Abstract
Purpose
The purpose of this study was to determine the biomechanical effects of simulated immediate motion and weightbearing during rehabilitation on different double-bundle posterior cruciate ligament reconstruction (DB-PCLR) graft options.
Methods
Nine each of commercially prepared (allograft) Achilles tendon allografts, fresh-frozen (autograft) bone-patellar tendon-bone grafts, and fresh-frozen quadriceps tendon grafts were paired with commercially prepared anterior tibialis allografts, fresh-frozen semitendinosus grafts, and fresh-frozen semitendinosus grafts, respectively. Graft pairs were loaded to simulate early range of motion on a stationary bicycle, partial weightbearing (30 %), and full weightbearing.
Results
Acquired laxity (displacement, mm) between graft pairs was not significantly different during simulated early range of motion. However, during simulated partial weightbearing, the median acquired laxity of the patellar tendon/semitendinosus pair (1.06 mm) was significantly less than that of the quadriceps tendon/semitendinosus (1.50 mm, p = 0.01) and Achilles/anterior tibialis (1.44 mm, p = 0.003) graft pairs. During simulated full weightbearing, significantly less acquired laxity was observed for the patellar tendon/semitendinosus graft pair (2.38 mm) compared to the Achilles/anterior tibialis pair (4.85 mm, p = 0.04), but a significant difference was not observed compared to the QT/semitendinosus graft pair (3.91 mm, n.s.). There were no significant differences in the ultimate loads between any of the graft pairs.
Conclusions
Simulated early range of motion and early partial weightbearing did not result in clinically significant acquired graft laxity in common graft options utilized for DB-PCLR. However, simulated full weightbearing did result in clinically significant acquired graft laxity, and therefore, early rehabilitation protocols should avoid implementing full weightbearing that could contribute to graft failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Historically, non-weightbearing and early immobilization in extension with limited prone range of motion post-operative rehabilitation protocols following posterior cruciate ligament (PCL) reconstructions have been advocated to protect the PCL graft from the posteriorly directed forces of gravity and the hamstrings [8, 36, 46]. Although these relatively conservative rehabilitation protocols have been implemented, the outcomes with regard to objective anteroposterior laxity with longer-term follow-up of single-bundle PCL reconstructions (SB-PCLRs) have revealed continued laxity compared to the contralateral knee of 4–6 mm in most studies [22, 26, 47]. Noting that a complete PCL tear is considered present with 8 mm of increased posterior tibial translation compared to the contralateral knee [24], it is concerning that between 50 and 75 % of this diagnostic measurement remains as residual PCL laxity. In an attempt to more accurately recreate native knee anatomy and kinematics and potentially decrease the likelihood of the development of unwanted anteroposterior laxity, anatomic double-bundle PCL reconstructions (DB-PCLRs) have been increasingly advocated [43, 51].
There is still some debate over the benefits of reconstructing both bundles of the PCL due to conflicting biomechanical [6, 11, 28, 38, 44, 50] and clinical evidence [11, 17, 48]. The roles of the two bundles of the PCL have been elucidated, and many authors have demonstrated that both the anterolateral bundle (ALB) and the posteromedial bundle (PMB) are functionally important and likely codominant to both anteroposterior stability and rotation throughout knee motion [19–21, 27, 51]. Additionally, Spiridonov et al. [43] have reported improved subjective outcomes and objective stability utilizing a DB-PCLR and a traditional rehabilitation protocol. Post-operatively, all patients were non-weightbearing for 6 weeks. Their physical therapy regimen emphasized immediate quadriceps activation and prone knee flexion to 90 degrees. All patients were also managed with a dynamic PCL functional brace post-operatively. Unlike traditional single-bundle techniques, concordance was predictably achieved in both subjective outcomes and objective stability. The potential benefits of immediate mobilization have also been demonstrated in the laboratory setting for various ligaments and showed improved mechanical and structural properties of the medial collateral ligament with mobilization [52, 53] and improved clinical outcomes following ACL reconstruction [23]. Moreover, the effects of a more aggressive post-operative rehabilitation protocol on a larger overall DB-PCLR graft volume that is more anatomic are unknown and merit further investigation.
The purpose of this study was twofold: (1) to determine whether a loading protocol designed to simulate an early range of motion and weightbearing rehabilitation protocol leads to acquired graft laxity and (2) to determine whether the laxity in the current, most commonly utilized reconstruction allograft options is comparable to readily available autograft options. It was hypothesized that there would be no clinically significant increase in the acquired laxity of either autograft or high-quality allograft options commonly used in DB-PCLRs due to simulated early knee range of motion and weightbearing.
Materials and methods
Allografts commonly used in PCL reconstructions were obtained from a commercially prepared source (AlloTrue, Allosource, Centennial, Colorado). Nine Achilles tendons (median age 57 years, range 49–61) and 9 anterior tibialis tendons (median age 51 years, range 21–65) were the allografts utilized in this study. Autografts potentially available for PCL reconstructions were harvested from fresh-frozen cadaveric specimens to simulate the use of autograft tissues. These grafts consisted of 9 bone-patellar tendon-bone (BTB) grafts (median age 56 years, range 48–65), 9 quadriceps tendon (QT) grafts (median age 61 years, range 48–65), and 18 semitendinosus grafts (median age 62 years, range 48–65).
Graft preparation
The calcaneal bone blocks of the Achilles grafts were trimmed to create bone plugs that were 11 mm in diameter and 25 mm in length, and the tendon was sized, if necessary, to pass through a tunnel diameter of 11 mm. Similarly, QT grafts and BTB grafts were trimmed to the same dimensions to fit through an 11-mm sizing block. All semitendinosus and anterior tibialis grafts were doubled and sized to pass through a 7-mm tunnel. Grafts sized to 11 mm and those sized to 7 mm were intended to replicate the anterolateral and posteromedial bundles, respectively, of a double-bundle PCL reconstruction construct.
Both the bone plugs and soft tissue ends of the various grafts were then fixed into rigid polyurethane foam blocks (Pacific Research Laboratories, Sawbones, Vashon Island, Washington) in clinically relevant reconstruction pairs (Table 1). Polyurethane foam blocks were chosen as a surrogate for human bone to allow for uniform modelling of the viscoelastic properties, strength, stiffness, and pullout testing of the chosen graft pairs to be conducted independent of potentially varying bone material properties and geometry shown to be present in cadaveric bone [12, 41]. Cancellous bone ranges in volumetric density between 0.09 and 1.26 g/cm3 (5.6–78.7 pcf) [42], and cortical bone densities have been reported between 2.0 and 2.2 g/cm3 (125–137 pcf) [7, 14]. Therefore, polyurethane blocks measuring 6 cm × 6 cm with a core of 20 pcf (0.32 g/cm3) foam laminated with a 1.5-mm layer of 50 pcf (0.8 g/cm3) were chosen to mimic the cortical and cancellous layers of human bone in a young athletic population with high bone mineral density [1, 2, 32, 33]. Both polyurethane densities chosen were greater than those previously reported in graft fixation research [4, 42] to replicate the population most likely to undergo PCLR [43] as opposed to a “worst-case scenario” of osteopenic bone.
Tunnel and graft sizes were chosen to be consistent with the technique for anatomic DB-PCLR described by Spiridonov et al. [43] and further validated by Wijdicks et al. [51]. Interference screw fixation was chosen for all interfaces to ensure consistency among graft pairs (Table 1). The ALB and PMB were first fixed in their “femoral” tunnels with a bone bridge gap distance of 3 mm [3, 43], and then, a second polyurethane testing block (“tibial” side) was clamped at a separation distance of 35 mm to replicate the native length of the PCL [37]. A graft tensioning device (Arthrex, Naples, Florida) was then used to independently tension each graft to 20 N, and the grafts were secured to the “tibial” block. Independent tensioning at a constant distance was performed to ensure that both bundles experienced equivalent fixation tension. Screw sizes were determined through pilot testing to ensure a balance of adequate fixation and to minimize the risk of graft laceration/amputation and are noted in Table 1.
Testing protocol
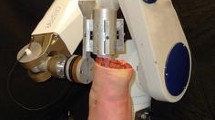
After initial graft tensioning and fixation, the polyurethane blocks were secured between custom loading fixtures attached to the actuator of a dynamic tensile testing machine (Instron E10000, Norwood, Massachusetts; Fig. 1). Measurement error of the testing machine was certified by Instron to be less than or equal to ±0.01 mm and ±0.3 % of the indicated force. Eight of nine graft pairs of each group were then subjected to an identical progressive cyclic uniaxial loading protocol. The loading protocol was developed to simulate the maximal forces the PCL could encounter at time zero with both a progressive range of motion and partial (30 %) and full weightbearing. Cyclic load values were based on estimates of the maximal potential posterior tibiofemoral shear forces reported during various activities. The initial cyclic loading phase simulated the forces of immediate range of motion on the PCL as previously demonstrated on a cycle ergometer to simulate the use of a stationary bicycle [9]. Posterior tibiofemoral shear forces were estimated to reach 0.05 times body weight during use of a standardized cycle ergometer [9]; hence, a 50 N force was applied for 600 cycles at 1 Hz (0.05 times body weight force of a 70-kg adult for 10 min of standardized ergometer cycling at 120 W and 60 rpm).
Previous reports have also demonstrated that maximal posterior tibiofemoral shear forces may reach approximately 275 N during normal gait on level ground (0.4 times body weight force [30, 31] of a 70-kg adult). This estimate was used to establish the maximum load for partial (30 %) and full weightbearing, and a cyclic loading protocol with incrementally increasing forces followed the cycle ergometer simulation. The number of cycles for the simulated partial and full weightbearing loading periods was based on pedometer data for the maximal steps a patient undergoing knee reconstruction might take daily in the first six post-operative weeks—approximately 3500 steps [40]. Therefore, paired graft constructs were subjected to cyclic loading over 3 phases at 1 Hz between 10 N and a progressively increasing maximum load that was incremented from 50 N (simulated range of motion cycling without resistance = phase 1) to 85 N (simulated partial weightbearing = phase 2) and to 275 N (simulated full weightbearing = phase 3) at cycle numbers 600, 4100, and 7600, respectively (Fig. 2). Displacement (mm), stiffness (N/mm), and the elastic limit (N; i.e. the load at which there is change from elastic to permanent deformation of the graft pairs [13]) for each of the constructs were continuously monitored and reported. After the first three loading phases, testing concluded with a pull to failure at 20 mm/min to determine the ultimate load (N) of each graft pair (phase 4). One additional non-cyclically loaded graft pair from each group was also pulled to failure at 20 mm/min to corroborate the load versus displacement curve of each construct.
Graphical representation of the cyclic loading protocol. Phase 1 simulated the forces of immediate range of motion on the PCL during stationary cycling: a 10–50 N force was applied for 600 cycles (i.e. 120 W and 60 rpm). Phase 2 simulated the forces of partial (30 %) weightbearing on the PCL during one post-operative day: a 10–85 N force was applied for 3500 cycles. Phase 3 simulated the forces of full weightbearing on the PCL during one post-operative day: a 10–275 N force was applied for 3500 cycles. Testing concluded with a pull to failure (Phase 4) at 20 mm/min where the elastic limit load (N) and ultimate load (N) of each of the graft pairs were determined
Statistical analysis
Displacement, stiffness, elastic limit, and ultimate load were summarized for each construct group with medians, minima, and maxima. Each group of graft pairs was compared to one another, and differences were assessed using the Kruskal–Wallis test and Dunn’s test for post hoc comparisons. Assuming 8 specimens per group and an α = 0.05 for nonparametric, two-tailed, pairwise comparisons, an effect size of d = 1.55 is detectable with 80 % power. Adjusted p values <0.05 were considered significant. All statistical analyses were performed using IBM SPSS Statistics, version 20 (Armonk, New York).
Results
Displacement
Acquired laxity (displacement, mm) between graft pairs was not significantly different during simulated early range of motion (phase 1; concluded at cycle 600), and the median and maximal displacement for all of the pairs were less than 0.9 and 1.4 mm, respectively (Table 2). During simulated partial weightbearing (phase 2; concluded at cycle 4100), the median acquired laxity of the BTB/semitendinosus pair (1.06 mm) was significantly less than the QT/semitendinosus (1.50 mm, p = 0.01) and Achilles/anterior tibialis (1.44 mm, p = 0.003) graft pairs. The maximal acquired laxity during phase 2 for the BTB/semitendinosus, QT/semitendinosus, and Achilles/anterior tibialis pairs was 1.85, 2.50, and 2.37 mm, respectively.
During simulated full weightbearing (phase 3; concluded at cycle 7600), significantly less acquired laxity was observed with the BTB/semitendinosus graft pair (2.38 mm) compared to the Achilles/anterior tibialis pair (4.85 mm, p = 0.04), but a significant difference was not observed compared to the QT/semitendinosus graft pair (3.91 mm, n.s.) or between the QT/semitendinosus and the Achilles/anterior tibialis pairs (p = 1.000; Table 2). The maximal acquired laxity during phase 3 for the BTB/semitendinosus, QT/semitendinosus, and Achilles/anterior tibialis pairs was 3.44, 15.29, and 20.95 mm, respectively.
Stiffness and pull-to-failure measurements
All stiffness and pull-to-failure data are contained in Table 2. The BTB/semitendinosus construct was stiffer than the Achilles/anterior tibialis pair throughout the testing protocol and stiffer than the QT/semitendinosus pair at the start of phase 3 (p = 0.03) and during the pull to failure (p = 0.02). There were no significant differences in the elastic limits or ultimate loads between any of the graft pairs. Furthermore, the elastic limits of all the graft pairs were greater than the forces applied to simulate early range of motion activities including cycle ergometry (50 N; phase 1), partial weightbearing (85 N; phase 2), and full weightbearing (275 N) (phase 3; Fig. 3). The non-cyclically loaded graft pairs of each type that were only subjected to pull-to-failure testing also demonstrated similar elastic modulus curves to the cyclically loaded constructs (Fig. 3). The mechanisms of failure and frequencies are given in Table 3.
Discussion
The most important finding of this study was that common DB-PCLR grafts did not acquire clinically significant laxity as a result of a simulated rehabilitation protocol representative of early range of motion on a stationary bicycle and partial weightbearing. In contrast, during simulated full weightbearing, clinically significant differences in the median and maximal acquired laxity were observed between graft options. These findings biomechanically validate the caution implemented with traditional rehabilitation protocols that avoid immediate full weightbearing following DB-PCLR; nonetheless, partial weightbearing may be a safe alternative for early rehabilitation. The BTB/semitendinosus graft pair exhibited the least acquired graft laxity under all loading conditions including simulated full weightbearing. However, during loading phases 1 and 2 (simulated early range of motion on a stationary bicycle and partial weightbearing, respectively), significant differences between the graft options were either not observed or not clinically significant. Additionally, there was no difference in the ultimate loads between the allograft (e.g. Achilles/anterior tibialis) and autograft (e.g. BTB/semitendinosus and QT/semitendinosus) construct options.
The in vitro tensile properties of single-bundle graft options for cruciate ligament reconstruction have been well documented [15, 34, 54, 55], while the tensile properties of common graft construct combinations used for DB-PCL reconstruction have not. In this study, the ultimate failure load of each construct (lowest median = 566 N) was much greater than the forces encountered with weightbearing and range of motion (full weightbearing ~275 N [29, 30]). Additionally, there were several consistent findings with pull-to-failure testing: the graft pairs most commonly failed at the bone–graft interface, the BTB/semitendinosus graft pair demonstrated the greatest stiffness, and the anticipated ultimate loads of the graft constructs (based on previous studies which reported tensile properties of the graft bundles in isolation [15, 34, 54, 55]) were not consistently reached. This likely indicates that interference screw fixation was superior for grafts containing bone plugs versus soft tissue grafts and that partial graft laceration in this model of dense bone may have been a contributing factor to graft failure and slippage, as previously reported [39, 49].
The level of evidence supporting different recommendations for rehabilitation following PCLR is lacking rigorous scientific basis. Rehabilitation protocols have largely been based on level 4 and expert opinion data following SB-PCLRs [8, 10, 25, 36] and on the premise that early range of motion is more likely to be associated with inferior stability outcomes. However, many authors have implemented these conservative immobilization protocols, and suboptimal stability has still been reported [26, 47]. Consequently, others have reported the requirement for manipulation under anaesthesia and/or lysis of adhesions for stiffness in patients utilizing post-operative immobilization [29]. The potential benefits of immediate mobilization have been demonstrated in the laboratory setting for various ligaments and showed improved mechanical and structural properties of the medial collateral ligament with mobilization [52, 53] and improved clinical outcomes following ACL reconstruction [23]. Although the dynamic function of the quadriceps musculature is protective of the healing PCL graft [36, 46], quadriceps inhibition is common following knee trauma and surgery [16]. Rehabilitation exercises that result in earlier reversal of quadriceps inhibition are potentially desirable assuming concomitant graft stretching can be avoided. The results of the current study demonstrate the cyclic forces placed on a PCL graft simulating early range of motion on a stationary bicycle and partial weightbearing do not result in clinically relevant laxity (displacement). Therefore, a more progressive but protected early rehabilitation protocol following DB-PCLR is not likely to result in additional joint laxity [35, 43] and may also lead to improved patient outcomes [23].
This study was not without limitations. A simulated in vitro biomechanical testing model substituting polyurethane foam for bone was utilized. The polyurethane foam was chosen because it was believed to more consistently mimic the cortical and cancellous layers of human bone in a young athletic population with high bone mineral densities [1, 2, 32, 33]. While the synthetic foam was successful in providing homogeneity of material characteristics, the results may not translate perfectly to the time zero in vivo environment. Furthermore, the results of this study are representative of a time zero, in vitro biomechanical model, and the effect of biological healing was correspondingly unable to be studied. Additionally, uniaxial testing is unable to simulate the 6 degrees of freedom of natural knee kinematics; therefore, the results obtained by this study may not yield a complete understanding of the effects of an aggressive rehabilitation protocol on the grafts in patients. Although the constructs were not preconditioned prior to testing, sub-millimetre (~0.5 mm) elongation was observed during the first cycle of loading for all groups and therefore likely did not affect the cyclic elongation behaviour of the constructs. In addition to the inability to load the grafts at different knee flexion angles, tissue–tunnel interactions throughout the flexion arc are also not accounted for, especially the potential for PCL graft abrasion by the tibial tunnel aperture, or the so-called killer turn [5, 18, 45]. Furthermore, a uniform method of PCLR graft fixation (interference screws) was utilized. As such, the use of soft tissue bone staples or fixation screws and washers for the soft tissue grafts could have resulted in different displacement and strength measurements. Nevertheless, this study presents a detailed and consistent biomechanical evaluation of common allograft and autograft options for DB-PCLR.
Conclusions
This study demonstrated that incrementally increased cyclic forces of simulated early range of motion on a stationary bicycle and partial weightbearing were unlikely to lead to acquired laxity in common graft pairs used for DB-PCL reconstruction. Additionally, a clinically relevant difference did not exist between the tensile properties of simulated common autograft and high-quality commercially prepared allograft PCL constructs at cyclic loads up to partial weightbearing. However, simulation of immediate full weightbearing following DB-PCL reconstruction may lead to clinically significant acquired graft laxity; therefore, early rehabilitation protocols should avoid implementing full weightbearing. In order to examine and improve early outcomes following PCL reconstruction, a prospective randomized trial of a traditional rehabilitation protocol versus an early range of motion and progressive weightbearing rehabilitation program is recommended.
References
Alfredson H, Nordstrom P, Lorentzon R (1996) Total and regional bone mass in female soccer players. Calcif Tissue Int 59(6):438–442
Alfredson H, Nordstrom P, Lorentzon R (1997) Bone mass in female volleyball players: a comparison of total and regional bone mass in female volleyball players and nonactive females. Calcif Tissue Int 60(4):338–342
Anderson CJ, Ziegler CG, Wijdicks CA, Engebretsen L, LaPrade RF (2012) Arthroscopically pertinent anatomy of the anterolateral and posteromedial bundles of the posterior cruciate ligament. J Bone Joint Surg Am 94(21):1936–1945
Barber FA (2013) Pullout strength of bone-patellar tendon-bone allograft bone plugs: a comparison of cadaver tibia and rigid polyurethane foam. Arthroscopy 29(9):1546–1551
Berg EE (1995) Posterior cruciate ligament tibial inlay reconstruction. Arthroscopy 11(1):69–76
Bergfeld JA, Graham SM, Parker RD, Valdevit AD, Kambic HE (2005) A biomechanical comparison of posterior cruciate ligament reconstructions using single- and double-bundle tibial inlay techniques. Am J Sports Med 33(7):976–981
Ding M, Dalstra M, Danielsen CC, Kabel J, Hvid I, Linde F (1997) Age variations in the properties of human tibial trabecular bone. J Bone Joint Surg Br 79(6):995–1002
Edson CJ, Fanelli GC, Beck JD (2010) Postoperative rehabilitation of the posterior cruciate ligament. Sports Med Arthrosc 18(4):275–279
Ericson MO, Nisell R (1986) Tibiofemoral joint forces during ergometer cycling. Am J Sports Med 14(4):285–290
Fanelli GC (2008) Posterior cruciate ligament rehabilitation: how slow should we go? Arthroscopy 24(2):234–235
Fanelli GC, Beck JD, Edson CJ (2012) Single compared to double-bundle PCL reconstruction using allograft tissue. J Knee Surg 25(1):59–64
Gardner MJ, Silva MJ, Krieg JC (2012) Biomechanical testing of fracture fixation constructs: variability, validity, and clinical applicability. J Am Acad Orthop Surg 20(2):86–93
Golish SR, Mihalko WM (2011) Principles of biomechanics and biomaterials in orthopaedic surgery. J Bone Joint Surg Am 93(2):207–212
Gong JK, Arnold JS, Cohn SH (1964) Composition of trabecular and cortical bone. Anat Rec 149:325–331
Hamner DL, Brown CHJ, Steiner ME, Hecker AT, Hayes WC (1999) Hamstring tendon grafts for reconstruction of the anterior cruciate ligament: biomechanical evaluation of the use of multiple strands and tensioning techniques. J Bone Joint Surg Am 81(4):549–557
Hart JM, Pietrosimone B, Hertel J, Ingersoll CD (2010) Quadriceps activation following knee injuries: a systematic review. J Athl Train 45(1):87–97
Hatayama K, Higuchi H, Kimura M, Kobayashi Y, Asagumo H, Takagishi K (2006) A comparison of arthroscopic single- and double-bundle posterior cruciate ligament reconstruction: review of 20 cases. Am J Orthop (Belle Mead NJ) 35(12):568–571
Huang TW, Wang CJ, Weng LH, Chan YS (2003) Reducing the “killer turn” in posterior cruciate ligament reconstruction. Arthroscopy 19(7):712–716
Kennedy NI, LaPrade RF, Goldsmith MT, Faucett SC, Rasmussen MT, Coatney GA, Engebretsen L, Wijdicks CA (2014) Posterior cruciate ligament graft fixation angles, part 1: biomechanical evaluation for anatomic single-bundle reconstruction. Am J Sports Med 42(10):2338–2345
Kennedy NI, LaPrade RF, Goldsmith MT, Faucett SC, Rasmussen MT, Coatney GA, Engebretsen L, Wijdicks CA (2014) Posterior cruciate ligament graft fixation angles, part 2: biomechanical evaluation for anatomic double-bundle reconstruction. Am J Sports Med 42(10):2346–2355
Kennedy NI, Wijdicks CA, Goldsmith MT, Michalski MP, Devitt BM, Aroen A, Engebretsen L, LaPrade RF (2013) Kinematic analysis of the posterior cruciate ligament, part 1: the individual and collective function of the anterolateral and posteromedial bundles. Am J Sports Med 41(12):2828–2838
Kim YM, Lee CA, Matava MJ (2011) Clinical results of arthroscopic single-bundle transtibial posterior cruciate ligament reconstruction: a systematic review. Am J Sports Med 39(2):425–434
Kruse LM, Gray B, Wright RW (2012) Rehabilitation after anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am 94(19):1737–1748
LaPrade CM, Civitarese DM, Rasmussen MT, LaPrade RF (2015) Emerging updates on the posterior cruciate ligament: a review of the current literature. Am J Sports Med. doi:10.1177/0363546515572770
Levy BA, Boyd JL, Stuart MJ (2011) Surgical treatment of acute and chronic anterior and posterior cruciate ligament and lateral side injuries of the knee. Sports Med Arthrosc 19(2):110–119
Lipscomb AB Jr, Anderson AF, Norwig ED, Hovis WD, Brown DL (1993) Isolated posterior cruciate ligament reconstruction. Long-term results. Am J Sports Med 21(4):490–496
Markolf KL, Feeley BT, Jackson SR, McAllister DR (2006) Biomechanical studies of double-bundle posterior cruciate ligament reconstructions. J Bone Joint Surg Am 88(8):1788–1794
Markolf KL, Graves BR, Sigward SM, Jackson SR, McAllister DR (2007) Effects of posterolateral reconstructions on external tibial rotation and forces in a posterior cruciate ligament graft. J Bone Joint Surg Am 89(11):2351–2358
Mook WR, Miller MD, Diduch DR, Hertel J, Boachie-Adjei Y, Hart JM (2009) Multiple-ligament knee injuries: a systematic review of the timing of operative intervention and postoperative rehabilitation. J Bone Joint Surg Am 91(12):2946–2957
Morrison JB (1969) Function of the knee joint in various activities. Biomed Eng 4(12):573–580
Morrison JB (1970) The mechanics of the knee joint in relation to normal walking. J Biomech 3(1):51–61
Nevill AM, Holder RL, Stewart AD (2003) Modeling elite male athletes’ peripheral bone mass, assessed using regional dual X-ray absorptiometry. Bone 32(1):62–68
Nordstrom P, Lorentzon R (1996) Site-specific bone mass differences of the lower extremities in 17-year-old ice hockey players. Calcif Tissue Int 59(6):443–448
Noyes FR, Butler DL, Grood ES, Zernicke RF, Hefzy MS (1984) Biomechanical analysis of human ligament grafts used in knee-ligament repairs and reconstructions. J Bone Joint Surg Am 66(3):344–352
Nyland J, Hester P, Caborn DN (2002) Double-bundle posterior cruciate ligament reconstruction with allograft tissue: 2-year postoperative outcomes. Knee Surg Sports Traumatol Arthrosc 10(5):274–279
Pierce CM, O’Brien L, Griffin LW, Laprade RF (2013) Posterior cruciate ligament tears: functional and postoperative rehabilitation. Knee Surg Sports Traumatol Arthrosc 21(5):1071–1084
Race A, Amis AA (1994) The mechanical properties of the two bundles of the human posterior cruciate ligament. J Biomech 27(1):13–24
Race A, Amis AA (1998) PCL reconstruction. In vitro biomechanical comparison of ‘isometric’ versus single and double-bundled ‘anatomic’ grafts. J Bone Joint Surg Br 80(1):173–179
Scheffler SU, Sudkamp NP, Gockenjan A, Hoffmann RF, Weiler A (2002) Biomechanical comparison of hamstring and patellar tendon graft anterior cruciate ligament reconstruction techniques: the impact of fixation level and fixation method under cyclic loading. Arthroscopy 18(3):304–315
Schmalzried TP, Szuszczewicz ES, Northfield MR, Akizuki KH, Frankel RE, Belcher G, Amstutz HC (1998) Quantitative assessment of walking activity after total hip or knee replacement. J Bone Joint Surg Am 80(1):54–59
Sell P, Collins M, Dove J (1988) Pedicle screws: axial pull-out strength in the lumbar spine. Spine (Phila Pa 1976) 13(9):1075–1076
Simonian PT, Sussmann PS, Baldini TH, Crockett HC, Wickiewicz TL (1998) Interference screw position and hamstring graft location for anterior cruciate ligament reconstruction. Arthroscopy 14(5):459–464
Spiridonov SI, Slinkard NJ, LaPrade RF (2011) Isolated and combined grade-III posterior cruciate ligament tears treated with double-bundle reconstruction with use of endoscopically placed femoral tunnels and grafts: operative technique and clinical outcomes. J Bone Joint Surg Am 93(19):1773–1780
Tsukada H, Ishibashi Y, Tsuda E, Fukuda A, Yamamoto Y, Toh S (2012) Biomechanical evaluation of an anatomic double-bundle posterior cruciate ligament reconstruction. Arthroscopy 28(2):264–271
Veltri DM, Deng XH, Torzilli PA, Warren RF, Maynard MJ (1995) The role of the cruciate and posterolateral ligaments in stability of the knee. A biomechanical study. Am J Sports Med 23(4):436–443
Veltri DM, Warren RF (1993) Isolated and combined posterior cruciate ligament injuries. J Am Acad Orthop Surg 1(2):67–75
Wang CJ, Chen HS, Huang TW (2003) Outcome of arthroscopic single bundle reconstruction for complete posterior cruciate ligament tear. Injury 34(10):747–751
Wang CJ, Weng LH, Hsu CC, Chan YS (2004) Arthroscopic single- versus double-bundle posterior cruciate ligament reconstructions using hamstring autograft. Injury 35(12):1293–1299
Weiler A, Hoffmann RF, Stahelin AC, Bail HJ, Siepe CJ, Sudkamp NP (1998) Hamstring tendon fixation using interference screws: a biomechanical study in calf tibial bone. Arthroscopy 14(1):29–37
Whiddon DR, Zehms CT, Miller MD, Quinby JS, Montgomery SL, Sekiya JK (2008) Double compared with single-bundle open inlay posterior cruciate ligament reconstruction in a cadaver model. J Bone Joint Surg Am 90(9):1820–1829
Wijdicks CA, Kennedy NI, Goldsmith MT, Devitt BM, Michalski MP, Aroen A, Engebretsen L, LaPrade RF (2013) Kinematic analysis of the posterior cruciate ligament, part 2: a comparison of anatomic single- versus double-bundle reconstruction. Am J Sports Med 41(12):2839–2848
Woo SL, Gomez MA, Seguchi Y, Endo CM, Akeson WH (1983) Measurement of mechanical properties of ligament substance from a bone-ligament-bone preparation. J Orthop Res 1(1):22–29
Woo SL, Gomez MA, Sites TJ, Newton PO, Orlando CA, Akeson WH (1987) The biomechanical and morphological changes in the medial collateral ligament of the rabbit after immobilization and remobilization. J Bone Joint Surg Am 69(8):1200–1211
Woo SL, Hollis JM, Adams DJ, Lyon RM, Takai S (1991) Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am J Sports Med 19(3):217–225
Woo SL, Wu C, Dede O, Vercillo F, Noorani S (2006) Biomechanics and anterior cruciate ligament reconstruction. J Orthop Surg Res 1:2
Acknowledgments
The authors thank Grant J. Dornan, MSc, for his assistance with statistical analysis. The authors thank Allosource (Centennial, Colorado) for the in-kind donation of allograft tissue.
Author information
Authors and Affiliations
Corresponding author
Additional information
Investigation performed at the Department of BioMedical Engineering, Steadman Philippon Research Institute, Vail, Colorado, USA.
Rights and permissions
About this article
Cite this article
Mook, W.R., Civitarese, D., Turnbull, T.L. et al. Double-bundle posterior cruciate ligament reconstruction: a biomechanical analysis of simulated early motion and partial and full weightbearing on common reconstruction grafts. Knee Surg Sports Traumatol Arthrosc 25, 2536–2544 (2017). https://doi.org/10.1007/s00167-016-4056-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-016-4056-7