Abstract
Purpose
The purpose of this study was to know which tunnel—the anteromedial (AM) bundle or the posterolateral (PL) bundle—should be prepared first to create the 2 femoral tunnels accurately in anatomic double-bundle (DB) anterior cruciate ligament (ACL) reconstruction.
Methods
Thirty-four patients were divided into 2 groups of 17 depending on the sequence of preparation of the 2 femoral tunnels. In group A, the AM tunnel was prepared first, whereas the PL tunnel was prepared first in group P. ACL reconstruction was performed using a three-dimensional (3-D) fluoroscopy-based navigation system to place the double femoral tunnels through an accessory medial portal. The double femoral socket positioning was evaluated by 3-D computed tomography (CT) scan image.
Results
The non-anatomical placement of the femoral sockets occurred in 5 patients (29%) in group A, whereas the 2 sockets were placed anatomically in all patients in group P (P < 0.05). Evaluation of the AM and the PL socket location on the 3-D CT images using the quadrant method showed more similar values to the laboratory data in a literature in group P than in group A. No complication occurred in group A, whereas complications such as socket communications or back wall blowout occurred in 5 patients (29%) in group P (P < 0.05).
Conclusion
The sequence of creating 2 femoral tunnels through accessory medial portal affected the resultant location of the sockets and the rate of the complications. When femoral tunnels are prepared with a transportal technique, PL tunnel first technique seems to be superior to AM first technique regarding anatomic placement. However, PL tunnel first technique accompanies the risk of socket communication.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Conventional single-bundle (SB) anterior cruciate ligament (ACL) reconstruction has demonstrated good clinical results [23]; however, patients with reconstructed knees occasionally develop a degenerative change including medial meniscus injury and osteoarthritis [15]. Recently, better knowledge of ACL anatomy and function has led to “anatomic” ACL reconstruction [4, 6, 8, 10, 25]. Anatomic double-bundle (DB) ACL reconstruction, reproducing anteromedial (AM) and posterolateral (PL) bundles within the native ACL insertion, has been introduced to offer better biomechanical outcomes, especially in controlling rotatory loads [24, 29]. Various surgical techniques for anatomic ACL reconstruction using hamstrings [21, 26, 32], bone-patellar tendon-bone [17, 18], or quadriceps tendon [14, 20] have been reported. One of the most important factors influencing the surgical outcome after ACL reconstruction might be the placement of the femoral socket. If the femoral sockets are not positioned within the native ACL insertion, rotatory instability and impingement on the roof or the posterior cruciate ligament might occur [5]. It is technically difficult to place the 2 femoral sockets in ideal positions reproducibly, even for experienced surgeons.
Many techniques have been reported to create the femoral tunnels including the traditional transtibial technique [11, 27], the outside-in technique in which the femoral guide pin is placed transfemorally from the outside [13, 28], and the transportal technique using a medial portal or an accessory medial portal [3, 12, 21]. We used a three-dimensional (3-D) fluoroscopy-based navigation system to place the 2 femoral tunnels accurately and reproducibly through an accessory medial portal [16]. With respect to the transportal technique, some authors prepared the AM tunnel first [12], whereas others prepared the PL tunnel first [30]; however, there has been no definite recommendation regarding which tunnel should be prepared first [30]. Therefore, the purpose of this study was to learn which one should be prepared first to create the 2 femoral tunnels, accurately in anatomic DB ACL reconstruction. We hypothesized that the sequence of preparing 2 femoral tunnels (AM first technique or PL first technique) would affect the accuracy and consistency of socket positioning in anatomic DB ACL reconstruction. Consecutive ACL reconstruction cases were divided into 2 groups depending on the sequence of preparing the femoral tunnels, i.e., AM first group or PL first group. We evaluated the position of the femoral sockets after anatomic DB ACL reconstruction using 3-D computed tomography (CT) scan images that were performed a week after surgery.
Materials and methods
Of the 37 consecutive patients on whom the ACL reconstruction was performed in our institute between July 2008 and August 2009, 34 met the inclusion criteria. In this study, these criteria were as follows: (1) using a 3-D fluoroscopy-based navigation system for operation, (2) using hamstring grafts for DB ACL reconstruction, (3) no prior intra-articular or extra-articular ligament reconstruction, and (4) no history of distal femoral fracture. The patients included 13 women and 21 men with an average age of 31 years (range 15–63). They were divided into 2 groups based on the sequence of preparing the 2 femoral tunnels. In group A, the AM tunnel was prepared prior to the PL tunnel and ACL reconstruction on these patients was performed between July 2008 and April 2009. In group P, the PL tunnel was prepared first when creating the 2 femoral tunnels and surgery on these patients was carried out between May 2009 and August 2009; there were 17 patients in each group. The mean AM socket diameter was 5.6 ± 0.3 mm in group A and was 5.5 ± 0.6 mm in group P, while the mean PL socket diameter was 5.3 ± 0.4 mm in group A, and was 5.0 ± 0.2 mm in group P. There was no significant difference in the stature of the patients in these 2 groups (n.s.). The surgeries were performed by 4 knee surgeons through this period. Information about patient is summarized in Table 1. The patients and their families were informed that data from their cases would be submitted for publication, and all gave their consent.
Surgical procedure
ACL reconstruction was arthroscopically performed using a 3-D fluoroscopy-based navigation system to place the double femoral tunnels [16]. Intra-operative 3-D images were acquired with the C-arm of Arcadis Orbic 3-D (Siemens AG, Erlangen, Germany) after the reference frame was attached rigidly to the femur. The acquired image data were downloaded to the navigation computer (StealthStation TRIA plus; Medtronic), and a 3-D image of the distal femur was reconstructed on the computer screen. The medial half of the 3-D reconstructed distal femur was deleted using computer software for a better view of the lateral wall and the roof of the femoral intercondylar notch. The hamstring tendons were harvested, and the doubled grafts were looped over EndoButton CLs (Smith & Nephew Endoscopy, Andover, MA). The anatomic insertion site of each native ACL bundle was marked on the femur and tibia by a radiofrequency device. The femoral insertion site of each bundle was determined in reference to the bony landmarks (i.e. the lateral intercondylar ridge and the lateral bifurcate ridge) both arthroscopically and on the 3-D reconstructed image [8, 9]. An arthroscope was introduced through a medial portal, and the tip of the femoral guide through an accessory medial portal was placed at the center of the AM and the PL bundle’s femoral footprint, respectively (Fig. 1). Keeping the femoral guide tip at the center of the AM or the PL footprint, the knee was flexed to 120° to 130°. On the navigation computer screen, the surgeon confirmed the anatomically correct placement of the tip of the femoral guide. The risk of a back wall blowout was also evaluated, and the virtual exit of the femoral tunnel on the monitor enabled us to estimate the length of the tunnel. After the guidewire for the femoral tunnel was placed, the wire was overdrilled for an adequate length using a cannulated drill, and the lateral femoral cortex was drilled through using an EndoButton drill (Smith & Nephew Endoscopy, Andover, MA). The AM tunnel was created first in group A, while the PL tunnel was created first in group P (Fig. 1). The other surgical methods were same in both groups.
a, c The tip of the femoral guide was placed at the center of the anteromedial (AM) and posterolateral (PL) bundle’s femoral footprint through an accessory medial portal. b, d The lateral wall and the roof of the femoral intercondylar notch, and the lateral intercondylar ridge (brokenline) on the three-dimensional (3-D) reconstructed image. Arrow shows the tip of the femoral guide placed at the center of the AM bundle and the PL bundle, respectively
Evaluation of the position of femoral sockets with 3-D CT scan
Three-dimensional CT scan image of the operated knee was done a week after surgery. Medical research center ethics committee of our institute approved this study including the use of a postoperative CT scan. The CT machine was a helical high-speed Aquilion™ 64 or Aquilion ONE™ (Toshiba Medical Systems Co., Japan). Then, 3-D reconstruction of the operated knee was performed using the ZIOSTATION software package (Ziosoft Inc., Tokyo, Japan). The tibia, the patella, and the medial femoral condyle were removed as necessary to visualize the lateral wall of the intercondylar notch. All measurements were taken on the surface of the lateral wall of the intercondylar notch completely in an orthogonal projection to the view angle of the surface being measured to ensure accuracy. According to Basdekis et al. [1], the measurement error of this evaluating method was very small.
We determined whether the 2 femoral sockets were positioned within the femoral insertion of the native ACL on the 3-D CT scan image. In a case where the lateral intercondylar ridge could be seen on the 3-D CT scan image, we defined both the AM and the PL sockets positioned posterior to the lateral intercondylar ridge as anatomic socket placements [8] (Fig. 2). In a case where the lateral intercondylar ridge could not be clearly seen on the image, we defined the 2 femoral sockets positioned posterior to the extended line from the posterior cortex of the distal femur as anatomic socket placements, in reference to the reports by Giron et al. [10] (Fig. 3). In addition, as the complication in preparing the femoral tunnels, the communication between the AM socket and the PL socket, and the back wall blowout were elucidated by the 3-D CT scan image (Fig. 4).
When the lateral intercondylar ridge could not be seen on the 3-D CT scan image, we defined the 2 femoral sockets positioned below the extended line from the posterior cortex as being correct socket within the native anterior cruciate ligament (ACL). a The 2 femoral sockets within the femoral insertion of the native ACL, b the PL socket protruded from the femoral insertion of the native ACL
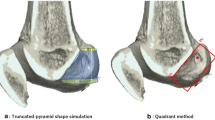
Morphometric assessment of the femoral socket positioning of the AM and the PL bundles was performed according to the quadrant technique as described by Bernard and Hertel [2]. The total sagittal diameter of the lateral condyle along Blumensaat’s line (D) and maximum lateral intercondylar notch height (H) were measured on the 3-D CT scan image. The distance from the center of the AM or the PL socket to the most dorsal subchondral contour of the lateral femoral condyle (d) and the distance from the center of each socket to Blumensaat’s line (h) were measured (Fig. 5). The length of distance d as a partial distance of D and the height of the distance h as a partial distance of H were expressed in percentages, such as d/D and h/H%, respectively. All measurements were taken by one orthopedic surgeon and were repeated at 3-month interval. For morphometric assessment using the quadrant technique, a mean of these repeated measurements was recorded and intra-observer variability was calculated.
Measurements of the femoral socket positioning using the quadrant method described by Bernard and Hertel [2]. The total sagittal diameter of the lateral condyle along Blumensaat’s line (D) and maximum lateral intercondylar notch height (H) were measured on the 3-D CT scan image. The distance from the center of the AM or the PL socket to the most dorsal subchondral contour of the lateral femoral condyle (d) and the distance from the center of the AM or the PL socket to Blumensaat’s line (h) were measured
Statistical analysis
Statistical analysis was done using the EXCEL statistics 2008 (SSRI Co., Ltd., Tokyo, Japan) software package for Microsoft Windows. Fisher’s exact test was used to compare the rate of complication and the rate of anatomical femoral socket placement. Paired t test was used to compare the femoral socket positioning using the quadrant technique and the stature, body weight, and body mass index of patients. The statistical significance level was set at P < 0.05.
Results
The non-anatomical placement of the femoral sockets was found in 5 patients (29%) in group A on the 3-D CT scan image evaluation, whereas both sockets were placed within the native femoral insertion in all patients in group P. The rate of anatomical femoral socket positioning was significantly higher in group P than in group A (P = 0.04). (Table 2) No complication in preparing the femoral sockets was found in group A; however, it was found in 5 patients (29%) in group P. The socket communications occur in 4 patients, and the back wall blowout was found in 1 patient. (Table 2) The rate of complication was significantly higher in group P than in group A (P = 0.04).
The assessment of the femoral socket positioning on the 3-D CT scan image according to the quadrant technique described by Bernard and Hertel [2] showed that the center of the AM socket was located at d/D = 22.4 ± 4.3% and h/H = 35.3 ± 10.6% in group A, whereas it was located at d/D = 20.1 ± 4.8% and h/H = 27.3 ± 6.1% in group P, respectively. In the same way, the center of the PL socket was located at d/D = 35.0 ± 7.9% and h/H = 59.5 ± 7.5% in group A, whereas it was located at d/D = 30.2 ± 6.5% and h/H = 53.6 ± 5.8% in group P, respectively (Table 3 and Fig. 6). The values of h/H for the AM socket and the PL socket were significantly larger in group A than in group P (P = 0.01 and P = 0.01, respectively). No significant difference was found in the values of d/D for the 2 sockets between group A and group P (n.s.). The intra-observer difference was 1.3 ± 1.2%. Laboratory data described by the quadrant method [31] are shown at the bottom of the Table 3.
Center of the AM (a) socket and the PL (b) socket location on the lateral wall of femoral notch was calculated using the quadrant method. Square dot showed the center of each socket in group A, round dot showed that in group P, and triangular dot showed the center of each bundle from the cadaveric data measured by Zantop et al. [31]
Discussion
The most important finding of the present study was that the sequence of drilling affected the position of the femoral sockets. When the AM tunnel was prepared prior to the PL tunnel preparation, the PL sockets were significantly apt to be positioned anterior (arthroscopically shallow) to the lateral intercondylar ridge with the knee in 90° of flexion. This shallow positioning of the PL socket might result from the intention to preserve a bony bridge and avoid socket communication with the pre-existing AM socket in group A. On the other hand, when the PL socket was prepared anatomically just posterior to the lateral intercondylar ridge with the knee in 90° of flexion, the 2 femoral sockets were positioned within the native ACL femoral insertion in all cases. However, the complications in preparing the femoral sockets occurred in 29% of the patients, which was statistically significant different from group A. It seemed that space for the AM socket was very limited in some patients because of the existence of the anatomically placed PL socket. As a result, the bony bridge could not be preserved in some cases, although the AM socket has been placed as arthroscopically deep as possible with the assistance of 3-D fluoroscopic navigation to avoid a back wall blowout. Colombet et al. suggested that with a 5-mm-diameter graft for the PL bundle and a 7-mm-diameter AM graft, it would be theoretically possible to place the centers of both grafts in their respective native attachment site positions, while leaving a 2-mm bony bridge between sockets [4]. Van Eck et al. recommended SB reconstruction to avoid a complication, if the femoral insertion site was less than 14 mm in diameter, in their recent literature [22]. According to Siebold et al. [19], the length of the femoral insertion varied between 10 and 19 mm, with a mean of 14 to 15 mm. Recognizing that there is an individual difference in the footprint sizes and that the figures of Japanese people are smaller than those of Europeans, there most likely would be cases in which no bony bridge can be preserved when both AM and PL sockets are placed within a narrow ACL femoral insertion.
The morphometric analysis of the femoral socket placements between group A and group P was done using the quadrant method described by Bernard and Hertel [2]. The values of h/H for the AM socket and the PL socket were significantly larger in group A than in group P. The values of d/D for the 2 sockets were larger in group A than in group P, even though no significant difference was found. Based on these calculated data, the 2 femoral sockets were positioned more posterior (arthroscopically deep) in group P than in group A. Zantop et al. reported the radiographic center of the AM and the PL attachment on the femoral side using the quadrant method in 20 human cadaveric knees [31]. According to them, the center of the AM bundle on the femoral side was 18.5 and 22.3%, whereas the center of the PL bundle on the femoral side was 29.3 and 53.6% of the depth of Blumensaat’s line and the height of the femoral condyle, respectively (Table 3 and Fig. 6). These data were very similar to our calculated value using the postoperative 3-D CT image in both groups. When the mean value was compared between group A and group P, the mean value in group P was more similar to the cadaveric data than in group A.
There is also possibility that the narrow femoral notch would make creating the two anatomical sockets difficult. Between the two groups in this study, the body height of the patients, which is reported to be most significant factor affecting ACL volume [7], was not different. Therefore, the difference of the femoral notch size seemed not to be a confounding factor to affect the results of this study. Overall, our results indicate that the femoral socket placements were more anatomic in group P than in group A, although rate of the complications was high. To achieve both the anatomic socket placement while avoiding socket communication, a navigation software to calculate and determine the appropriate position of the double femoral sockets on 3-D navigation monitor might be helpful in future. The arthroscopic caliper to measure accurately the length of lateral wall of femoral notch might be also useful.
There were some limitations to this study. The first was that the patients were not assigned into 2 groups randomly. Surgery was performed on the patients in group A at an earlier period than those in group P. The learning curve might have affected the results of the study. In other words, there is a possibility that an improvement in surgical techniques might have decreased the rate of the non-anatomical socket placement in group P. Another limitation is that the 3-D CT scan was not taken on the contralateral knee being used as control. It is difficult to evaluate the 3-D CT scan image of the operated knee alone, because the natural morphology of the lateral wall of the intercondylar notch was destroyed by the reconstructive surgery. The lateral intercondylar ridge could not be identified, and accurate evaluation of the femoral insertion was impossible in some patients. Finally, the navigation system that was used as guidance to determine the position of the femoral tunnels was a confounding factor. Therefore, there is possibility that the results of this study could not be directly applied to DB ACL reconstruction without the navigation system.
In our retrospective observation of the socket location between the two techniques, i.e. AM first technique and PL first technique, there was difference in the resultant femoral socket locations between the two techniques. Further randomized prospective studies are required in future to answer which technique—the AM first technique or the PL first technique—would be more appropriate to prepare two anatomical femoral sockets in ACL reconstruction.
Conclusion
The results of this study revealed that the sequence of drilling affected the position of the femoral sockets. Preparing the PL tunnel prior to the AM tunnel enabled us to make the double sockets consistent inside the femoral insertion of native ACL. On the other hand, the rate of complications such as socket communication or back wall blowout was significantly higher in PL tunnel first technique. We believe that it is necessary to take into account the sequence of preparing the two femoral tunnels in DB ACL reconstruction.
References
Basdekis G, Christel P, Anne F (2009) Validation of the position of the femoral tunnels in anatomic double-bundle ACL reconstruction with 3-D CT scan. Knee Surg Sports Traumatol Arthrosc 17:1089–1094
Bernard M, Hertel P, Hornung H, Cierpinski T (1997) Femoral insertion of the ACL. Radiographic quadrant method. Am J Knee Surg 10:14–22
Christel P, Sahasrabudhe A, Basdekis G (2008) Anatomic double-bundle anterior cruciate ligament reconstruction with anatomic aimers. Arthroscopy 24:1146–1151
Colombet P, Robinson J, Christel P, Franceschi J, Djian P, Bellier G, Sbihi A (2006) Morphology of anterior cruciate ligament attachments for anatomic reconstruction: a cadaveric dissection and radiographic study. Arthroscopy 22:984–992
Colvin A, Shen W, Musahl V, Fu F (2009) Avoiding pitfalls in anatomic ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 17:956–963
Farrow L, Chen M, Cooperman D, Victoroff B, Goodfellow D (2007) Morphology of the femoral intercondylar notch. J Bone Joint Surg Am 89:2150–2155
Fayad L, Rosenthal E, Morrison W, Carrino J (2008) Anterior cruciate ligament volume: analysis of gender differences. J Magn Reson Imaging 27:218–223
Ferretti M, Ekdahl M, Shen W, Fu F (2007) Osseous landmarks of the femoral attachment of the anterior cruciate ligament: an anatomic study. Arthroscopy 23:1218–1225
Fu F, Jordan S (2007) The lateral intercondylar ridge–a key to anatomic anterior cruciate ligament reconstruction. J Bone Joint Surg Am 89:2103–2104
Giron F, Cuomo P, Aglietti P, Bull A, Amis A (2006) Femoral attachment of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc 14:250–256
Giron F, Cuomo P, Edwards A, Bull A, Amis A, Aglietti P (2007) Double-bundle “anatomic” anterior cruciate ligament reconstruction: a cadaveric study of tunnel positioning with a transtibial technique. Arthroscopy 23:7–13
Harner C, Honkamp N, Ranawat A (2008) Anteromedial portal technique for creating the anterior cruciate ligament femoral tunnel. Arthroscopy 24:113–115
Kaseta M, DeFrate L, Charnock B, Sullivan R, Garrett WJ (2008) Reconstruction technique affects femoral tunnel placement in ACL reconstruction. Clin Orthop Relat Res 466:1467–1474
Kim S, Jung K, Song D (2006) Arthroscopic double-bundle anterior cruciate ligament reconstruction using autogenous quadriceps tendon. Arthroscopy 22: 797.e791–795
Lidén M, Sernert N, Rostgård-Christensen L, Kartus C, Ejerhed L (2008) Osteoarthritic changes after anterior cruciate ligament reconstruction using bone-patellar tendon-bone or hamstring tendon autografts: a retrospective, 7-year radiographic and clinical follow-up study. Arthroscopy 24:899–908
Nakagawa T, Takeda H, Nakajima K, Nakayama S, Fukai A, Kachi Y, Kawano H, Miura T, Nakamura K (2008) Intraoperative 3-dimensional imaging-based navigation-assisted anatomic double-bundle anterior cruciate ligament reconstruction. Arthroscopy 24:1161–1167
Pujol N, Fong O, Karoubi M, Beaufils P, Boisrenoult P (2010) Anatomic double-bundle ACL reconstruction using a bone-patellar tendon-bone autograft: a technical note. Knee Surg Sports Traumatol Arthrosc 18:43–46
Shino K, Nakata K, Nakamura N, Toritsuka Y, Nakagawa S, Horibe S (2005) Anatomically oriented anterior cruciate ligament reconstruction with a bone-patellar tendon-bone graft via rectangular socket and tunnel: a snug-fit and impingement-free grafting technique. Arthroscopy 21:1402
Siebold R, Ellert T, Metz S, Metz J (2008) Femoral insertions of the anteromedial and posterolateral bundles of the anterior cruciate ligament: morphometry and arthroscopic orientation models for double-bundle bone tunnel placement–a cadaver study. Arthroscopy 24:585–592
Sonnery-Cottet B, Chambat P (2006) Anatomic double bundle: a new concept in anterior cruciate ligament reconstruction using the quadriceps tendon. Arthroscopy 22: 1249.e1241–1244
Toritsuka Y, Amano H, Kuwano M, Iwai T, Mae T, Ohzono K, Shino K (2009) Outcome of double-bundle ACL reconstruction using hamstring tendons. Knee Surg Sports Traumatol Arthrosc 17:456–463
van Eck C, Lesniak B, Schreiber V, Fu F (2010) Anatomic single- and double-bundle anterior cruciate ligament reconstruction flowchart. Arthroscopy 26:258–268
Williams Rr, Hyman J, Petrigliano F, Rozental T, Wickiewicz T (2005) Anterior cruciate ligament reconstruction with a four-strand hamstring tendon autograft. Surgical technique. J Bone Joint Surg Am 87(Suppl 1):51–66
Yagi M, Wong E, Kanamori A, Debski R, Fu F, Woo S (2002) Biomechanical analysis of an anatomic anterior cruciate ligament reconstruction. Am J Sports Med 30:660–666
Yasuda K, Ichiyama H, Kondo E, Miyatake S, Inoue M, Tanabe Y (2008) An in vivo biomechanical study on the tension-versus-knee flexion angle curves of 2 grafts in anatomic double-bundle anterior cruciate ligament reconstruction: effects of initial tension and internal tibial rotation. Arthroscopy 24:276–284
Yasuda K, Kondo E, Ichiyama H, Kitamura N, Tanabe Y, Tohyama H, Minami A (2004) Anatomic reconstruction of the anteromedial and posterolateral bundles of the anterior cruciate ligament using hamstring tendon grafts. Arthroscopy 20:1015–1025
Yasuda K, Kondo E, Ichiyama H, Tanabe Y, Tohyama H (2006) Clinical evaluation of anatomic double-bundle anterior cruciate ligament reconstruction procedure using hamstring tendon grafts: comparisons among 3 different procedures. Arthroscopy 22:240–251
Yu J, Garrett WE (2006) Femoral tunnel placement in anterior cruciate ligament reconstruction. Oper Tech Sports Med 14:45–49
Zantop T, Diermann N, Schumacher T, Schanz S, Fu F, Petersen W (2008) Anatomical and nonanatomical double-bundle anterior cruciate ligament reconstruction: importance of femoral tunnel location on knee kinematics. Am J Sports Med 36:678–685
Zantop T, Kubo S, Petersen W, Musahl V, Fu F (2007) Current techniques in anatomic anterior cruciate ligament reconstruction. Arthroscopy 23:938–947
Zantop T, Wellmann M, Fu F, Petersen W (2008) Tunnel positioning of anteromedial and posterolateral bundles in anatomic anterior cruciate ligament reconstruction: anatomic and radiographic findings. Am J Sports Med 36:65–72
Zelle B, Vidal A, Brucker P, Fu F (2007) Double-bundle reconstruction of the anterior cruciate ligament: anatomic and biomechanical rationale. J Am Acad Orthop Surg 15:87–96
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Taketomi, S., Nakagawa, T., Takeda, H. et al. Anatomical placement of double femoral tunnels in anterior cruciate ligament reconstruction: anteromedial tunnel first or posterolateral tunnel first?. Knee Surg Sports Traumatol Arthrosc 19, 424–431 (2011). https://doi.org/10.1007/s00167-010-1246-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-010-1246-6