Abstract
Obtaining symmetric and balanced gaps under equilateral loads is a common goal in posterior cruciate ligament (PCL)-retaining and -sacrificing TKAs. Owing to limitations in existing surgical tensors, however, tensing knee ligaments with standardized and symmetric loads has been possible only with the patella subluxated or everted. We therefore determined the influences of (1) patellar eversion versus complete reduction, (2) PCL resection, and (3) load magnitude on gap symmetry and balance in the anterior cruciate ligament (ACL)-deficient knee. We used a novel computer-controlled tensioner to measure gaps in 10 cadavers with an applied force of 50 N, 75 N, and 100 N per side. Gap data were acquired at 0º, 30º, 60º, 90º, and 120º flexion with the patella reduced and everted and with the PCL intact and resected. Everting the patella tightened the medial and lateral flexion gaps between 90º and 120º by 0.7 mm to 2.7 mm. PCL resection increased gaps from 30° to 120° by 1 mm to 3 mm. Increasing the force from 50 N to 100 N increased the mean gap by 0.5 mm. Everting the patella and resecting the PCL influenced gap balance and symmetry. Surgeons should be aware of how these conditions affect gaps during assessment and balancing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The operative goal of tissue balancing in TKA has been defined classically by equal gaps medially and laterally (symmetry) and in flexion and extension (balance) [12, 15, 17, 19]. Although this principle generally is well established in TKA, the clinical process often is considered a subjective intraoperative measurement technique judged primarily by the surgeon’s feel [16, 17]. Assessment of the ligament tension and the overall soft tissue balance requires surgical experience, which can be unreliable owing to lack of standardized, quantitative measurement techniques and tools [12, 33].
Various forms of ligament-tensioning devices have been introduced to help control the applied stress when planning and balancing gaps in TKA. Spacer blocks [28] and mechanical tensioning devices [21] typically are used to improve reproducibility of the measurements; however, even very experienced surgeons have had difficulties in obtaining perfectly balanced TKAs with these mechanical tools [3, 33]. Most tensioning and force-sensing devices necessitate either complete eversion or subluxation of the patella to be inserted in the knee [8, 9]. However, everting the patella during surgery influences leg alignment [21] and the load distribution [10] across the knee, suggesting gap assessment should be performed with the patella in situ [9]. Other devices that allow for a reduced patella and even partially closed soft tissues [29, 33] are not capable of actively applying forces to the femorotibial joint or to the medial and lateral compartments independently [9]. Computer navigation has enabled accurate intraoperative measurement of gap kinematics, ie, quasistatic gap heights for the complete arc of knee motion, and often is used to control ligament balancing [5, 6]. Yet ligament tensioning usually is assessed qualitatively with manual trials [12] or mechanical tools [6, 29]. To improve ligament balancing, force-sensing devices have been introduced and tested clinically, although intraoperative stressing still is applied manually [9, 10, 33]. None of the described devices that realistically and easily can be applied clinically are capable of quantifying gap kinematics with independent force application in the medial and lateral compartments with a reduced patella and closed extensor mechanism.
A question arises regarding whether medial and lateral gaps in the open knee where the patella is everted are similar to those in the closed knee where the patella is reduced [9, 21]. A difference in these two conditions would suggest the gaps we aim to achieve intraoperatively in the open knee may change once the knee is closed, resulting in a postoperatively tighter or looser knee than planned. Investigating these relationships in PCL-retaining and PCL-substituting TKAs [13, 25] at 90° flexion [1, 19] and over a range of motion (ROM) [11, 32] and applied loads [1, 8, 24, 29] would be relevant for a wide range of TKA surgical techniques and philosophies. To quantify gaps under these different conditions, we used a novel computer-integrated tensioning tool that can apply loads independently in the medial and lateral sides of the knee in the open and closed states.
Our specific research questions were: (1) Does everting the patella decrease the medial and lateral gaps in flexion? (2) Does PCL resection increase the flexion gap equally in the patella-everted and in the patella-reduced knee? (3) Are the gaps equal and symmetric when the knee is loaded and passively flexed through a ROM? (4) Do the gaps increase uniformly with increasing load?
Materials and Methods
We obtained 10 intact cadaveric lower limbs (nine males and one female) for this study. The average age of the specimens was 71.5 years (range, 60–89 years). To evaluate gap symmetry, balance, and laxity through a ROM in each knee, we used a novel computerized ligament-tensioning and force-sensing device that was designed for TKA and allowed standardized application of loads (in Newtons) and measurement of gap heights (in millimeters) on the medial and lateral sides independently with either the patella completely reduced or everted and with the PCL intact or resected. The setup also permitted control of the amount of load applied, allowing us to measure the influence of the magnitude of force on the gaps. Finally, the setup permitted these measurements to be made at any angle of knee flexion, which was monitored by the system (in degrees). We tested 60 different conditions of varying force, flexion, and surgical scenarios: 50 N, 75 N, and 100 N of force applied independently to the medial and lateral compartments (ie, 100 N, 150 N, and 200 N) in full extension; in 30°, 60°, 90°, and 120° flexion; and with the (1) patella reduced and PCL intact, (2) patella everted and PCL intact, (3) patella reduced and PCL excised, and (4) patella everted and PCL excised. We examined eight right knees and two left knees. None of the specimens had a history of any knee disorder. All specimens were fresh-frozen and thawed to room temperature for at least 12 hours before use. We clamped the specimens to the table in a standardized position with the femoral axis parallel to the floor.
We performed a standard midline skin incision. A medial parapatellar approach was used in all cases. The deep incision was carried up approximately 5 cm proximal from the proximal tip of the patella. We everted the patella and the fat pad was excised. The ACL was excised.
Femoral and tibial reference arrays were fixed rigidly in the diaphysis with two 4-mm-diameter Schanz pins per array (Fig. 1) for the Praxim computer navigation system (Praxim Inc, Santa Clara, CA). We registered the three-dimensional anatomy of the distal femur and proximal tibia using the image-free bone morphing protocol of the Praxim software. Reference points on the medial and lateral tibial plateaus also were digitized.
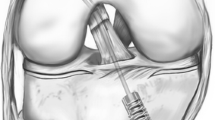
The tibial cut was planned to remove approximately 10 mm of bone from the medial plateau and 10 mm of bone from the lateral plateau and navigated with a conventional tibial jig (Smith and Nephew, Inc, Memphis, TN). This gave us an average posterior slope of 3° and an average of 3° varus when compared with the anatomic axis of the tibia when the resection was checked with the navigation system. The tibial cut was made to make space for the tensioner in the knee. The measurement system, however, reported the gap between the uncut femoral bone and the deepest point digitized on each uncut tibial plateau (as described in more detail subsequently) (Fig. 2). At this point, a combined intraarticular tensioning and force-sensing device was inserted into the knee (Fig. 3).
Gap kinematics were acquired on the medial and lateral sides of the knee with the computer navigation system. (A) Gap heights (black vertical lines) were calculated dynamically as a function of the flexion angle; they are calculated as the distance between the digitized center of the tibial plateau and the nearest point on the femoral condyle, measured normal to the tibial cut (dotted line). An equal amount of bone (approximately 10 mm) was removed on the medial and lateral sides of the tibia to allow sufficient space for the tensor on either side. (B) Gaps were acquired at 0°, 30°, 60°, 90°, and 120° flexion.
The ligament-tensioning and force-sensing device (NanoSpacer®; Praxim Inc) was designed to be inserted in the knee after the tibial cut is made but before any of the femoral cuts are performed [23]. The system is comprised of two independent (medial and lateral) fluid-filled bladders (Fig. 3) that are sandwiched between two parallel plates and are connected to a pressure-monitoring and motion control box. The control box is connected to the Praxim navigation station, which allows digital setting and automatic control of the amount of applied force in Newtons exerted by each bladder. We applied forces of 50 N, 75 N, and 100 N per medial and lateral side. These were chosen based on the range of force typically applied during TKA [24, 29] and the pressure limit of the device. We measured gap heights with the navigation system by computing the smallest distance between the deepest point digitized on the tibial joint surface and the femoral condyle (in the direction normal to the tibial cut) (Fig. 3). Each gap measurement was repeated three times and averaged for additional analysis.
Independent tensioning of the medial and lateral compartments without external handles or grips allowed for complete reduction of the patella and controlled joint loading and gap measurement throughout the full ROM of the knee.
The first scenario was patella reduced (sutured) and PCL intact (Fig. 4A). This was followed by the patella everted and PCL intact (Fig. 4B). The PCL then was excised and the procedure was repeated. The knee was tested with the patella reduced and the PCL excised and the patella everted and the PCL excised. In one specimen, the PCL was resected while performing the tibial cut, and in four specimens, it was not possible to flex the leg to 120°.
We used a generalized linear model to determine differences in gap heights for each side, flexion angle, and scenario with the remaining scenarios, sides, flexion angles, and/or forces included as covariables. An independent covariance matrix was assumed in the generalized estimating equation. The statistical analysis was performed using the SAS® System for Windows, Version 9.1 (SAS Institute Inc, Cary, NC).
Results
Everting the patella tightened the medial and lateral gaps in flexion (Figs. 5, 6). When the patella was everted at 90° and 120° flexion in the PCL-intact knee (Fig. 5A–B), the medial gap decreased by 1.0 mm (p < 0.0001) and by 2.5 mm (p = 0.0001), respectively, and the lateral gap decreased by 0.7 mm (p = 0.0011) and 1.7 mm (p = 0.0203), respectively (Table 1). When the patella was everted at 90° and 120° flexion in the PCL-resected knee (Fig. 5C–D), there was a mean decrease in the medial gap of 0.9 mm (p < 0.0001) and 2.7 mm (p < 0.0001), respectively, and in the lateral gap of 1.1 mm (p < 0.0001) and 3 mm (p < 0.0001), respectively. Everting the patella in extension did not have a major effect on the gaps (0.1–0.3 mm, p = 0.041 to p = 0.212).
The mean medial and lateral gap kinematics as a function of the flexion angle for all scenarios are shown: [(A) patella reduced, PCL intact; (B) patella everted, PCL intact; (C) patella reduced, PCL resected; (D) patella everted, PCL resected] and forces (50 N, 75 N, 100 N) tested (n = 10). We observed differences in the medial and lateral gap heights between the patella-reduced and -everted scenarios at 60°, 90°, and 120° flexion (p < 0.0001 to p = 0.0203) and between the PCL-intact and -resected scenarios at 30°, 60°, 90°, and 120° flexion (p < 0.0001 to p = 0.0028).
The mean medial and lateral gap heights averaged over 50 N, 75 N, and 100 N of force for all 10 cadavers at 90° flexion are shown. Differences were found in the gap heights between the patella-reduced and -everted scenarios (p < 0.0001 to p = 0.0011), between the PCL-intact and -resected scenarios (p < 0.0001), and between the medial and lateral sides (p < 0.0001) at 90° flexion. Error bars indicate ± 1 standard deviation.
Resecting the PCL resulted in larger (p < 0.0001 for all conditions) gap heights from 30° to 120° flexion by approximately 1 mm to 3 mm for both sides (Fig. 5, A versus C, B versus D). At 90° flexion, when the PCL was resected in the patella-reduced knee, there was a mean gap increase (p < 0.0001) of 2.1 mm and 1.6 mm on the medial and lateral sides, respectively (Table 1). When the PCL was resected in the patella-everted knee, there were mean gap increases of 2.1 mm and 1.2 mm on the medial (p < 0.0001) and lateral sides (p = 0.0028) at 90° flexion, respectively (Fig. 6). When the knee was flexed from 30° to 90° and the PCL was intact (Fig. 5A–B), there were reductions (p < 0.0001) in the medial compartment gaps by 1 mm (patella reduced) and by 2 mm (patella everted) in comparison to the PCL-resected knee.
The medial gap was consistently smaller (p < 0.0001) than the lateral gap at 90° flexion for all scenarios and forces tested, indicating more laxity on the lateral side of the knee in flexion (Fig. 6). Looking at the variation in the gaps from extension to flexion (Fig. 5), the medial and lateral compartments were larger (p < 0.0001 to p = 0.0012) at 30°, 60°, 90°, and 120° flexion than in 0° extension for all conditions. The amount of increase ranged from approximately 2 mm to 6 mm and was dependent on the scenario. Both sides increased (p < 0.0001) by approximately 4 mm when the leg was brought from extension into 30° flexion for all scenarios. In the PCL-intact knee, the PCL decreased (p < 0.0001) the medial compartment gap by 1 mm to 2 mm when the knee was flexed from 30° to 90° as described above. As the knee was flexed from 90° to 120°, the mean medial gap increased (p < 0.0001), whereas the mean lateral gap decreased (p < 0.0001 to p = 0.0012) for all cases.
The change in gap height with increasing load was generally similar for all flexion angles, scenarios, and sides, with an increase of 0.25 ± 0.1 mm (mean ± standard deviation) per 25-N increase in force.
Discussion
Ligament balancing and gap assessment are crucial intraoperative steps for achieving joint stability. Although manual trials and basic mechanical tools have been implemented to qualitatively assess the balancing procedure, most of these tools do not yet allow application of symmetric load to the joint with reduced soft tissue. Consequently, evaluation of gap kinematics with reduced soft tissue, active control of applied tension, and simultaneous force sensing has not been established. The purpose of this study was to use a novel TKA tensioning device to determine what effect everting the patella and resecting the PCL had on the medial and lateral joint spaces of the ACL-deficient knee. We also sought to quantify how the joint space varies from side to side when the knee is loaded and passively flexed and how the gaps varied over a range of applied load.
Although this study has provided new information about ligament balance in the ACL-deficient knee and the effects of some commonly performed techniques, it does have some limitations. We did not study arthritic knee conditions, which might alter the results, especially regarding ligament status. Data obtained with fresh-frozen specimens might not be easily transferable to intraoperative conditions. We believe our results are nevertheless of interest because they provide a baseline for comparison with these other conditions. In addition, only one knee tested was from a female cadaver, whereas the other nine were from male cadavers. Although this was a limitation of the specimens available to us, we do not expect this affected our results, as a published report suggests there are no significant differences in the biomechanical properties of the soft tissue envelope for male and female knees [35]. In addition, the gap height data of the female patient were similar to the male data.
Our data suggest an everted patella affects the medial and lateral gaps in flexion. We believe reducing the patella more realistically reproduces in vivo conditions, whereas an everted position might also affect physiologic tibial rotation and translation, and overall leg alignment. Lüring et al. [21] reported eversion of the patella, compared with subluxation, affects the mean overall limb alignment by as much as 2.9° valgus and as much as 3.0° flexion, although they were not able to investigate the patella completely reduced scenario using their tensioning device. Using a knee-loading apparatus and a passive force- and moment-monitoring device, Crottet et al. [9] reported patella eversion shifts distribution of the contact force laterally and may bias knee stability tests. To our knowledge, their cadaver study was the first to allow for reduced soft tissue with a force-sensing device designed for TKA. A limitation of passive devices, however, is load application can be performed only manually by stressing the leg, making precise load application difficult. Furthermore, height augmentation must be realized with manual shims, which adds time and complexity [9, 10, 28].
Resecting the PCL in general led to an increase in gap height with flexion, as expected from previous reports [4, 18, 25]. PCL resection caused the tibia to drop with respect to the femur in flexion, opening up the medial and lateral compartments and generally loosening the knee.
Our results showed the newly developed force-sensing device allows for combined ligament tensioning and force sensing in our experimental setup and confirms the ACL-deficient knee does not have equal gaps throughout a full ROM and does not have equal medial and lateral gaps [22, 25, 26, 29, 30, 32]. Tanaka et al. [29] reported, in their cadaver study, medial and lateral joint gaps in extension were smaller than in any degree of flexion, whereas the lateral gap showed a greater increase during flexion than the medial gap. We also found increased gaps in 90° flexion compared with full extension for all testing scenarios with differences of as much as 6 mm. The PCL reduced the mean medial compartment gap height at 90° flexion by as much as 2 mm. In agreement with the study of Tanaka et al. [29], we also observed a larger opening of the lateral gap with intact PCL conditions of as much as 90° flexion. However, this phenomenon is inversed by flexing the knee 120°. In addition to the general influence of the PCL and position of the patella, the flexion angle also influences balancing of the extension gap. The largest change in overall gap height occurred between full extension and 30° flexion. This confirms the important findings of previous studies [19, 25, 29].
We found varying the magnitude of the applied force from 50 N to 100 N per side resulted in an average increase in the medial and lateral gaps of approximately 0.5 mm over all conditions. We chose to apply loads in this range as it corresponded to reports of forces applied with other tensioning devices used in TKA [1, 3, 8, 29]. It was difficult to directly compare the effect of increasing force with those in other studies, however, as some authors only report gaps at one load magnitude or they use devices that do not apply load in the same manner [23, 28, 33]. We might expect a more appreciable change than what we measured in the gap height could occur in surgery after releasing certain ligament structures around the knee [2, 3, 35].
Current TKA techniques are designed for equal medial and lateral gaps in flexion and extension. Although modern TKA has had good clinical results and a low failure rate if well aligned [7, 20] and balanced [31, 34], the majority of these TKAs do not recreate physiologic knee motions [13, 14] and do not feel normal to patients [27]. Intraoperative data obtained with instrumented tools capable of quantifying loads, gap heights, and flexion angles, such as the one used in this study, will allow us to better document and interpret the effects of component positioning and releases on gap kinematics and ligament tension [11, 33]. Navigation systems already are capable of capturing patient-specific joint morphologic features, gaps, and load-bearing axes [6]. Additional data on the soft tissue envelope and relative positions of the three-dimensional implant surfaces, combined with computerized tools that automatically can control knee gaps, could help us achieve more predicable joint stability. Instrumented tibial trial components allow for dynamic evaluation of compartmental loads in the knee through a passive ROM with the femoral trial in place [11, 33]. Using an active spacer with dynamic height control and force-sensing capabilities could allow for this dynamic evaluation of compartmental loads during the femoral planning stage before the femur is resected, as the spacer height can be controlled to equal the combined theoretical thickness of the planned tibial and femoral components at different flexion angles. Thus the surgeon would be able to evaluate the tightness or looseness of the medial and lateral compartments in flexion based on a planned rotational position of the femoral component and a planned tibial thickness, for example. Ultimately, with such data and tools, we may be able to provide a more complete picture and move toward a more universal solution to the balancing problem.
The described instrumented distracter allows for controlled application of forces and quantification of gap kinematics at any flexion angle of the knee and avoids the confounding effect of patellar eversion. These data create new questions that now can be examined. For example, we can compare the motion of TKAs implanted with equally balanced gaps with that of TKAs with gaps adjusted to match the normal knee. Computer navigation systems are beginning to allow us to document these kinematics intraoperatively. Before making any suggestion that we change the goals of ligament balance for TKA, the kinematic results of reproducing normal gaps should be assessed in the laboratory to investigate the performance of existing and new implant designs in such conditions.
References
Asano H, Hoshino A, Wilton TJ. Soft-tissue tension total knee arthroplasty. J Arthroplasty. 2004;19:558–561.
Asano H, Muneta T, Hoshino A. Stiffness of soft tissue complex in total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2008;16:51–55.
Asano H, Muneta T, Sekiya I. Soft tissue tension in extension in total knee arthroplasty affects postoperative knee extension and stability. Knee Surg Sports Traumatol Arthrosc. 2008;16:999–1003.
Baldini A, Scuderi GR, Aglietti P, Chalnick D, Insall JN. Flexion-extension gap changes during total knee arthroplasty: effect of posterior cruciate ligament and posterior osteophytes removal. J Knee Surg. 2004;17:69–72.
Bathis H, Perlick L, Tingart M, Luring C, Perlick C, Grifka J. Flexion gap configuration in total knee arthroplasty following high tibial osteotomy. Int Orthop. 2004;28:366–369.
Bathis H, Perlick L, Tingart M, Luring C, Zurakowski D, Grifka J. Alignment in total knee arthroplasty: a comparison of computer-assisted surgery with the conventional technique. J Bone Joint Surg Br. 2004;86:682–687.
Choong PF, Dowsey MM, Stoney JD. Does accurate anatomical alignment result in better function and quality of life? A prospective randomized controlled trial comparing conventional and computer-assisted total knee arthroplasty. J Arthroplasty. 2008 May 17. [Epub ahead of print].
Christen B, Heesterbeek PV, Wymenga A, Wehrli U. Posterior cruciate ligament balancing in total knee replacement: the quantitative relationship between tightness of the flexion gap and tibial translation. J Bone Joint Surg Br. 2007;89:1046–1050.
Crottet D, Kowal J, Sarfert SA, Maeder T, Bleuler H, Nolte LP, Durselen L. Ligament balancing in TKA: evaluation of a force-sensing device and the influence of patellar eversion and ligament release. J Biomech. 2007;40:1709–1715.
Crottet D, Maeder T, Fritschy D, Bleuler H, Nolte LP, Pappas IP. Development of a force amplitude- and location-sensing device designed to improve the ligament balancing procedure in TKA. IEEE Trans Biomed Eng. 2005;52:1609–1611.
D’Lima DD, Patil S, Steklov N, Colwell CW Jr. An ABJS Best Paper: Dynamic intraoperative ligament balancing for total knee arthroplasty. Clin Orthop Relat Res. 2007;463:208–212.
Griffin FM, Insall JN, Scuderi GR. Accuracy of soft tissue balancing in total knee arthroplasty. J Arthroplasty. 2000;15:970–973.
Hamai S, Miura H, Higaki H, Matsuda S, Shimoto T, Sasaki K, Yoshizumi M, Okazaki K, Tsukamoto N, Iwamoto Y. Kinematic analysis of kneeling in cruciate-retaining and posterior-stabilized total knee arthroplasties. J Orthop Res. 2008;26:435–442.
Hamai S, Miura H, Higaki H, Shimoto T, Matsuda S, Okazaki K, Iwamoto Y. Three-dimensional knee joint kinematics during golf swing and stationary cycling after total knee arthroplasty. J Orthop Res. 2008;26:1556–1561.
Insall J, Tria AJ, Scott WN. The total condylar knee prosthesis: the first 5 years. Clin Orthop Relat Res. 1979;145:68–77.
Insall JN. Total knee arthroplasty in rheumatoid arthritis. Ryumachi. 1993;33:472.
Insall JN, Binazzi R, Soudry M, Mestriner LA. Total knee arthroplasty. Clin Orthop Relat Res. 1985;192:13–22.
Kadoya Y, Kobayashi A, Komatsu T, Nakagawa S, Yamano Y. Effects of posterior cruciate ligament resection on the tibiofemoral joint gap. Clin Orthop Relat Res. 2001;391:210–217.
Laskin RS. Flexion space configuration in total knee arthroplasty. J Arthroplasty. 1995;10:657–660.
Longstaff LM, Sloan K, Stamp N, Scaddan M, Beaver R. Good alignment after total knee arthroplasty leads to faster rehabilitation and better function. J Arthroplasty. 2008 May 17. [Epub ahead of print].
Luring C, Hufner T, Kendoff D, Perlick L, Bathis H, Grifka J, Krettek C. Eversion or subluxation of patella in soft tissue balancing of total knee arthroplasty? Results of a cadaver experiment. Knee. 2006;13:15–18.
Markolf KL, Mensch JS, Amstutz HC. Stiffness and laxity of the knee—the contributions of the supporting structures: a quantitative in vitro study. J Bone Joint Surg Am. 1976;58:583–594.
Marmignon C, Leimnei A, Lavallee S, Cinquin P. Automated hydraulic tensor for total knee arthroplasty. Int J Med Robot. 2005;1:51–57.
Matsueda M, Gengerke TR, Murphy M, Lew WD, Gustilo RB. Soft tissue release in total knee arthroplasty: cadaver study using knees without deformities. Clin Orthop Relat Res. 1999;366:264–273.
Mihalko WM, Krackow KA. Posterior cruciate ligament effects on the flexion space in total knee arthroplasty. Clin Orthop Relat Res. 1999;360:243–250.
Moore TM, Meyers MH, Harvey JP Jr. Collateral ligament laxity of the knee: long-term comparison between plateau fractures and normal. J Bone Joint Surg Am. 1976;58:594–598.
Suggs JF, Hanson GR, Park SE, Moynihan AL, Li G. Patient function after a posterior stabilizing total knee arthroplasty: cam-post engagement and knee kinematics. Knee Surg Sports Traumatol Arthrosc. 2008;16:290–296.
Swank M, Romanowski IR, Korbee LL, Bignozzi S. Ligament balancing in computer-assisted total knee arthroplasty: improved clinical results with a spring-loaded tensioning device. Proc Inst Mech Eng H. 2007;221:755–761.
Tanaka K, Muratsu H, Mizuno K, Kuroda R, Yoshiya S, Kurosaka M. Soft tissue balance measurement in anterior cruciate ligament-resected knee joint: cadaveric study as a model for cruciate-retaining total knee arthroplasty. J Orthop Sci. 2007;12:149–153.
Tokuhara Y, Kadoya Y, Nakagawa S, Kobayashi A, Takaoka K. The flexion gap in normal knees: an MRI study. J Bone Joint Surg Br. 2004;86:1133–1136.
Unitt L, Sambatakakis A, Johnstone D, Briggs TW. Short-term outcome in total knee replacement after soft-tissue release and balancing. J Bone Joint Surg Br. 2008;90:159–165.
Van Damme G, Defoort K, Ducoulombier Y, Van Glabbeek F, Bellemans J, Victor J. What should the surgeon aim for when performing computer-assisted total knee arthroplasty? J Bone Joint Surg Am. 2005;87(suppl 2):52–58.
Wasielewski RC, Galat DD, Komistek RD. An intraoperative pressure-measuring device used in total knee arthroplasties and its kinematics correlations. Clin Orthop Relat Res. 2004;427:171–178.
Wyss T, Schuster AJ, Christen B, Wehrli U. Tension controlled ligament balanced total knee arthroplasty: 5-year results of a soft tissue orientated surgical technique. Arch Orthop Trauma Surg. 2008;128:129–135.
Zalzal P, Papini M, Petruccelli D, de Beer J, Winemaker MJ. An in vivo biomechanical analysis of the soft-tissue envelope of osteoarthritic knees. J Arthroplasty. 2004;19:217–223.
Acknowledgments
We thank Stephen Lyman, PhD, Director, Epidemiology and Biostatistics Core, Hospital for Special Surgery, for assistance with the statistical analysis, and Timothy Wright, PhD, Director of the Biomechanics Laboratory, Hospital for Special Surgery, and Clara Hilario of the Hospital for Special Surgery CAS Laboratory for support in this study and for providing use of the laboratory facilities. We also thank Anne-Thérèse Bourreau for assistance with the data processing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Richard Laskin—Deceased.
One of the authors (CP) is employed by Praxim Inc, Walpole, MA.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
About this article
Cite this article
Mayman, D., Plaskos, C., Kendoff, D. et al. Ligament Tension in the ACL-deficient Knee: Assessment of Medial and Lateral Gaps. Clin Orthop Relat Res 467, 1621–1628 (2009). https://doi.org/10.1007/s11999-009-0748-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-009-0748-3