Abstract
Purpose
The best renal replacement therapy (RRT) modality remains controversial. We compared mortality and short- and long-term renal recovery between patients treated with continuous RRT and intermittent hemodialysis.
Methods
Patients of the prospective observational multicenter cohort database OUTCOMEREA™ were included if they underwent at least one RRT session between 2004 and 2014. Differences in patients’ baseline and daily characteristics between treatment groups were taken into account by using a marginal structural Cox model, allowing one to substantially reduce the bias resulting from confounding factors in observational longitudinal data analysis. The composite primary endpoint was 30-day mortality and dialysis dependency.
Results
Among 1360 included patients with RRT, 544 (40.0 %) and 816 (60.0 %) were initially treated by continuous RRT and intermittent hemodialysis, respectively. At day 30, 39.6 % patients were dead. Among survivors, 23.8 % still required RRT. There was no difference between groups for the primary endpoint in global population (HR 1.00, 95 % CI 0.77–1.29; p = 0.97). In patients with higher weight gain at RRT initiation, mortality and dialysis dependency were significantly lower with continuous RRT (HR 0.54, 95 % CI 0.29–0.99; p = 0.05). Conversely, this technique appeared to be deleterious in patients without shock (HR 2.24, 95 % CI 1.24–4.04; p = 0.01). Six-month mortality and persistent renal dysfunction were not influenced by the RRT modality in patients with dialysis dependence at ICU discharge.
Conclusion
Continuous RRT did not appear to improve 30-day and 6-month patient outcomes. It seems beneficial for patients with fluid overload, but might be deleterious in the absence of hemodynamic failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute kidney injury (AKI) occurs in one intensive care unit (ICU) patient out of three, of whom 20 % will require renal replacement therapy (RRT) [1]. AKI is associated with a mortality excess which increases dramatically with its severity [2]. Moreover, patients requiring RRT in ICUs present a substantial risk of end-stage kidney disease [3]. Improving the outcome for these patients is an ongoing challenge.
Optimal initial RRT modality remains debated. Continuous renal replacement therapy (CRRT) has long been preferred to intermittent hemodialysis (IHD), because this technique was expected to be associated with an improved hemodynamic tolerance. However, CRRT is less efficient for hydroelectrolytic correction, requires regional or systemic anticoagulation, and is more expensive.
Recent results of both randomized trials and meta-analyses comparing these techniques have failed to demonstrate superiority of CRRT in terms of mortality or hemodynamic stability [4–8]. However, the conclusions of these studies are limited by several shortcomings which need to be taken into account. Among these, the insufficient statistical power of several of the aforementioned studies, the restricted studied population which limits external applicability of the results, including the exclusion of hemodynamically unstable patients in some studies [7, 9], the variability of hemodynamic instability definition [5], the absence of standardization of RRT initiation criteria [6, 10], or the lack of information regarding dialysis dose [7] deserve to be mentioned.
Additionally, concerns regarding a higher rate of dialysis dependency after AKI in patients treated with IHD have been underlined by several studies. In a recent meta-analysis [11], a propensity-matched analysis showed a higher rate of dialysis dependence among survivors after IHD (RR 1.99, 95 % CI 1.53–2.59) conversely to CRRT (HR 0.75, 95 % CI 0.65–0.87) [12]. As consequences of these uncertainties, the recent Kidney Disease—Improving Global Outcomes (KDIGO) guidelines recommend CRRT in patients with hemodynamic instability, while underlining the need for additional studies and the complementarities of these technique in other groups of patients [13].
As regards to the high mortality among AKI patients requiring RRT and the difficulty to reach a sufficient number of patients, performing a randomized controlled trial (RCT) to assess the influence of initial RRT modality on renal outcome is believed to be both difficult and inefficient. In this situation, the use of observational longitudinal data is a possible alternative [14]. Marginal structural models (MSM) were recently developed for such data to take into account time-dependent confounders impacting both the choice of treatment and the outcome. Hence, they are able to substantially decrease biases resulting from such confounders in observational longitudinal data analysis [15].
The aim of our study was to compare the influence of RRT modality in terms of mortality and short- and long-term renal recovery in a high-quality multicenter prospective cohort.
Methods
Study population
The OUTCOMEREA™ cohort has already been extensively described elsewhere [16]. In this observational prospective multicenter cohort, patients over 16 years of age admitted in French ICUs were randomly included. Their clinical and biologic data were registered in the database each day of their ICU stay. This database has been approved by the French Advisory Committee for Data Processing in Health Research (CCTIRS) and the French Informatics and Liberty Commission (CNIL). The study was approved by the ethics committee of Clermont-Ferrand, France.
With regards to the RRT evaluation, patients from 19 centers of the French OUTCOMEREA™ cohort were included if they underwent RRT during their hospitalization.
To ensure homogeneity in RRT procedures, the study period was limited to 1 January 2004 to 1 September 2014. The day of RRT initiation was taken as the study inclusion day for a patient (details concerning RRT modalities are provided in Online Resource 1).
Exclusion criteria were decision to forgo life-sustaining therapies in the first 24 h of ICU admission, past history of kidney transplantation, preexisting chronic kidney disease requiring RRT (whether peritoneal dialysis or hemodialysis), specific IHD indications (i.e., biguanide intoxication or hyperkalemia over 8 mmol/l [17, 18]), and formal IHD contraindication (i.e., brain injury [19]).
Outcomes
The main outcome was a composite criterion composed of mortality or dialysis dependency 30 days after the beginning of RRT.
The secondary outcome was the 30-day mortality comparatively between the two groups.
To identify a potential category of patients who could benefit more specifically from one of these techniques, the following subgroup analyses were planned in the experimental design: (1) chronic renal or heart disease; (2) liver cirrhosis; (3) diabetes; (4) hypertension; (5) age subgroups; (6) hemodynamic status at RRT initiation defined according to SOFA hemodynamic component (strictly inferior to 3, and equal or above 3); (7) invasive mechanical ventilation at RRT initiation; (8) early insufficient dialysis intensity, as defined as a first RRT session resulting in a predialysis urea over 25 mmol/L before the next session [20]; and (9) extent of daily weight gain between ICU admission and inclusion defined as the upper quartile of the study population.
Finally, the 6-month prognosis of patients alive and still requiring RRT at ICU discharge was compared between the two modalities with a composite criterion: mortality and persistent renal dysfunction (definitions are provided in Online Resource 2).
Statistical analysis
Quantitative variables are presented as median and interquartile range and compared between groups with the Wilcoxon test. Qualitative variables are presented as frequency and corresponding percentage and compared with the Chi square test. Both variables identified in the literature as confounding factors and variables associated with RRT techniques selected by the univariate analysis (p value threshold = 0.2) were used for calculating the predicted probability of receiving CRRT or IHD for each time period (data collection and management details are provided in Online Resource 2).
Marginal structural models
These models use inverse probability of treatment weighting (IPTW) estimators to create a pseudo-population where the treatment is independent of baseline and time-dependent confounding factors introduced into the weights. MSM allow an estimation which is asymptotically unbiased of longitudinal treatment effect. The link between treatment and primary outcome can be determined in the pseudo-population. Provided that all model assumptions are satisfied, the link can be considered as causal and extrapolated to the first population [15, 21]. To note, patients discontinuing RRT were considered as staying under the last RRT modality they received (see Online Resource 3 and Supplementary Figs. S1–4 for a detailed methodology description).
Independently to the MSM analysis, 6 months after ICU discharge, the prognosis of ICU survivors was assessed via a weighted logistic regression. Treatment groups were defined according to the most received modality within the first 7 days after RRT initiation. A unique IPTW estimator for each patient was estimated as the inverse of probability of receiving CRRT given the following patient characteristics: baseline characteristics previously included in IPTW estimators, weight gain between ICU admission and RRT initiation, length of ICU stay, dialysis quality the first 7 days, occurrence of an infection, an adverse event, a nephrotoxic drug administration, mechanical invasive ventilation requirement, or limiting therapeutic effort decision.
All statistical analyses were conducted with SAS 9.3 (SAS Institute Inc., Cary, NC, USA). Code was implemented according to Hernan et al. [22].
Results
Baseline characteristics
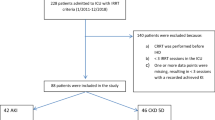
Among 1913 patients treated by RRT, our final population comprised 1360 patients (Fig. 1).
The initial RRT modality was CRRT in 544 patients (40.0 %) and IHD in 816 patients (60.0 %). Main characteristics of the population are presented in Table 1 and Supplementary Table S1.
A total of 202 (14.9 %) patients were discharged alive and lost to follow-up prior to day 30: 22 were transferred to another ICU, 143 to another acute hospital ward, 11 were discharged home, and no destination data was available for 26. Characteristics of censored patients are available in Supplementary Table S2.
30-day mortality/dialysis dependency
Day-30 mortality was 39.6 % (539 patients) including 286 patients (35.0 %) in the IHD group and 253 patients (46.5 %) in the CRRT group.
Dialysis dependency at day 30 was present among 23.8 % of surviving patients, including 24.9 % in the IHD group and 21.8 % in the CRRT group. IPTW estimators are provided in Supplementary Table S3 and Supplementary Fig. S3.
There was no significant difference for the primary endpoint (alive without RRT) between the two groups in the global population (HR 1.00, 95 % CI 0.77–1.29; p = 0.97) (Fig. 2; Supplementary Table S4). The main prognostic factor appeared to be a life support limitation decision (HR 12.44, 95 % CI 7.38–20.96).
30-day mortality and dialysis dependency according to renal replacement therapy technique received, in the global population and subgroups analyses. a At first RRT session. b Between ICU entrance and inclusion. CI confidence interval, IMV invasive mechanical ventilation, SOFA sequential organ failure assessment, RRT renal replacement therapy, IHD intermittent hemodialysis, CRRT continuous renal replacement therapy
Results were similar for subgroups of chronic kidney or heart diseases, cirrhosis, diabetes, and hypertension. Patients with higher weight gain (daily mean weight gain greater than 2 kg between ICU admission and inclusion) presented a significantly lower death rate and dialysis dependency when CRRT was chosen as initial modality (HR 0.54, 95 % CI 0.29–0.99; p = 0.05). Conversely, the use of CRRT as initial modality in patients without hemodynamic instability was associated with a higher mortality (HR 2. 24, 95 % CI 1.24–4.04; p = 0.01) (Supplementary Table S5). The results of the final model were robust (Supplementary Tables S6 and S7).
The 30-day mortality model did not show differences between techniques for the global population (Supplementary Table S8 and Fig. S5).
Six-month mortality/persistent renal dysfunction
A total of 295 patients were alive and required RRT at ICU discharge. In this subgroup of patients, the 6-month mortality was 18.6 % (16.4 % in the IHD group and 25.4 % in the CRRT group). Among survivors, 126 (52.5 %) patients had a persistent renal dysfunction: 108 (57.1 %) in the IHD group and 18 (35.3 %) in the CRRT group. When confounding factors were taken into account, no significant difference was noted between the two treatment groups (OR 0.70, 95 % CI 0.36–1.37; p = 0.29). The weighted survival curve is presented in Fig. 3.
Six-month mortality according to main type of renal replacement therapy received during the first 7 days: survival curves are weighted with patient IPTW estimators. Survival curve initial time is ICU discharge. IHD intermittent hemodialysis, CRRT continuous renal replacement therapy, ICU intensive care unit
Discussion
In a high-quality large multicenter database using an innovative statistical approach, we were not able to demonstrate any benefit of RRT modality in terms of 30-day mortality and dialysis dependency, even in patients with hemodynamic instability. However, the initial use of CRRT was associated with decreased 30-day mortality and dialysis dependency among patients with fluid overload at RRT initiation. Interestingly, CRRT as initial RRT modality was found to be detrimental in patients without any hemodynamic instability. In this study, long-term prognosis was not modified by initial RRT modality.
With regards to long-term prognosis, several studies suggested a decreased risk of long-term dialysis dependency when CRRT was used as initial modality [11, 23], whereas in our study no improvement of prognosis could be observed. However, this apparent discrepancy may be explained by several factors. First of all, few data exist regarding renal recovery ad integrum. In our study, we aimed to assess long-term prognosis in the global patient, i.e., in terms of mortality and persistent renal dysfunction, instead of limiting it to dialysis dependency. Secondly, we considered the most frequently received RRT modality in the first 7 days, rather than the first treatment received, because of the frequent change of technique in this context. Results usually differ between observational studies, which are in favor of a long-term benefit of CRRT, and RCTs, which failed to show a difference between techniques. This difference may be explained by confounding factors and allocation bias limiting observational studies [11]. In our study, thanks to the IPTW estimator, known factors associated with RRT prescription and main prognostic factors were taken into account, resulting in a decreased bias due to confounders.
Whereas CRRT was supposed to be associated with a higher hemodynamic stability [24, 25], this assumption was not confirmed by recent randomized trials or meta-analyses [5–7, 17]. Recent studies demonstrated that adequate IHD prescription resulted in improved hemodynamic tolerance. Thus, the simple implementation of guidelines for the management of IHD sessions was proved to limit hemodynamic instability during sessions [26] and translate into similar results of tolerance between IHD and CRRT in randomized trials [6]. In keeping with these findings, our study results demonstrate that RRT modality has little influence on outcome, even in patients with hemodynamic instability. Improvements in terms of hemodynamic tolerance may reflect either improvement of generators, of prescription modalities, or of quality of care. Nevertheless, the advantage of CRRT in terms of hemodynamic stability is hardly supported by recent evidence, at least in experienced centers.
Although hemodynamic instability in itself seems to be a poor criterion of choice for initial modality, one of the striking findings of our study is the influence of the RRT modality when fluid balance is taken into account. Although insufficiently studied, previous research suggested that fluid control might be easier to achieve using CRRT [25]. An increasing body of evidence suggests that fluid overload is associated with both poor renal outcome and survival [27, 28]. Beyond renal congestion, the kidney being a capsulated organ, fluid overload may translate into interstitial edema, increased intracapsular pressure, ultimately leading to decreased renal perfusion and renal function worsening [29, 30]. This seems to be also true at the time of RRT initiation [31]. Being a potent modality in optimizing fluid balance, CRRT in our study was interestingly associated with improved 30-day mortality and decreased dialysis dependency in patients with high daily weight gain at the time of RRT initiation. Although further studies in this field are needed to confirm our findings, this may explain the influence of RRT modality on dialysis dependency in previous studies [11].
Another interesting result of our study is the increased mortality and dialysis dependency when CRRT was used in the absence of hemodynamic failure, even when dialysis dose was taken into account. Delayed recognition of sepsis due to temperature lowering induced by CRRT could worsen patients’ prognosis [32]. This quite surprising result must be confirmed by further studies.
Several strengths of our study should be noted. Observational studies on the subject were limited by the lack of comparability between groups, and meta-analyses have pointed out numerous limitations of RCTs [4]. Limited availability of one dialysis modality [17] or practitioner resistance to randomize hypotensive patients [7] have contributed to insufficient patient numbers in RCTs. This study, by using an MSM, managed to bypass these limitations. MSM enabled us to include a great number of patients from an observational multicenter cohort, thereby ensuring the possible generalization of our results. Provided that all confounders were taken into account, a causal conclusion could be inferred from this study. Indeed, in our study, we considered factors associated with both the choice of treatment and prognosis factors influencing the outcome. Furthermore, in routine practice, both techniques are applied in the same patients throughout the ICU stay. The rate of crossover is high between the two techniques in meta-analyses [4]. The impact of the switch from one technique to the other is improperly taken into account by intention-to-treat analyses in RCTs. Switches from one technique to the other are mainly due to coagulation disorders, insufficient hemodynamic tolerance, or decreased severity of illness. In our study, the global crossover rate was 19.5 % and was more frequent in the CRRT group (24.1 vs 16.4 %), potentially because of a decrease of patients’ severity of illness. MSM allows us to conduct an as-treated analysis, by regularly renewing IPTW estimators, and therefore is not limited by crossover [33].
Our study presents some limitations. We recognize that RRT prescriptions were not standardized in our study, which may result in heterogeneity of administration of RRT techniques. Since 2008, the interest in higher doses of RRT was questioned in several studies [34, 35] and could have resulted in a change in administered doses during the study period, especially for CRRT. Of note, the actual delivered dose of CRRT is not easy to assess, and some studies showed a discrepancy between prescribed and effective clearance [36]. As a result, in our study, we chose to represent RRT efficiency by adjusting according to dialysis quality, defined as a serum urea under 25 mmol/L prior to the next RRT session [20].
Another possible heterogeneity in our study is the timing of RRT initiation. However, knowing whether early RRT initiation has an impact on mortality is still under debate [23, 37], and conclusions are hard to draw from existing studies because of the variability of early initiation definitions. Still, in our study, early initiation was defined as one starting on the same day as the renal injury class was reached and was introduced in weights and adjustment in the final model.
Some limitations specific to MSM should be discussed. The first concerns the estimator’s propriety: longitudinal treatment effect estimation is asymptotically unbiased only under the condition that both models for weights and treatment effect estimations are correctly specified. By renewing our main analysis with double robust IPTW, we confirmed that our results were stable. Secondly, as stated in the “Methods” section, the interpretation of the estimation could be considered as causal, provided that all model assumptions were satisfied (see Supplementary material 1 for details). On the contrary, other assumptions, such as exchangeability, cannot be tested. Even if all known confounding factors were introduced in the model, unknown confounding factors might not have been considered, thus resulting in a residual bias. By a sensitivity analysis, we concluded that it was highly unlikely that an unknown factor could substantially change our final result. The consistency assumption, also untestable, relied on a sufficient description of treatment exposure. By introducing into the model the timing of RRT initiation and dialysis quality, we believed that most important treatment parameters were taken into account. Independently of model assumptions, the hypothesis made with regards to discharged patients illustrates the difficulty in appropriately taking into account competitive risk in MSM.
This study must be seen as complementary to available literature. Its aims were multiple: first, to confirm results concerning short-term outcomes while including a large number of patients; secondly, to translate results obtained in specialized centers into results in routine practice; lastly, to point out new avenues of inquiry by subgroup analysis and the long-term outcome, considering not only dialysis dependency but also persisting renal dysfunction. Conclusions regarding some subgroup analyses can be limited by the lack of power, and, on the contrary, multiple comparisons can also explain why some subgroups reach statistical significance. In consequence, our results need further confirmation by subsequent studies.
Lastly, concerning the secondary 6-month outcome, two limitations must be noted. First, this outcome was gathered retrospectively and was limited to patients discharged from ICU and still requiring RRT. In consequence, results concerning the 6-month outcome, especially persistent renal dysfunction, might not be applicable to patients experiencing at least a partial renal recovery allowing discontinuation of RRT in the ICU. Second, eGFR criterion might have overestimated renal recovery due to muscle wasting during ICU stay [38].
In conclusion, our study suggests that RRT modality has little influence on both survival and renal outcome at 30 days and 6 months. In addition, in an unselected population of patients, RRT modalities do not seem to influence outcome of patients with hemodynamic instability. However, our study suggests a benefit from CRRT among patients with positive fluid balance. The association between recent evidence suggesting deleterious effects of positive fluid balance, benefits of CRRT in controlling fluid balance, and our findings should encourage physicians to prefer CRRT in these patients. Last, although unexplained, our results suggest that IHD in patients without hemodynamic instability is less deleterious. These results deserve to be confirmed in additional studies.
References
Clec’h C, Gonzalez F, Lautrette A, Nguile-Makao M, Garrouste-Orgeas M, Jamali S, Golgran-Toledano D, Descorps-Declere A, Chemouni F, Hamidfar-Roy R, Azoulay E, Timsit JF (2011) Multiple-center evaluation of mortality associated with acute kidney injury in critically ill patients: a competing risks analysis. Crit Care 15:R128
Clec’h C, Darmon M, Lautrette A, Chemouni F, Azoulay E, Schwebel C, Dumenil AS, Garrouste-Orgeas M, Goldgran-Toledano D, Cohen Y, Timsit JF (2012) Efficacy of renal replacement therapy in critically ill patients: a propensity analysis. Crit Care 16:R236
Gammelager H, Christiansen CF, Johansen MB, Tonnesen E, Jespersen B, Sorensen HT (2013) Five-year risk of end-stage renal disease among intensive care patients surviving dialysis-requiring acute kidney injury: a nationwide cohort study. Crit Care 17:R145
Bagshaw SM, Berthiaume LR, Delaney A, Bellomo R (2008) Continuous versus intermittent renal replacement therapy for critically ill patients with acute kidney injury: a meta-analysis. Crit Care Med 36:610–617
Rabindranath K, Adams J, Macleod AM, Muirhead N (2007) Intermittent versus continuous renal replacement therapy for acute renal failure in adults. Cochrane Database Syst Rev: CD003773
Vinsonneau C, Camus C, Combes A, Costa de Beauregard MA, Klouche K, Boulain T, Pallot JL, Chiche JD, Taupin P, Landais P, Dhainaut JF (2006) Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: a multicentre randomised trial. Lancet 368:379–385
Lins RL, Elseviers MM, Van der Niepen P, Hoste E, Malbrain ML, Damas P, Devriendt J (2009) Intermittent versus continuous renal replacement therapy for acute kidney injury patients admitted to the intensive care unit: results of a randomized clinical trial. Nephrol Dial Transplant 24:512–518
Misset B, Timsit JF, Chevret S, Renaud B, Tamion F, Carlet J (1996) A randomized cross-over comparison of the hemodynamic response to intermittent hemodialysis and continuous hemofiltration in ICU patients with acute renal failure. Intensive Care Med 22:742–746
Mehta RL, McDonald B, Gabbai FB, Pahl M, Pascual MT, Farkas A, Kaplan RM (2001) A randomized clinical trial of continuous versus intermittent dialysis for acute renal failure. Kidney Int 60:1154–1163
Gasparovic V, Filipovic-Grcic I, Merkler M, Pisl Z (2003) Continuous renal replacement therapy (CRRT) or intermittent hemodialysis (IHD)–what is the procedure of choice in critically ill patients? Ren Fail 25:855–862
Schneider AG, Bellomo R, Bagshaw SM, Glassford NJ, Lo S, Jun M, Cass A, Gallagher M (2013) Choice of renal replacement therapy modality and dialysis dependence after acute kidney injury: a systematic review and meta-analysis. Intensive Care Med 39:987–997
Wald R, Shariff SZ, Adhikari NK, Bagshaw SM, Burns KE, Friedrich JO, Garg AX, Harel Z, Kitchlu A, Ray JG (2014) The association between renal replacement therapy modality and long-term outcomes among critically ill adults with acute kidney injury: a retrospective cohort study. Crit Care Med 42:868–877
KDIGO (2011) Section 5: dialysis interventions for treatment of AKI. Kidney Int Suppl 2:89–115
Benson K, Hartz AJ (2000) A comparison of observational studies and randomized, controlled trials. N Engl J Med 342:1878–1886
Robins JM, Hernan MA, Brumback B (2000) Marginal structural models and causal inference in epidemiology. Epidemiology 11:550–560
Clec’h C, Alberti C, Vincent F, Garrouste-Orgeas M, de Lassence A, Toledano D, Azoulay E, Adrie C, Jamali S, Zaccaria I, Cohen Y, Timsit JF (2007) Tracheostomy does not improve the outcome of patients requiring prolonged mechanical ventilation: a propensity analysis. Crit Care Med 35:132–138
Uehlinger DE, Jakob SM, Ferrari P, Eichelberger M, Huynh-Do U, Marti HP, Mohaupt MG, Vogt B, Rothen HU, Regli B, Takala J, Frey FJ (2005) Comparison of continuous and intermittent renal replacement therapy for acute renal failure. Nephrol Dial Transplant 20:1630–1637
John S, Griesbach D, Baumgartel M, Weihprecht H, Schmieder RE, Geiger H (2001) Effects of continuous haemofiltration vs intermittent haemodialysis on systemic haemodynamics and splanchnic regional perfusion in septic shock patients: a prospective, randomized clinical trial. Nephrol Dial Transplant 16:320–327
Davenport A (2009) Continuous renal replacement therapies in patients with acute neurological injury. Semin Dial 22:165–168
Palevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D, Finkel K, Kellum JA, Paganini E, Schein RM, Smith MW, Swanson KM, Thompson BT, Vijayan A, Watnick S, Star RA, Peduzzi P (2008) Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 359:7–20
Bailly S, Pirracchio R, Timsit JF (2016) What’s new in the quantification of causal effects from longitudinal cohort studies: a brief introduction to marginal structural models for intensivists. Intensive Care Med 42:576–579
Hernan MA, Brumback B, Robins JM (2000) Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 11:561–570
Vaara ST, Reinikainen M, Wald R, Bagshaw SM, Pettila V (2014) Timing of RRT based on the presence of conventional indications. Clin J Am Soc Nephrol 9:1577–1585
Bellomo R, Ronco C (1999) Continuous renal replacement therapy in the intensive care unit. Intensive Care Med 25:781–789
Augustine JJ, Sandy D, Seifert TH, Paganini EP (2004) A randomized controlled trial comparing intermittent with continuous dialysis in patients with ARF. Am J Kidney Dis 44:1000–1007
Schortgen F, Soubrier N, Delclaux C, Thuong M, Girou E, Brun-Buisson C, Lemaire F, Brochard L (2000) Hemodynamic tolerance of intermittent hemodialysis in critically ill patients: usefulness of practice guidelines. Am J Respir Crit Care Med 162:197–202
Grams ME, Estrella MM, Coresh J, Brower RG, Liu KD (2011) Fluid balance, diuretic use, and mortality in acute kidney injury. Clin J Am Soc Nephrol 6:966–973
Teixeira C, Garzotto F, Piccinni P, Brienza N, Iannuzzi M, Gramaticopolo S, Forfori F, Pelaia P, Rocco M, Ronco C, Anello CB, Bove T, Carlini M, Michetti V, Cruz DN (2013) Fluid balance and urine volume are independent predictors of mortality in acute kidney injury. Crit Care 17:R14
Herrler T, Tischer A, Meyer A, Feiler S, Guba M, Nowak S, Rentsch M, Bartenstein P, Hacker M, Jauch KW (2010) The intrinsic renal compartment syndrome: new perspectives in kidney transplantation. Transplantation 89:40–46
Prowle JR, Echeverri JE, Ligabo EV, Ronco C, Bellomo R (2010) Fluid balance and acute kidney injury. Nat Rev Nephrol 6:107–115
Heung M, Wolfgram DF, Kommareddi M, Hu Y, Song PX, Ojo AO (2012) Fluid overload at initiation of renal replacement therapy is associated with lack of renal recovery in patients with acute kidney injury. Nephrol Dial Transplant 27:956–961
Stoneking LR, Winkler JP, DeLuca LA, Stolz U, Stutz A, Luman JC, Gaub M, Wolk DM, Fiorello AB, Denninghoff KR (2015) Physician documentation of sepsis syndrome is associated with more aggressive treatment. West J Emerg Med 16:401–407
Yang S, Eaton CB, Lu J, Lapane KL (2014) Application of marginal structural models in pharmacoepidemiologic studies: a systematic review. Pharmacoepidemiol Drug Saf 23:560–571
Jun M, Heerspink HJ, Ninomiya T, Gallagher M, Bellomo R, Myburgh J, Finfer S, Palevsky PM, Kellum JA, Perkovic V, Cass A (2010) Intensities of renal replacement therapy in acute kidney injury: a systematic review and meta-analysis. Clin J Am Soc Nephrol 5:956–963
Van Wert R, Friedrich JO, Scales DC, Wald R, Adhikari NK (2010) High-dose renal replacement therapy for acute kidney injury: systematic review and meta-analysis. Crit Care Med 38:1360–1369
Lyndon WD, Wille KM, Tolwani AJ (2012) Solute clearance in CRRT: prescribed dose versus actual delivered dose. Nephrol Dial Transplant 27:952–956
Jun M, Bellomo R, Cass A, Gallagher M, Lo S, Lee J (2014) Timing of renal replacement therapy and patient outcomes in the randomized evaluation of normal versus augmented level of replacement therapy study. Crit Care Med 42:1756–1765
Schetz M, Gunst J, De Vlieger G, Van den Berghe G (2015) Recovery from AKI in the critically ill: potential confounders in the evaluation. Intensive Care Med 41:1648–1657
Acknowledgments
The authors thank Celine Feger, M.D. (EMIBiotech), for her editorial support.
Members of the Outcomerea Study Group
Scientific Committee: Jean-François Timsit (Medical and Infectious Diseases ICU, Bichat-Claude Bernard Hospital, Paris, France; UMR 1137 Inserm–Paris Diderot University IAME, F75018, Paris); Elie Azoulay (Medical ICU, Saint Louis Hospital, Paris, France); Maïté Garrouste-Orgeas (ICU, Saint-Joseph Hospital, Paris, France); Jean-Ralph Zahar (Infection Control Unit, Angers Hospital, Angers, France); Christophe Adrie (ICU, Delafontaine Hospital, Saint Denis, and Physiology, Cochin Hospital, Paris, France); Michael Darmon (Medical ICU, Saint Etienne University Hospital, St Etienne, France); and Christophe Clec’h (ICU, Avicenne Hospital, Bobigny, and UMR 1137 Inserm–Paris Diderot university IAME, F75018, Paris, France).
Biostatistical and Information System Expertise: Jean-Francois Timsit (Medical and Infectious Diseases ICU, Bichat-Claude Bernard Hospital, Paris, France; UMR 1137 Inserm–Paris Diderot university IAME, F75018, Paris); Corinne Alberti (Medical Computer Sciences and Biostatistics Department, Robert Debré Hospital, Paris, France); Adrien Français (Integrated Research Center U823, Grenoble, France); Aurélien Vesin (OUTCOMEREA organization and Integrated Research Center U823, Grenoble, France); Stephane Ruckly (OUTCOMEREA organization and Inserm UMR 1137 IAME, F75018, Paris); Sébastien Bailly (Grenoble University Hospital Inserm UMR 1137 IAME, F75018, Paris) and Christophe Clec’h (ICU, Avicenne Hospital, Bobigny, and Inserm UMR 1137 IAME, F75018, Paris, France); Frederik Lecorre (Supelec, France); Didier Nakache (Conservatoire National des Arts et Métiers, Paris, France); and Aurélien Vannieuwenhuyze (Tourcoing, France).
Investigators of the OUTCOMEREA Database: Christophe Adrie (ICU, Delafontaine Hospital, Saint Denis, and Physiology, Cochin Hospital, Paris, France); Bernard Allaouchiche (ICU, Pierre Benite Hospital, Lyon, France); Laurent Argaud (Medical ICU, Hospices Civils de Lyon, Lyon, France); Claire Ara-Somohano (Medical ICU, University Hospital, Grenoble, France); Elie Azoulay (Medical ICU, Saint Louis Hospital, Paris, France); Francois Barbier (medical-surgical ICU, Orleans, France), Jean-Pierre Bedos (ICU, Versailles Hospital, Versailles, France); Julien Bohé (ICU, Hôpital Pierre Benite, Lyon France), Lila Bouadma (ICU, Bichat Hospital, Paris, France); Christine Cheval (ICU, Hyeres Hospital, Hyeres, France); Christophe Clec’h (ICU, Avicenne Hospital, Bobigny, France); Michael Darmon (ICU, Saint Etienne Hospital, Saint Etienne, France); Anne-Sylvie Dumenil (Antoine Béclère Hospital, Clamart, France); Claire Dupuis (Bichat hospital and UMR 1137 Inserm–Paris Diderot University IAME, F75018, Paris, France), Marc Gainier hôpital la Timone, Marseille, France), Akim Haouache (Surgical ICU, H Mondor Hospital, Creteil, France); Samir Jamali (ICU, Dourdan, Dourdan Hospital, Dourdan, France); Hatem Khallel (ICU, Cayenne General Hospital, Cayenne, France); Alexandre Lautrette (ICU, G Montpied Hospital, Clermont-Ferrand, France); Guillaume Marcotte (Surgical ICU, Hospices Civils de Lyon, Lyon, France); Eric Le Miere (ICU, Louis Mourier Hospital, Colombes, France); Maxime Lugosi (Medical ICU, University Hospital Grenoble, Grenoble, France); Bruno Mourvillier (ICU, Bichat Hospital, Paris, France); Benoît Misset (ICU, Saint-Joseph Hospital, Paris, France); Delphine Moreau (ICU, Saint-Louis Hospital, Paris, France); Bruno Mourvillier (ICU, Bichat Hospital, Paris, France); Laurent Papazian (Hopital Nord, Marseille, France), Benjamin Planquette (pulmonology ICU, George Pompidou Hospital, Versailles, France); Bertrand Souweine (ICU, G Montpied Hospital, Clermont-Ferrand, France); Carole Schwebel (ICU, A Michallon Hospital, Grenoble, France); Gilles Troché (ICU, Antoine Béclère Hospital, Clamart, France); Marie Thuong (ICU, Delafontaine Hospital, Saint Denis, France); Guillaume Thierry (ICU, Saint-Louis Hospital, Paris, France); Dany Toledano (ICU, Gonesse Hospital, Gonesse, France); and Eric Vantalon (SICU, Saint-Joseph Hospital, Paris, France).
Study Monitors: Julien Fournier, Caroline Tournegros, Stéphanie Bagur, Mireille Adda, Vanessa Vindrieux, Loic Ferrand, Nadira Kaddour, Boris Berthe, Samir Bekkhouche, Kaouttar Mellouk, Sylvie Conrozier, Igor Theodose, Veronique Deiler, and Sophie Letrou.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Funding
The study was entirely funded by the OUTCOMEREA research network. AST received an educational grant from the French Kidney Foundation under the aegis of the French Medical Research Foundation; code DEA2014FDR/FRM04_FdR-SdN-SFD_FRM_TRUCHE.
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Take-home message: Optimal renal replacement therapy (RRT) technique in the ICU remains controversial, in patients with shock or fluid overload. Cohort studies suggested increased risk of persistent acute kidney injury or dialysis dependency with intermittent hemodialysis. In a MSM Cox model in a cohort of 1360 patients adjusted on daily patients’ characteristics, we found that RRT modality did not influenced neither 30-day mortality nor renal outcome. In subgroups, continuous RRT benefits patients with hemodynamic instability and is deleterious in patients with hemodynamic instability.
This article was presented at the 2016 congress of the French-Language Society of Intensive Care.
The members of the OUTCOMEREA Study Group are listed at the end of this article and in the electronic supplementary material (file ESM 2).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Truche, AS., Darmon, M., Bailly, S. et al. Continuous renal replacement therapy versus intermittent hemodialysis in intensive care patients: impact on mortality and renal recovery. Intensive Care Med 42, 1408–1417 (2016). https://doi.org/10.1007/s00134-016-4404-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-016-4404-6