axis of the tibial component with a root mean squared error of 4° from the F–E plane of the knee [27]. The V–V cut for the tibial component was adjusted after inserting trial components until there was minimal V–V laxity at 0° flexion [28, 51]. The F–E cut or posterior slope for the tibial component was adjusted after inserting trial components until the A–P distance or offset between the distal medial condyle of the femoral component and the anterior cortex of the tibia measured with a caliper at 90° flexion matched that of the native knee at the time of exposure [28]. After the correctly sized trial components were determined, the reference 3D-printed femoral component was coated with petroleum jelly and cemented to the distal femur. A size D Persona tibial baseplate was coated with petroleum jelly and cemented into the proximal tibia after which the correctly sized tibial insert was attached. The petroleum jelly allowed the components to be released from the cemented surfaces which enabled accurate exchanges of femoral components and the tibial force sensor described below. After the cement hardened, the components were removed. The patella was not resurfaced.
Tibial forces in the medial and lateral compartments were measured with each of the five 3D-printed femoral components using a custom tibial force sensor [52]. The tibial force sensor had the same exterior size and shape as the correctly sized Persona tibial component and insert. The tibial force sensor measured force independently in the medial and lateral compartments and over the full area of the liner with a maximum root mean squared error of 6 N [50, 52].
The testing order of the five 3D-printed femoral components was randomized. After inserting a 3D-printed femoral component, the patellar osteotomy was closed with two transverse bone screws. To stabilize the TKA knee during flexion, constant forces of 26, 80, and 15 N were applied to the semimembranosus/semitendinosus, quadriceps, and biceps femoris tendons, respectively, which were proportional to the muscle cross-sectional area [61] and smaller than forces used to stabilize the TKA in other studies [12, 17, 29, 33, 35, 56, 58, 60]. The tibial forces in the medial and lateral compartments were measured at 30° increments as the TKA knee was moved passively from 0° to 120° flexion and back to 0°. The tibial force difference was computed as the medial tibial force minus the lateral tibial force and the tibial force imbalance was the magnitude of the difference. After a test was completed for a 3D-printed femoral component, the patellar osteotomy was opened, a different 3D-printed femoral component was inserted, and the test was repeated.
Eight laxities were measured in four degrees of freedom with each of the five 3D-printed femoral components using the load application system and methods described previously [53]. Because a size D keel was used in the tibia during the TKA but the correctly sized tibial baseplate might be greater than size D depending on the knee specimen, a set of tibial baseplates was 3D printed that had a size D keel with proximal mating features in sizes E–H. These 3D-printed tibial baseplates were used during laxity testing. With the correctly sized components implanted, the knee was subjected to a preconditioning protocol consisting of first passively flexing and extending the knee five times from 0° to 120° flexion. Next, the knee was moved to a flexion angle randomly selected from 0°, 60°, and 120° and then cycled five times between prescribed load limits for each degree of freedom in a random order [5]. The prescribed load limits were ± 3 N m for I–E rotation [8], ± 5 N m for V–V rotation [38], ± 45 N for A–P translation [15], and ± 100 N for C–D translation [39]. The limits of each load were selected to engage the soft tissues sufficiently to load them beyond the initial toe region of the tibiofemoral joint’s load–deformation curve [15, 38]. The protocol was repeated for the remaining two flexion angles. After completing preconditioninig, the order of flexion angle–degree of freedom combinations was randomized for measuring laxities. For each combination, the knee was loaded to the positive limit, loaded to the negative limit, unloaded, loaded to the negative limit, loaded to the positive limit, and unloaded. The positive laxity was the average of the two positive limits minus the average of the two unloaded positions. The negative laxity was the average of the two negative limits minus the average of the two unloaded positions. The positive laxity and negative laxity were measured over a range of flexion angles from 0° to 120° in 30° increments. Repeatability of laxities and contact forces was determined by measuring each variable over five trials for each of three specimens. The greatest standard deviations of the laxities were 0.5° for V–V and I–E rotations and 0.3 mm for A–P and C–D translations. The greater standard deviation of the contact forces was 12 N.
Following University of California policies, this study did not require institutional review board (IRB) approval because de-identified cadaveric specimens were used.
Statistical analysis
A preliminary power analysis was performed using the standard deviations from the first five specimens to detect changes in laxities, which might be undesirable in TKA, taken from the literature. The change in V–V laxity of 1.5° was based on a study that showed patients with osteoarthritis who reported having an unstable knee had 1.5° more V–V laxity than those that did not report instability [13]. The changes in A–P laxity and I–E laxity of 1.8 mm and 3.6°, respectively, were based on a study that showed a 40% increase in polyethylene wear when A–P translation increased by 1.8 mm and I–E rotation increased by 3.6° [32]. The change in C–D laxity of 1 mm was based on a study which reported changes in the A–P, I–E, and V–V laxities due to a change in liner thickness of 1 mm [44]. The preliminary power analysis showed that a sample size of ten specimens was necessary [α = 0.05, (1 − β) = 0.95] to detect the changes in laxities above. A post hoc power analysis using the standard deviations from all ten specimens confirmed that a power of at least 0.97 was achieved for all laxities measured.
To determine the change in tibial force difference caused by 2° and 4° of V–V malalignment of the femoral component, a simple linear regression was performed which related the mean change in tibial force difference to the degree of V–V malalignment at the flexion angle where the effect of V–V malalignment was the greatest. The regression was performed separately for varus malalignments and valgus malalignments as each is likely to affect the medial and lateral structures differently due to the differences in stiffness of the soft tissue restraints [8, 23, 60].
To determine whether V–V malalignment of the femoral component in kinematically aligned TKA caused statistically significant changes in laxities, a two-factor repeated measures ANOVA was performed for each laxity. The two factors were femoral component malalignment at five levels (2° varus, 4° varus, 2° valgus, 4° valgus, and 0° reference) and flexion angle at five levels (0° to 120° in 30° increments). Tukey’s test was used to compare the means of each of eight laxities using each of four increments of malalignment to those of the reference component. The level of significance, α, was set at 0.05.
Results
Varus and valgus malalignments caused significant increases in tibial force imbalance which were greatest at 0° flexion where the increase for varus malalignments was due to the medial tibial force increasing relative to the lateral tibial force and the increase for valgus malalignments was due to the lateral tibial force increasing relative to the medial tibial force (Fig. 3). The simple linear regressions between the average increase in tibial force imbalance (i.e., magnitude of tibial force difference) at 0° flexion and degree of V–V malalignment indicated that the average increases lie nearly on a straight line (R2 = 1) with sensitivities (i.e., slopes) of 54 N/deg (p = 0.0024) and 44 N/deg (p = 0.0077) for varus and valgus malalignments, respectively (Fig. 4).
Bar graph showing the mean (bar) and standard deviation (error bar) of the change in tibial force difference for each of four malaligned femoral components as a function of flexion angle. Tibial force difference was computed as medial tibial force minus lateral tibial force. The tibial force imbalance was the magnitude of the difference
Column graph showing the mean (bars), standard deviation (error bars), and simple linear regressions of change in tibial force difference of each malaligned femoral component from the reference component at 0° of flexion. The tibial force difference was computed as the medial tibial force minus the lateral tibial force and the tibial force imbalance was the magnitude of the difference. Regressions were done separately for valgus malalignments and varus malalignments. Regressions were forced through zero for the reference component. A linear fit though the mean changes in tibial force differences yielded strong relationships (R2 = 1.0 for both)
There were no statistically significant changes in seven of the eight laxities between the malaligned femoral components and the reference femoral component (Figs. 5, 6, 7, 8). The only statistically significant change in the laxities was in valgus laxity at 30° flexion (1.0° ± 0.4°, p = 0.0014).
Bar graphs showing the mean (bar) and standard deviation (error bar) of the a change in the varus laxity of each malaligned femoral component from the reference femoral component, and b change in the valgus laxity of each malaligned femoral component from the reference femoral component. Statistically significant differences based on Tukey’s test (p < 0.05) are marked with an asterisk. The only statistically significant change was an increase in valgus laxity of 1.0° at 30° flexion for the 4° malaligned varus femoral component
Bar graphs showing the mean (bar) and standard deviation (error bar) of the a change in the internal axial rotation laxity of each malaligned femoral component from the reference femoral component, and b change in the external axial rotation laxity of each malaligned femoral component from the reference femoral component. There were no statistically significant differences between any of the malaligned femoral components and the reference femoral component
Bar graphs showing the mean (bar) and standard deviation (error bar) of the a change in the anterior laxity of each malaligned femoral component from the reference femoral component, and b change in the posterior laxity of each malaligned femoral component from the reference femoral component. There were no statistically significant differences between any of the malaligned femoral components and the reference femoral component
Bar graphs showing the mean (bar) and standard deviation (error bar) of the a change in the compression laxity of each malaligned femoral component from the reference femoral component, and b change in the distraction laxity of each malaligned femoral component from the reference femoral component. There were no statistically significant differences between any of the malaligned femoral components and the reference femoral component
Discussion
One key finding is that V–V malalignment of the femoral component caused statistically significant increases in tibial force imbalance which were most pronounced at 0° flexion. The relationship between tibial force imbalance and patient-reported outcomes has been studied recently for mechanically aligned TKA. One study reported that patients with a tibial force imbalance less than 67 N at 10°, 45°, and 90° had better patient-reported outcome scores [22]. A second study found that tibial force imbalance (i.e., medial > lateral) greater than 45 N at extension was associated with significantly better patient-reported outcome scores [30]. A final study found that greater increase in activity level was associated with tibial force imbalance less than 67 N when averaged at 0°, 45°, and 90° [40]. Assuming that tibial force imbalance is related to patient-reported outcomes in kinematically aligned TKA, surgeons performing kinematically aligned TKA should strive to minimize V–V malalignment of the femoral component to limit tibial force imbalance.
During kinematically aligned TKA, the intraoperative check to verify the V–V alignment of the femoral component is to use calipers to measure the thicknesses of the two distal condylar bone resections [28, 46]. Ideally, the thicknesses of the bone resections should match the thicknesses of the corresponding regions on the femoral component after accounting for cartilage wear and kerf of the saw blade [28, 45]. By measuring the thicknesses of the bone resections with a caliper (Zimmer Biomet, 1 mm increments, 0.5 mm resolution) and comparing that measurement to the thicknesses of the corresponding regions of the femoral component, a difference of 0.5 mm using kinematically aligned TKA with manual instruments is achievable. Using the 54 N/deg slope of the regression line for varus malalignment (which is larger than that for valgus malalignment hence conservative) and recognizing that 0.8 mm added and subtracted from opposite distal condyles of the femoral component produces 2° of V–V malalignment, a difference of 0.5 mm on each distal femoral condyle limits the increase in tibial force imbalance to 68 N on average. Limiting the error to 0.5 mm for the thicknesses of the distal femoral resections in kinematically aligned TKA is critical; if the error was to exceed 0.5 mm and was not corrected, then any adjustment would need to be made in the V–V angle of the tibial component which would result in malalignment of this component as well as the femoral component.
A second key finding is that 2° or 4° of V–V malalignment generally did not cause statistically significant changes in laxities. Of the four degrees of freedom tested (V–V, I–E, A–P, and C–D), arguably the V–V laxity is most clinically relevant since soft tissue balancing addresses primarily this degree of freedom [2, 3, 7, 21, 48, 49, 63]. As expected, varus malalignment caused an increase in valgus laxity (Fig. 5) because material was removed on the medial distal femoral condyle (Fig. 2) and a decrease in varus laxity (Fig. 5) because material was added on the lateral distal femoral condyle (Fig. 2). In contrast, valgus malalignment caused an increase in varus laxity and a decrease in valgus laxity. Significant effects on V–V laxity at 60° and beyond were not expected and were not observed because the posterior surfaces of the femoral condyles come into play and these surfaces were not malaligned.
Comparing the findings reported herein to those of previous studies is difficult because those studies that have investigated V–V malalignment of the femoral component have been computational involving weight bearing. One study used finite element analysis to determine polyethylene stresses under a 3000 N compressive load at 0° flexion while varying the varus angle of the femoral component [36]. Three other studies used whole body models to investigate the effect of V–V malalignments during gait [11, 34, 55]. Consistent with the findings of the present study, these studies reported that V–V malalignment of the femoral component caused significant changes in the tibial force imbalance.
Several methodological issues should be discussed because of their potential to affect the results. One issue concerns the coefficient of friction of the femoral components on the ultra-high molecular weight polyethylene (UHMWPE) tibial liner. The 3D-printed femoral components were printed using an acrylic-like plastic, whereas femoral components used in TKA are typically made of cobalt–chrome. The difference in the coefficients of friction could affect the laxities. However, any effect was systematic and would likely not change our findings because differences from the reference component were of interest. Additionally, each femoral component was wet sanded with super fine (1000-grit) sandpaper and a thin film of bovine serum was applied as a lubricant before testing. A pilot study showed that the static coefficient of friction of a lubricated 3D-printed femoral component on UHMWPE (µ = 0.18) was close to that of the cobalt–chrome femoral component on UHMWPE (µ = 0.14).
A second methodological issue concerns the forces applied to the muscles. Using muscle forces with different magnitudes would likely change the tibial force imbalance and laxities. Because of this, the muscles forces were kept as small as possible while still maintaining stability of the knee. This allowed the soft tissue restraints to have as much relative contribution to knee stability as possible. The muscle forces used in the present study were small relative to muscle forces used in previously published in vitro studies [12, 17, 29, 33, 35, 56, 58, 60]. Moreover, because changes (i.e., differences) in tibial forces and laxities were of interest, the effect of muscle forces was systematic and negated in computing differences. Accordingly, the use of small muscle forces in conjunction with analysis of differences minimized the effect of muscle forces on results.
Also, the use of small load limits might have affected the changes in laxities. The load limits of ± 5 N m for V–V moment [38], ± 3 N m for I–E torque [8], ± 45 N for A–P force [15], and ± 100 N for C–D force confined the laxity measurements to the low stiffness region of the load–displacement curve [15]. Confining the laxity measurements to the low stiffness region was done purposely because any instability as a result of increasing laxity should manifest in the low stiffness regions.
The V–V malalignments were created by rotating about the center of the reference femoral component. Creating V–V malalignments by rotating about the peripheral edge of one condyle of the femoral component instead of the center could affect the results. However rotating about the center of the reference femoral component isolated the V–V angular error as an independent variable for study. Rotating about the peripheral edge would have introduced not only V–V angular error, but also a proximal–distal translation error.
Because the number of specimens tested from males far outweighed that number tested for females (9 versus 1), the quantitative results may not apply to females. Although there are anatomic differences between males and females primarily in size and intercondylar width [10, 19, 57], each specimen was treated as its own control in which case the results would not be expected to differ fundamentally. However, the specific values might be affected in which case the slopes of regression lines might differ.
A final methodologic issue, which is intrinsic in any study that malaligns the femoral component and determines the effects on tibiofemoral laxities and tibial forces, is that the effects do not apply when the alignment of the tibial component is different from that in the study. Hence the results and their interpretation in the present study apply only when the tibial component is kinematically aligned. The requirements and corresponding procedures for kinematically aligning the tibial component were described earlier in the “Methods”.
The clinical relevance of our results is that, with the tibial component kinematically aligned, V–V malalignment of the femoral component in kinematically aligned TKA should be avoided to limit increases in tibial force imbalance. High tibial force imbalance can be effectively prevented by measuring the thicknesses of the distal femoral resections with a caliper and insuring that these thicknesses are within 0.5 mm of the corresponding thickness of the distal regions of the femoral component. Because laxities were largely unaffected, instability resulting from V–V malalignment of the femoral component within the ± 4° range studied is not a clinical concern.
Conclusion
Based on the slope for the regression line for varus malalignments which was greater than that for valgus malalignments, the increase in imbalance can be effectively limited to 68 N by keeping differences in resection thickness to within ± 0.5 mm of the thickness of the respective distal region of the femoral component. Additionally, of eight increases in laxities only the increase in valgus laxity for the 4° varus malalignment at 30° flexion was statistically significant but this increase was limited to 1° which is not of clinical concern.
References
Anouchi YS, Whiteside LA, Kaiser AD, Milliano MT (1993) The effects of axial rotational alignment of the femoral component on knee stability and patellar tracking in total knee arthroplasty demonstrated on autopsy specimens. Clin Orthop Relat Res 287:170–177
Athwal KK, Hunt NC, Davies AJ, Deehan DJ, Amis AA (2014) Clinical biomechanics of instability related to total knee arthroplasty. Clin Biomech 29(2):119–128
Babazadeh S, Stoney JD, Lim K, Choong PFM (2009) The relevance of ligament balancing in total knee arthroplasty: how important is it? A systematic review of the literature. Orthop Rev 1(2):70–78
Bach JM, Hull ML (1995) A new load application system for in vitro study of ligamentous injuries to the human knee joint. J Biomech Eng 117(4):373–382
Bach JM, Hull ML, Patterson HA (1997) Direct measurement of strain in the posterolateral bundle of the anterior cruciate ligament. J Biomech 30(3):281–283
Bäthis H, Perlick L, Tingart M, Perlick C, Lüring C, Grifka J (2005) Intraoperative cutting errors in total knee arthroplasty. Arch Orthop Trauma Surg 125(1):16–20
Bellemans J, Vandenneucker H, Van Lauwe J, Victor J (2010) A new surgical technique for medial collateral ligament balancing: multiple needle puncturing. J Arthroplasty 25(7):1151–1156
Blankevoort L, Huiskes R, De Lange A (1988) The envelope of passive knee joint motion. J Biomech 21(9):705–709, 711–720
Brar AS, Howell SM, Hull ML (2016) What are the bias, imprecision, and limits of agreement for finding the flexion-extension plane of the knee with five tibial reference lines? Knee 23(3):406–411
Charlton WP, St John TA, Ciccotti MG, Harrison N, Schweitzer M (2002) Differences in femoral notch anatomy between men and women: a magnetic resonance imaging study. Am J Sports Med 30(3):329–333
Chen Z, Wang L, Liu Y, He J, Lian Q, Li D, Jin Z (2015) Effect of component mal-rotation on knee loading in total knee arthroplasty using multi-body dynamics modeling under a simulated walking gait. J Orthop Res 33(9):1287–1296
Churchill DL, Incavo SJ, Johnson CC, Beynnon BD (2001) The influence of femoral rollback on patellofemoral contact loads in total knee arthroplasty. J Arthroplasty 16(7):909–918
Creaby MW, Wrigley TV, Lim BW, Hinman RS, Bryant AL, Bennell KL (2013) Self-reported knee joint instability is related to passive mechanical stiffness in medial knee osteoarthritis. BMC Musculoskelet Disord 14(1):326
Dalury DF, Pomeroy DL, Gorab RS, Adams MJ (2013) Why are total knee arthroplasties being revised? J Arthroplast 28(8):120–121
Eagar P, Hull ML, Howell SM (2001) A method for quantifying the anterior load–displacement behavior of the human knee in both the low and high stiffness regions. J Biomech 34(12):1655–1660
Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M (2001) Early failures in total knee arthroplasty. Clin Orthop Relat Res 392:315–318
Ghosh K, Blain A, Longstaff L, Rushton S, Amis A, Deehan D (2014) Can we define envelope of laxity during navigated knee arthroplasty? Knee Surg Sports Traumatol Arthrosc 22(8):1736–1743
Ghosh KM, Merican AM, Iranpour F, Deehan DJ, Amis AA (2010) The effect of femoral component rotation on the extensor retinaculum of the knee. J Orthop Res 28(9):1136–1141
Gillespie RJ, Levine A, Fitzgerald SJ, Kolaczko J, DeMaio M, Marcus RE, Cooperman DR (2011) Gender differences in the anatomy of the distal femur. J Bone Jt Surg Br 93(3):357–363
Grood ES, Suntay WJ (1983) A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng 105(2):136–144
Gu Y, Roth JD, Howell SM, Hull ML (2014) How frequently do four methods for mechanically aligning a total knee arthroplasty cause collateral ligament imbalance and change alignment from normal in white patients? J Bone Jt Surg Am 96-A(12):e101(101)–e101(109)
Gustke KA, Golladay GJ, Roche MW, Elson LC, Anderson CR (2014) A new method for defining balance: promising short-term clinical outcomes of sensor-guided TKA. J Arthroplast 29(5):955–960
Heesterbeek PJC, Verdonschot N, Wymenga AB (2008) In vivo knee laxity in flexion and extension: a radiographic study in 30 older healthy subjects. Knee 15(1):45–49
Hohmann E, Tetsworth K (2016) Do manual cutting guides for total knee arthroplasty introduce systematic error? Int Orthop 40(2):277–284
Hollister AM, Jatana S, Singh AK, Sullivan WW, Lupichuk AG (1993) The axes of rotation of the knee. Clin Orthop Relat Res 290:259–268
Howell SM, Chen J, Hull ML (2013) Variability of the location of the tibial tubercle affects the rotational alignment of the tibial component in kinematically aligned total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 21(10):2288–2295
Howell SM, Hull ML (2016) Kinematic Alignment in Total Knee Arthroplasty. In: Scott S (ed) Insall and Scott surgery of the knee, 6th edn. Elsevier, Philadelphia
Howell SM, Papadopoulos S, Kuznik KT, Hull ML (2013) Accurate alignment and high function after kinematically aligned TKA performed with generic instruments. Knee Surg Sports Traumatol Arthrosc 21(10):2271–2280
Hunt NC, Ghosh KM, Blain AP, Athwal KK, Rushton SP, Amis AA, Longstaff LM, Deehan DJ (2014) How does laxity after single radius total knee arthroplasty compare with the native knee? J Orthop Res 32(9):1208–1213
Jacobs CA, Christensen CP, Karthikeyan T (2016) Greater medial compartment forces during total knee arthroplasty associated with improved patient satisfaction and ability to navigate stairs. J Arthroplast 31(9 Suppl):87–90
Kim TK, Chang CB, Kang YG, Chung BJ, Cho HJ, Seong SC (2010) Execution accuracy of bone resection and implant fixation in computer assisted minimally invasive total knee arthroplasty. Knee 17(1):23–28
Kretzer JP, Jakubowitz E, Sonntag R, Hofmann K, Heisel C, Thomsen M (2010) Effect of joint laxity on polyethylene wear in total knee replacement. J Biomech 43(6):1092–1096
Kwak SD, Ahmad CS, Gardner TR, Grelsamer RP, Henry JH, Blankevoort L, Ateshian GA, Mow VC (2000) Hamstrings and iliotibial band forces affect knee kinematics and contact pattern. J Orthop Res 18(1):101–108
Lerner ZF, DeMers MS, Delp SL, Browning RC (2015) How tibiofemoral alignment and contact locations affect predictions of medial and lateral tibiofemoral contact forces. J Biomech 48(4):644–650
Li G, Rudy TW, Sakane M, Kanamori A, Ma CB, Woo SL (1999) The importance of quadriceps and hamstring muscle loading on knee kinematics and in-situ forces in the ACL. J Biomech 32(4):395–400
Liau JJ, Cheng CK, Huang CH, Lo WH (2002) The effect of malalignment on stresses in polyethylene component of total knee prostheses—a finite element analysis. Clin Biomech (Bristol Avon) 17(2):140–146
Markolf KL, Gorek JF, Kabo JM, Shapiro MS (1990) Direct measurement of resultant forces in the anterior cruciate ligament. An in vitro study performed with a new experimental technique. J Bone Jt Surg Am 72-A(4):557–567
Markolf KL, Mensch JS, Amstutz HC (1976) Stiffness and laxity of the knee—the contributions of the supporting structures. J Bone Jt Surg Am 58-A(5):583–594
Mayman D, Plaskos C, Kendoff D, Wernecke G, Pearle AD, Laskin R (2009) Ligament tension in the acl-deficient knee: assessment of medial and lateral gaps. Clin Orthop Relat Res 467(6):1621–1628
Meneghini RM, Ziemba-Davis MM, Lovro LR, Ireland PH, Damer BM (2016) Can intraoperative sensors determine the “target” ligament balance? early outcomes in total knee arthroplasty. J Arthroplast 31(10):2181–2187
Merican AM, Ghosh KM, Deehan DJ, Amis AA (2009) The transpatellar approach for the knee in the laboratory. J Orthop Res 27(3):330–334
Miller MC, Berger RA, Petrella AJ, Karmas A, Rubash HE (2001) Optimizing femoral component rotation in total knee arthroplasty. Clin Orthop Relat Res 392:38–45
Mont MA, Urquhart MA, Hungerford DS, Krackow KA (1997) Intramedullary goniometer can improve alignment in knee arthroplasty surgery. J Arthroplast 12(3):332–336
Mueller JK, Wentorf FA, Moore RE (2014) Femoral and tibial insert downsizing increases the laxity envelope in TKA. Knee Surg Sports Traumatol Arthrosc 22(12):3003–3011
Nam D, Lin KM, Howell SM, Hull ML (2014) Femoral bone and cartilage wear is predictable at 0 degrees and 90 degrees in the osteoarthritic knee treated with total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 22(12):2975–2981
Nedopil AJ, Howell SM, Hull ML (2016) Does malrotation of the tibial and femoral components compromise function in kinematically aligned total knee arthroplasty? Orthop Clin N Am 47(1):41–50
Otani T, Whiteside LA, White SE (1993) Cutting errors in preparation of femoral components in total knee arthroplasty. J Arthroplast 8(5):503–510
Roche M, Elson L, Anderson C (2014) Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin N Am 45(2):157–165
Romero J, Duronio JF, Sohrabi A, Alexander N, MacWilliams BA, Jones LC, Hungerford DS (2002) Varus and valgus flexion laxity of total knee alignment methods in loaded cadaveric knees. Clin Orthop Relat Res 394:243–253
Roth JD (2016) How Well Does Kinematically Aligned Total Knee Arthroplasty Prevent Clinically Important Changes in Passive Knee Function? An In Vitro Biomechanical Study of Tibiofemoral Laxities and Contact. Ph.D. Dissertation in Biomedical Engineering, University of California, Davis
Roth JD, Howell SM, Hull ML (2015) Native knee laxities at 0°, 45°, and 90° of flexion and their felationship to the goal of the gap-balancing alignment method of total knee arthroplasty. J Bone Jt Surg Am 97-A(20):1678–1684
Roth JD, Howell SM, Hull ML (2017) An improved tibial force sensor to compute contact force and contact location in vitro after total knee arthroplasty. J Biomech Eng (In press)
Roth JD, Hull ML, Howell SM (2015) The limits of passive motion are variable between and unrelated within normal tibiofemoral joints. J Orthop Res 33(11):1594–1602
Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM (2002) Why are total knee arthroplasties failing today? Clin Orthop Relat Res 404(404):7–13
Smith CR, Vignos MF, Lenhart RL, Kaiser J, Thelen DG (2016) The influence of component alignment and ligament properties on tibiofemoral contact forces in total knee replacement. J Biomech Eng 138(2):021017
Stoddard JE, Deehan DJ, Bull AM, McCaskie AW, Amis AA (2013) The kinematics and stability of single-radius versus multi-radius femoral components related to mid-range instability after TKA. J Orthop Res 31(1):53–58
Terzidis I, Totlis T, Papathanasiou E, Sideridis A, Vlasis K, Natsis K (2012) Gender and side-to-side differences of femoral condyles morphology: osteometric data from 360 caucasian dried femori. Anat Res Int 2012, Article ID 679658, 1–6.
Victor J, Labey L, Wong P, Innocenti B, Bellemans J (2010) The influence of muscle load on tibiofemoral knee kinematics. J Orthop Res 28(4):419–428
Walker PS, Meere PA, Bell CP (2014) Effects of surgical variables in balancing of total knee replacements using an instrumented tibial trial. Knee 21(1):156–161
Wang XN, Malik A, Bartel DL, Wickiewicz TL, Wright T (2014) Asymmetric varus and valgus stability of the anatomic cadaver knee and the load sharing between collateral ligaments and bearing surfaces. J Biomech Eng 136(8):081005(081001)–081005(081006)
Ward SR, Eng CM, Smallwood LH, Lieber RL (2009) Are current measurements of lower extremity muscle architecture accurate? Clin Orthop Relat Res 467(4):1074–1082
Werner FW, Ayers DC, Maletsky LP, Rullkoetter PJ (2005) The effect of valgus/varus malalignment on load distribution in total knee replacements. J Biomech 38(2):349–355
Whiteside LA, Kasselt MR, Haynes DW (1987) Varus–valgus and rotational stability in rotationally unconstrained total knee arthroplasty. Clin Orthop Relat Res 219:147–157
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
J.D. Roth has a postdoctoral fellowship from THINK Surgical, S.M. Howell is a paid consultant for THINK Surgical and Medacta and receives royalties from Zimmer-Biomet. M.L. Hull receives research funding from Zimmer-Biomet.
Funding
The authors acknowledge the support of the National Science Foundation (Grant no. CBET-1067527) and support of Zimmer (Award no. CW88095).
Ethical approval
The cadaveric specimens were obtained through the UC Davis Donated Body Program and were approved for use by this program. The authors would like to thank individuals who donate their remains and tissues for the advancement of education and research.
Rights and permissions
About this article
Cite this article
Riley, J., Roth, J., Howell, S.M. et al. Increases in tibial force imbalance but not changes in tibiofemoral laxities are caused by varus–valgus malalignment of the femoral component in kinematically aligned TKA. Knee Surg Sports Traumatol Arthrosc 26, 3238–3248 (2018). https://doi.org/10.1007/s00167-018-4841-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-018-4841-6