Abstract
Purpose
Central line-associated bloodstream infection (CLABSI) is an important cause of complications in paediatric intensive care units (PICUs). Peripherally inserted central catheters (PICCs) could be an alternative to central venous catheters (CVCs) and the effect of PICCs compared with CVCs on CLABSI prevention is unknown in PICUs. Therefore, we aimed to evaluate whether PICCs were associated with a protective effect for CLABSI when compared to CVCs in critically ill children.
Methods
We have carried out a retrospective multicentre study in four PICUs in São Paulo, Brazil. We included patients aged 0–14 years, who needed a CVC or PICC during a PICU stay from January 2013 to December 2015. Our primary endpoint was CLABSI up to 30 days after catheter placement. We defined CLABSI based on the Center for Disease Control and Prevention’s National Healthcare Safety Networks (NHSN) 2015 surveillance definitions. To account for potential confounders, we used propensity scores with inverse probability weighting.
Results
A total of 1660 devices (922 PICCs and 738 CVCs) in 1255 children were included. The overall CLABSI incidence was 2.28 (95% CI 1.70–3.07)/1000 catheter-days. After covariate adjustment using propensity scores, CVCs were associated with higher risk of CLABSI (adjHR 2.20, 95% CI 1.05–4.61; p = 0.037) compared with PICCs. In a sensitivity analysis, CVCs remained associated with higher risk of CLABSI (adjHR 2.18, 95% CI 1.02–4.64; p = 0.044) after adding place of insertion and use of parenteral nutrition to the model as a time-dependent variable.
Conclusions
PICC should be an alternative to CVC in the paediatric intensive care setting for CLABSI prevention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Central line-associated bloodstream infection (CLABSI) is an important cause of complications in paediatric intensive care units (PICUs). Even though the rate of CLABSI has decreased in developed countries, mainly due to the large-scale implementation of bundles for its prevention [1, 2], it is still a common problem, especially in the most severely ill population. The burden is even worse in undeveloped and developing countries, and becomes more relevant if we consider that CLABSI increases length of stay (LOS), antibiotic use, hospital costs and morbidity [3, 4].
The use of peripherally inserted central catheters (PICCs), inside and outside the PICU, is increasing worldwide, probably due to the low risk of mechanical complications during placement [5]. It has also been suggested that PICC use is associated with a reduction in the incidence of CLABSI. PICCs have had the advantage over central venous catheters (CVCs) in reducing CLABSI in specific paediatric populations, such as outpatients who need total parenteral nutrition (TPN) or chemotherapy [6]. However, no advantage regarding the prevention of CLABSI has been observed in hospitalised and critically ill adult patients [7, 8]. There is a lack of studies regarding the role of PICCs in reducing the risk of CLABSI compared to CVCs in paediatric critical care settings. The objective of our study was to evaluate whether PICCs were associated with a protective effect for CLABSI when compared to CVCs in children admitted to four PICUs in Brazil.
Methods
Patients and devices
We performed a retrospective multicentre study in four PICUs (total 56 beds) at four non-teaching private hospitals in São Paulo, Brazil. The four PICUs were embedded in a network, had a closed model of organisation and staff, and were under central administration. All PICUs received clinical (including oncological, neurological and cardiological patients) and surgical patients, aged 0–14 years. We included patients admitted to any of the 4 PICUs who received a CVC or PICC during their PICU stay from January 2013 to December 2015. We excluded patients under the age of 30 days at the time of catheter insertion, catheters removed on the same day as placement, long-term use catheters and those whose catheters were inserted by dissection, because these catheters have specific characteristics and risk factors associated with CLABSI. There were no haemodialysis catheters as we only have peritoneal dialysis in our units.
The data were extracted from the Epimed Monitor System® and electronic medical records. The Epimed Monitor System® (Epimed Solution®, Rio de Janeiro, Brazil), is a commercial cloud-based registry for quality improvement, performance evaluation, and benchmarking purposes. Data were entered in the Epimed Monitor System by registered nurses trained in case-management of critically ill patients [9, 10]. We retrieved data for age, gender, weight, main reason for admission, type of admission (i.e. medical/surgical), underlying diseases, PIM and PRISM scores, and organ support during PICU stay. We also extracted details of the site of catheter insertion, placement and removal dates and daily use of parenteral nutrition during the entire PICU stay.
Our exposure was defined as the type of catheter: PICC and CVC. The use of PICC or CVC was at the discretion of the attending physician. Our units have standard operating procedures (SOPs) for device placements, and the PICC and CVC placement technique was standardised across the 4 units and audited by a trained nurse with a checklist. CVCs were placed either by a paediatric intensive care physician or a paediatric surgeon, and PICCs were placed by a specially trained nurse. Both CVC and PICC were placed using maximal sterile barrier precautions and alcoholic chlorhexidine for skin asepsis. All PICUs have infection control policies based on Institute of Healthcare Improvement bundles for CLABSI. This bundle has five main pillars: hand hygiene; maximal barrier precautions; chlorhexidine skin asepsis; optimal catheter site selection; and daily review of line necessity [11]. The staff had continuing training on aseptic precautions during device placement and maintenance. All catheter dressings were audited daily by a trained nurse, and devices were evaluated daily in a multidisciplinary round and removed as soon as possible. All four hospitals have a centrally coordinated hospital infection committee; healthcare-associated infection rates are analysed monthly by the committee and every positive case is discussed, promoting educational feedback and identifying improvement action [10].
Our primary endpoint was CLABSI up to 30 days after catheter placement. We defined CLABSI based on the Center for Disease Control and Prevention’s National Healthcare Safety Networks (NHSN) 2015 surveillance definitions [12]. We measured the primary endpoint as the CLABSI rate, defined as the number of CLABSI occurrences divided by the total days of catheter use, multiplied by 1000. The Hospital Infection Surveillance Committee from each hospital prospectively tracked all blood cultures (Vitek® XL and Vitek® MS; bioMérieux, France) collected in the respective hospital. For patients with positive blood cultures, CLABSI was defined if the criteria from the NHSN 2015 definition were fulfilled [12]. CLABSI cases were prospectively entered in the hospital-acquired infections module of the Epimed Monitor System®. For this study, we revised the electronic medical records of all previously defined CLABSI cases to check if all NHSN criteria were fulfilled. The reviewing process was blinded as to whether the patients had received a PICC or CVC.
Statistical analysis
Continuous data are presented as means (standard deviation) or as medians and interquartile range (IQR), as appropriate. Categorical variables are shown as percentages. For comparison of categorical variables, Fisher’s exact test or a Chi square test were used; for continuous variables, an unpaired t test or the Mann–Whitney U test was used, as appropriate.
As the choice between PICC and CVC was not randomised, we used a propensity score strategy to correct for potential confounding factors, indication, and unbalanced variables upon device insertion [13,14,15]. We used the covariate-balancing propensity score (CBPS) method, which concurrently maximises the covariate balance and the treatment assignment prediction. CBPS is a new and robust method which outperformed other propensity score methods in simulations and empirical data [16, 17]. We defined a priori the variables listed in Table 1 to include in the CBPS model, allowing for a non-parsimonious model and aiming for an optimal covariate balance, as recommended by the propensity score literature [13, 18]. To avoid selection- and immortal-time bias, we included only variables from ICU admission up to the day of device insertion. The dependent variable for the CBPS model was every catheter used for a patient, and we accounted for clustering at the unit and patient levels [15, 19]. To evaluate the association between our exposure (device type) and the occurrence of CLABSI, we fitted Cox proportional hazards regression models. First, we ran a model only with the exposure. Second, we applied the optimal weights from the CBPS and fitted the Cox model in the weighted population [15, 20]. Additionally, we fitted the same Cox model in the weighted population, adjusting for place of insertion and total parenteral nutrition as a time-dependent variable. In a sensitivity analysis, we re-estimated the propensity score model and fitted the previously Cox models after exclusion of patients with bronchiolitis/asthma as baseline comorbidities, because of the potential different reasons for central venous line indication in these patients. The assumption of proportionality was verified with an interaction term in the model and graphically by a log–log plot. The models accounted for multiple catheters per patient using a robust variance estimator [15, 21]. To evaluate the covariate balance, we assessed the absolute standardised difference before and after weighting.
Statistical analysis was performed using Stata 13.1 (StataCorp, Texas) and R statistical software (R Foundation for Statistical Computing, Vienna), packages cbpss and cobalt.
The study was reviewed and approved by the Ethics Committee of every hospital and informed consent was waived.
Results
Patients
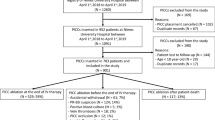
From January 2013 to December 2015, we identified 1786 catheters placed in 1316 children. We excluded 23 long-term use catheters, 56 catheters inserted by dissection and 47 catheters removed on the same day as placement, resulting in a total of 1660 catheters implanted in 1255 children (eFig. 1).
The demographic and clinical characteristics of the population are described in Table 1. There were similar distributions of demographic variables in the CVC and PICC groups but there was a shift for using PICCs in older children (p = 0.063). We observed higher prevalence of cardiologic (CVC 9.6% × PICC 4.5%, p < 0.001) and chronic neurological diseases (CVC 2.0% × PICC 0.4%, p 0.007) in the CVC group but lower prevalence of acute respiratory diseases (bronchiolitis and asthma) (CVC 13.9% × PICC 20.8%, p = 0.001). We also observed more frequent use of CVCs in more severe patients, measured by the prognostic scores (PRISM and PIM), use of mechanical ventilation and vasoactive drugs, and history of cardiac arrest, both in admission and during PICU stay. The main reasons for PICU admission were similar in both groups, with infection and respiratory diseases being the most common. The crude ICU mortality was higher in the CVC group (CVC 9.4% × PICC 6.3%; p = 0.038); however no difference was observed for crude in-hospital mortality (CVC 9.8% × PICC 7.2%; p = 0.089). Median ICU LOS [CVC 10 (4–20) × PICC 9 (5–17) days; p = 0.83] and median hospital LOS [CVC 18 (9–39) × PICC 19 (10–37) days; p = 0.37] were similar in both the CVC and PICC groups.
Catheter characteristics
A total of 1660 devices were included in the study, 738 (44.5%) CVCs and 922 (55.5%) PICCs. The distribution of number of catheters per patient during the ICU stay was as follows: 977 (77.8%) children had only 1 central line catheter, 198 (15.8%) had 2, 51 (4.0%) had 3, 17 (1.4%) had 4, 7 (0.6%) had 5, 4 (0.3%) had 6 and 1 (0.1%) had 7. The catheter characteristics are shown in Table 2. Overall, the median time from ICU admission to catheter insertion was comparable between devices. PICCs and CVCs were mainly implanted in supra-diaphragmatic sites (PICC ~97% × CVC ~ 80%), with superior limbs and jugular accesses as the preferred sites of insertion for PICCs and CVCs, respectively. The median catheter duration was comparable between devices.
CLABSI incidence
We observed 44 CLABSIs in 1660 catheters (2.6%), and higher incidence in the CVC group (CVC 4.2% × PICC 1.4%; p < 0.001). There was no difference in median time between catheter placement and infectious event [CVC 9 (6–16) × PICC 10 (6–15) days; p = 0.95]. The pathogens isolated in all infectious events are presented in eTable 1. More than half of the CLABSIs (56.8%) were caused by Gram-negative bacteria, with Klebsiella pneumoniae as the main agent. The second most isolated agent was fungus (27.3%), especially Candida parapsilosis, followed by Gram-positive bacteria (15.9%).
The overall rate of CLABSI was 2.28 (95% CI 1.70–3.07) per 1000 catheter-days (44 events in 19,278 catheter-days). The rate for CVCs was 3.72 (95% CI 2.61–5.29) compared with 1.19 (95% CI 0.69–2.05) per 1000 catheter-days for PICCs. The unadjusted analysis showed a higher risk of CLABSI in the CVC group compared with PICC (HR 3.13, 95% CI 1.64–5.98; p < 0.001) (Table 3). The distributions of the propensity scores and the standardised differences before and after weighting are shown in eFigs. 2 and 3. After weighting, we observed an important reduction on the standardised differences for all covariates, remaining below 0.10 as recommended (eFig. 4). The positive association between CVC and CLABSI remained significant in the weighted population (adjHR 2.20, 95% CI 1.05–4.61; p = 0.037; Fig. 1; eFig. 5). This association was also observed after adding place of insertion and use of parenteral nutrition into the model (Table 3).
In the sensitivity analysis, we analysed 1389 catheters from 1031 children. The positive association between CVC and CLABSI remained significant (adjHR 2.25, 95% CI 1.01–5.03; p = 0.047; eTable 2, propensity score and model diagnosis: eFigs. 6, 7, 8 and 9), after adjusting for the inverse probability of treatment weighting (IPTW), place of insertion and use of parenteral nutrition.
Discussion
This is the first multicentre study to compare the incidence of CLABSI in PICCs and CVCs in the paediatric critical care setting. We observed that PICCs have been used for a large proportion of critically ill children (around 56%) in our study, and that the use of CVCs, compared to PICCs, increased the risk for CLABSI after adjusting for several potential confounding factors.
We observed an overall incidence of 2.28 CLABSIs per 1000 catheter-days, similar to incidences currently reported in PICUs in high-income countries (from 0.3 to 5.2 infections per 1000 catheter-days) [1, 22,23,24,25]. In contrast, middle- and low-income countries reported higher incidences, ranging from 1.6 to 44.6 events per 1000 catheter-days [26,27,28]; in Brazil, specifically, the incidence reported in PICUs was 8.46 [26]. In recent decades, there have been efforts to improve practice in ICUs regarding the prevention of CLABSI, but it remains one of the most important infectious complications during hospital, and especially ICU, stay [29,30,31,32]. Importantly, the reduction in CLABSI incidence associated with these measures seems to have reached a plateau, so additional actions are needed to further reduce the rate of CLABSI [25, 33].
Two systematic reviews and meta-analyses in adult patients showed controversial results regarding the role of PICCs in CLABSI prevention. Maki et al. found a slightly lower risk of CLABSI in PICCs when compared to CVCs [6], while Chopra et al. observed this protective effect only in outpatients [8]. In the paediatric population, three studies showed a protective [34] or no effect [35, 36] of PICC on the incidence of CLABSI in non-intensive care patients. Our study contributes in a singular way to the literature showing that, in a large sample of critically ill children, PICC use was associated with lower risk of CLABSI after adjusting for several potential confounders in a setting with a low incidence of CLABSI. Some possible reasons for this result can be highlighted. First, the choice of catheter insertion site is usually inherited in the choice between a PICC or a CVC. Because one of the pathogenesis pathways of CLABSI is the extra luminal migration of bacteria from the skin [37], the longer length of the PICC, and the lower density of bacteria in extremities [7], it is expected that PICCs may lead to a lower incidence of CLABSI. Second, the number of lumens, a risk factor for CLABSI [38, 39], is also intrinsic to the catheter type, because in a paediatric population CVCs are usually double-lumen and PICCs single-lumen [5]. Finally, gauge is another physical characteristic that could contribute to our results. In younger children, PICCs have a reduced gauge, which leads to less manipulation of the device because it is virtually impossible to take blood samples and transfuse blood products due to the increased resistance.
Our data have strengths that should be mentioned. The use of PICCs has been increasing recently, thus it is worth noting that more than half the children in our study received only a PICC, placed soon after PICU admission. Therefore, our multicentre study with a large sample can provide information with a potential clinical application in the paediatric population. Second, we used a robust method to adjust for confounders, such as the covariate-balancing propensity score, also accounting for the number of devices used. Additionally, we adjusted for the use of parenteral nutrition through each device on a daily basis, one of the most important risk factors for CLABSI [40].
However, our study has limitations. First, this is a retrospective observational study and the device choice was not randomised. Thus, although we use a robust method to adjust for confounding, there is still the risk of residual confounding. Second, we had no individual data regarding the compliance to the CLABSI prevention bundle. However, the four units were centrally coordinated and received similar training, material supply and routinely audit and feedback about the unit compliance to the prevention measures. Thereby, it would be unexpected that PICC and CVC were unequally exposed to the recommended prevention measures. Third, we retrieved data about blood transfusion for all patients, but were not able to identify if the transfusion occurred after device insertion, thus, to avoid bias, we did not use this variable in our analysis. Fourth, we could not retrieve information about the number of lumens of all catheters placed, a risk factor already described in the literature [38, 39]. Fifth, we did not study the incidence of thrombosis, an important complication commonly associated with PICCs, nor other mechanical complications associated with the use of central lines devices and did not apply a cost-effectiveness analysis. Finally, we included PICUs with low incidence of CLABSI and located in private/non-teaching centrally coordinated hospitals in Brazil. Because local practices and resources availability could be different in other PICUs, other studies should be conducted to assess the generalisability our findings.
Conclusion
CLABSI is an important infectious complication in PICU patients. The role of PICC use in the prevention of CLABSI is unknown in paediatric critical care patients. Our study confirms the hypothesis that PICCs have a protective role in CLABSI prevention when compared to CVCs, in PICUs with low incidence of CLABSI, and their use should be considered instead of CVCs, whenever possible, in the paediatric intensive care setting.
References
Edwards JD, Herzig CT, Liu H et al (2015) Central line–associated blood stream infections in pediatric intensive care units: longitudinal trends and compliance with bundle strategies. Am J Infect Control 43:489–493. doi:10.1016/j.ajic.2015.01.006
Randolph AG (2016) Pragmatic trials in critically ill children are CATCHing on. Lancet 387:1697–1698. doi:10.1016/S0140-6736(16)00566-3
Nowak JE, Brilli RJ, Lake MR et al (2010) Reducing catheter-associated bloodstream infections in the pediatric intensive care unit: business case for quality improvement. Pediatr Crit Care Med 11:579–587. doi:10.1097/PCC.0b013e3181d90569
Blot SI, Depuydt P, Annemans L et al (2005) Clinical and economic outcomes in critically ill patients with nosocomial catheter-related bloodstream infections. Clin Infect Dis 41:1591–1598
Gibson C, Connolly BL, Moineddin R et al (2013) Peripherally inserted central catheters: use at a tertiary care pediatric center. J Vasc Interv Radiol 24:1323–1331. doi:10.1016/j.jvir.2013.04.010
Maki DG, Kluger DM, Crnich CJ (2006) The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc 81:1159–1171. doi:10.4065/81.9.1159
Safdar N, Maki DG (2005) Risk of catheter-related bloodstream infection with peripherally inserted central venous catheters used in hospitalized patients. Chest 128:489–495. doi:10.1378/chest.128.2.489
Chopra V, O’Horo JC, Rogers MAM et al (2013) The risk of bloodstream infection associated with peripherally inserted central catheters compared with central venous catheters in adults: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 34:908–918. doi:10.1086/671737
Soares M, Bozza FA, Angus DC et al (2015) Organizational characteristics, outcomes, and resource use in 78 Brazilian intensive care units: the ORCHESTRA study. Intensive Care Med 41:2149–2160. doi:10.1007/s00134-015-4076-7
Ranzani OT, Simpson ES, Augusto TB et al (2014) Evaluation of a minimal sedation protocol using ICU sedative consumption as a monitoring tool: a quality improvement multicenter project. Crit Care 18:580. doi:10.1186/s13054-014-0580-3
How-to Guide: Prevent Central Line-Associated Bloodstream Infections. Cambridge, MA: Institute for Healthcare Improvement; 2012. (Available at https://www.ihi.org)
Bloodstream Infection Event (Central Line-Associated Bloodstream Infection and non-central line-associated Bloodstream Infection)—Device associated module. Updated January 2017. https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf:
Austin PC (2014) The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments: propensity scores and survival analysis. Stat Med 33:1242–1258. doi:10.1002/sim.5984
Truche A-S, Darmon M, OUTCOMEREA Study Group et al (2016) Continuous renal replacement therapy versus intermittent hemodialysis in intensive care patients: impact on mortality and renal recovery. Intensive Care Med 42:1408–1417. doi:10.1007/s00134-016-4404-6
Pages J, Hazera P, 3SITES Study Group et al (2016) Comparison of alcoholic chlorhexidine and povidone–iodine cutaneous antiseptics for the prevention of central venous catheter-related infection: a cohort and quasi-experimental multicenter study. Intensive Care Med 42:1418–1426. doi:10.1007/s00134-016-4406-4
Wyss R, Ellis AR, Brookhart MA et al (2014) The role of prediction modeling in propensity score estimation: an evaluation of logistic regression, bCART, and the covariate-balancing propensity score. Am J Epidemiol 180:645–655. doi:10.1093/aje/kwu181
Imai K, Ratkovic M (2014) Covariate balancing propensity score. J R Stat Soc B 76:243–263. doi:10.1111/rssb.12027
Brookhart MA (2006) Variable selection for propensity score models. Am J Epidemiol 163:1149–1156. doi:10.1093/aje/kwj149
Arpino B, Cannas M (2016) Propensity score matching with clustered data. An application to the estimation of the impact of caesarean section on the Apgar score: propensity score matching with clustered data. An application to the estimation of the impact of caesarean section on the Apgar score. Stat Med 35:2074–2091. doi:10.1002/sim.6880
Cole SR, Hernán MA (2004) Adjusted survival curves with inverse probability weights. Comput Methods Progr Biomed 75:45–49. doi:10.1016/j.cmpb.2003.10.004
Parienti J-J, Mongardon N, Mégarbane B et al (2015) Intravascular complications of central venous catheterization by insertion site. N Engl J Med 373:1220–1229. doi:10.1056/NEJMoa1500964
Dudeck MA, Edwards JR, Allen-Bridson K et al (2015) National healthcare safety network report, data summary for 2013, device-associated module. Am J Infect Control 43:206–221. doi:10.1016/j.ajic.2014.11.014
Fontela PS, Platt RW, Rocher I et al (2012) Epidemiology of central line–associated bloodstream infections in Quebec intensive care units: a 6-year review. Am J Infect Control 40:221–226. doi:10.1016/j.ajic.2011.04.008
Miller MR, Niedner MF, Huskins WC et al (2011) Reducing PICU central line-associated bloodstream infections: 3-year results. Pediatrics 128:e1077–e1083. doi:10.1542/peds.2010-3675
Patrick SW, Kawai AT, Kleinman K et al (2014) Health care-associated infections among critically ill children in the US, 2007–2012. Pediatrics 134:705–712
Rosenthal VD, Al-Abdely HM, El-Kholy AA et al (2016) International nosocomial infection control consortium report, data summary of 50 countries for 2010–2015: device-associated module. Am J Infect Control 44:1495–1504. doi:10.1016/j.ajic.2016.08.007
Leblebicioglu H, Erben N, Rosenthal VD et al (2014) International Nosocomial Infection Control Consortium (INICC) national report on device-associated infection rates in 19 cities of Turkey, data summary for 2003–2012. Ann Clin Microbiol Antimicrob 13:1
Rosenthal VD (2009) Central line-associated bloodstream infections in limited-resource countries: a review of the literature. Clin Infect Dis 49:1899–1907. doi:10.1086/648439
Marschall J, Mermel LA, Fakih M et al (2014) Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 35:753–771. doi:10.1086/676533
O’Grady NP, Alexander M, Dellinger EP et al (2002) Guidelines for the prevention of intravascular catheter-related infections. Pediatrics 110:e51. doi:10.1542/peds.110.5.e51
Advani S, Reich NG, Sengupta A et al (2011) Central line-associated bloodstream infection in hospitalized children with peripherally inserted central venous catheters: extending risk analyses outside the intensive care unit. Clin Infect Dis 52:1108–1115. doi:10.1093/cid/cir145
Jumani K, Advani S, Reich NG et al (2013) Risk factors for peripherally inserted central venous catheter complications in children. JAMA Pediatr 167:429. doi:10.1001/jamapediatrics.2013.775
Gilbert RE, Mok Q, Dwan K et al (2016) Impregnated central venous catheters for prevention of bloodstream infection in children (the CATCH trial): a randomised controlled trial. Lancet 387:1732–1742
Goes-Silva E, Abreu TF, Frota ACC et al (2009) Use of peripherally inserted central catheters to prevent catheter-associated bloodstream infection in children. Infect Control Hosp Epidemiol 30:1024–1026. doi:10.1086/606040
Al Raiy B, Fakih MG, Bryan-Nomides N et al (2010) Peripherally inserted central venous catheters in the acute care setting: a safe alternative to high-risk short-term central venous catheters. Am J Infect Control 38:149–153. doi:10.1016/j.ajic.2009.06.008
Hord JD, Lawlor J, Werner E et al (2016) Central line associated blood stream infections in pediatric hematology/oncology patients with different types of central lines: cLABSI in patients with different central line types. Pediatr Blood Cancer 63:1603–1607. doi:10.1002/pbc.26053
Safdar N, Maki DG (2004) The pathogenesis of catheter-related bloodstream infection with noncuffed short-term central venous catheters. Intensive Care Med 30:62–67. doi:10.1007/s00134-003-2045-z
Chopra V, Ratz D, Kuhn L et al (2014) PICC-associated bloodstream infections: prevalence, patterns, and predictors. Am J Med 127:319–328. doi:10.1016/j.amjmed.2014.01.001
Carter JH, Langley JM, Kuhle S, Kirkland S (2016) Risk factors for central venous catheter-associated bloodstream infection in pediatric patients: a cohort study. Infect Control Hosp Epidemiol 37:939–945. doi:10.1017/ice.2016.83
Touré A, Chambrier C, Vanhems P et al (2013) Propensity score analysis confirms the independent effect of parenteral nutrition on the risk of central venous catheter-related bloodstream infection in oncological patients. Clin Nutr 32:1050–1054. doi:10.1016/j.clnu.2012.12.006
Acknowledgements
We thank all the staff working at the participating ICUs. We also thank the Americas Research and Education Institute, São Paulo, Brazil. This research was supported by funding from the Americas Research and Education Institute, São Paulo, Brazil. The sponsor had no role in the acquisition, analysis or interpretation of the data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Take-home message: PICCs could be used as an alternative to CVCs in Paediatric ICUs. We observed that in four PICUs from Brazil, PICCs were commonly used instead of CVCs.
We showed for the first time in a multicentre study that PICCs were associated with a protective effect in CLABSI prevention in paediatric critical care setting.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yamaguchi, R.S., Noritomi, D.T., Degaspare, N.V. et al. Peripherally inserted central catheters are associated with lower risk of bloodstream infection compared with central venous catheters in paediatric intensive care patients: a propensity-adjusted analysis. Intensive Care Med 43, 1097–1104 (2017). https://doi.org/10.1007/s00134-017-4852-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-017-4852-7