Abstract
Purpose
Compare the effectiveness of different cutaneous antiseptics in reducing risk of catheter-related infection in intensive care unit (ICU) patients.
Methods
We compared the risk of central venous catheter-related infection according to four-step (scrub, rinse, dry, and disinfect) alcoholic 5 % povidone–iodine (PVI-a, n = 1521), one-step (disinfect) alcoholic 2 % chlorhexidine (2 % CHX-a, n = 1116), four-step alcoholic <1 % chlorhexidine (<1 % CHX-a, n = 357), and four-step aqueous 10 % povidone–iodine (PVI, n = 368) antiseptics used for cutaneous disinfection and catheter care during the 3SITES multicenter randomized controlled trial. Within this cohort, we performed a quasi-experimental study (i.e., before–after) involving the four ICUs which switched from PVI-a to 2 % CHX-a. We used propensity score matching (PSM, n = 776) and inverse probability weighting treatment (IPWT, n = 1592). The end point was the incidence of catheter-related infection (CRI) defined as catheter-related bloodstream infection (CRBSI) or a positive catheter tip culture plus clinical sepsis on catheter removal.
Results
In the cohort analysis and compared with PVI-a, the incidence of CRI was lower with 2 % CHX-a [adjusted hazard ratio (aHR), 0.51; 95 % confidence interval (CI) (0.28–0.96), p = 0.037] and similar with <1 % CHX-a [aHR, 0.73; (0.36–1.48), p = 0.37] and PVI [aHR, 1.50; 95 % CI (0.85–2.64), p = 0.16] after controlling for potential confounders. In the quasi-experimental study and compared with PVI-a, the incidence of catheter-related infection was again lower with 2 % CHX-a after PSM [HR, 0.35; 95 % CI (0.15, 0.84), p = 0.02] and in the IPWT analysis [HR, 0.31; 95 % CI (0.14, 0.70), p = 0.005]. The incidence of CRBSI or adverse event was not significantly different between antiseptics in all analyses.
Conclusions
In comparison with PVI-a, the use of 2 % CHX-a for cutaneous disinfection of the central venous catheter insertion site and maintenance catheter care was associated with a reduced risk of catheter infection, while the benefit of <1 % CHX-a was uncertain.
Clinical trials identifier: NCT01479153.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Critically ill patients often require a short-term central venous catheter to administrate emergent lifesaving drugs. By offering the microorganisms colonizing the cutaneous surface a route into the sterile intravascular compartment, these devices increase the risk of catheter-related bloodstream infection [1]. Strong consensus exists regarding the need to disinfect the site of insertion before central venous catheterization and during catheter care to prevent catheter-related infection [2]. Which antiseptic is best to achieve this goal has been the subject of controversy [3].
Pioneered by Maki and colleagues in 1991 [4], chlorhexidine-based and iodine-based products have been compared in several randomized controlled trials with apparent superiority of chlorhexidine [5]. However, chlorhexidine was more frequently used as an alcoholic solution while iodine was more frequently used as an aqueous solution [5], raising concerns about the role of alcohol [6] in chlorhexidine versus povidone–iodine comparisons [7]. The Centers for Disease Control and Prevention (CDC) 2011 guidelines for the prevention of intravascular catheter-related infections [2] stated: “No comparison has been made between using chlorhexidine preparations with alcohol and povidone–iodine in alcohol to prepare clean skin. Unsolved issue”. This gap in knowledge has been addressed by Mimoz and colleagues [8] who carried out the first randomized controlled trial comparing alcoholic 2 % chlorhexidine and alcoholic 5 % povidone–iodine to prevent catheter-related infections. However, only 2 % chlorhexidine concentration was studied. In addition, replication of a single study result in a large independent sample is important for validating a new finding before widespread implementation.
The aim of this study was to compare the risk of catheter infection according to the use of cutaneous antiseptics to clean central venous catheter insertion sites and for catheter care thereafter among adult patients included in the 3SITES study [9]. We were particularly interested by the comparison between alcoholic 5 % povidone–iodine and alcoholic 2 % chlorhexidine among ICUs which switched during the trial.
Methods
Study design and participants
We conducted a cohort and quasi-experimental study using the 3SITES trial data [9], a multicenter randomized controlled trial aimed at comparing central venous catheter intravascular complications according to the anatomic site of insertion in ten French ICUs. The 3SITES study was conducted between December 2011 and June 2014 in four university and five general hospitals involving 3027 patients and 3471 central venous catheters. Demographics, patients, and catheter risk factors for catheter infection and specific laboratory testing such as catheter tip culture and peripheral blood cultures were prospectively monitored. The choice of the antiseptic used for cutaneous disinfection and catheter care was left to the discretion of each participating ICU (Supplemental Appendix Fig. S1) and included 5 % povidone–iodine/69 % ethanol (PVI-a, Betadine alcoolique®, Meda Pharma), colored 2 % chlorhexidine/70 % isopropyl alcohol (2 % CHX-a, Chloraprep® with tint, Carefusion BD), 10 % povidone–iodine (PVI, Betadine aqueuse®, Meda Pharma), 0.25 % chlorhexidine, 0.025 % benzalkonium chloride, and 4 % benzylic alcohol (<1 % CHX-a, Biseptine®, Bayer), 0.5 % chlorhexidine/75 % ethanol (<1 % CHX-a, Gluconate de chlorhexidine Alcoolique 0.5 %®, Gifrer Barbezat). These data were prospectively collected for each central venous catheter.
Patients 18 years of age or older were eligible for the study if they were admitted to the ICU and required non-tunneled central venous vascular access through a new venipuncture. The cohort study included all the 3SITES catheters. The quasi-experimental study included only consecutive catheters for which cutaneous disinfection and catheter care were performed in the participating ICUs using PVI-a at the start of the trial and switched to colored 2 % CHX-a during the trial. The inclusion of ICU which used the two products in the quasi-experimental study allowed us to control for a potential center effect that could affect both groups.
The research ethics committee at Caen University approved the study protocol for all the participating centers. Informed written consent was obtained from all participants or their proxies in cases of impaired decision-making capacity at the time of enrollment.
Infection control procedures
All participating ICU followed the French Haute Autorité de Santé checklist [10] and US guidelines for preventing catheter-related infections [2]. Maximal sterile barrier precautions were employed, including use of surgical hand antisepsis, sterile gloves, surgical long-sleeved gowns, masks, and caps. Patients were covered by sterile drapes. None of the study catheters were antiseptic-impregnated, antibiotic-impregnated, or tunneled. Impregnated dressings were not used. Central venous catheters were not used for routine blood sampling or renal replacement therapy.
Decisions to remove catheters were made independently by the physicians caring for each patient. After aseptic removal, catheter tips were sent for quantitative culture. Peripheral blood cultures were systematically drawn at the time of catheter removal. Patients discharged from the ICU with the catheter in place had simultaneous blood cultures drawn from a peripheral vein and the central venous catheter to determine the differential time to positivity.
Antiseptics were used according to the manufacturer’s recommendation, i.e., a one-step protocol was used for 2 % CHX-a and a four-step protocol was used for other antiseptics, i.e., scrub, rinse, dry, and disinfect as described elsewhere [8].
Intervention
During the 3SITES study, one-step 2 % CHX-a became available in France and was provided free of charge to the participating ICUs. The rationale to introduce 2 % CHX-a was to increase compliance with the CDC guideline which recommends: “Prepare clean skin with a > 0.5 % chlorhexidine preparation with alcohol before central venous catheter and peripheral arterial catheter insertion and during dressing changes”. Because the use of a four-step protocol was recommended by the French Hygiene and Infection Control Society, the adoption of one-step protocol 2 % CHX-a required the approval of the local infection control team, in each participating ICU, and occurred sequentially in four participating ICUs which were previously using four-step PVI-a (Supplemental Appendix Fig. S1). Product training was provided on the novel one-step alcoholic chlorhexidine applicator to each participating ICU prior to patient enrollment.
End-point definitions
For the purpose of this study, we defined catheter-related infection (CRI) as catheter-related bloodstream infection (CRBSI) or the combination of catheter tip colonization plus clinical signs of sepsis on catheter removal without other cause of infection identified. Clinical signs of sepsis included fever (body temperature >38.5 °C) or hypothermia (body temperature <36.5 °C) [8].
Catheter colonization required a catheter tip quantitative culture with ≥103 CFU per millimeter of growth [11]. CRBSI required catheter tip colonization with the same phenotypic microorganism isolated from a peripheral blood culture [2]. For the diagnosis of CRBSI with a potential skin contaminant, two separate peripheral blood cultures had to grow the same microorganism causing catheter tip colonization. Differential time-to-positivity was used in patients discharged from the ICU [12]. A blinded adjudication committee reviewed all suspected cases of CRBSI.
Statistical analysis
The sample size was based on the principal hypothesis of the 3SITES trial. No post hoc power calculation was performed for this study.
Characteristics of patients and catheters were described by group of antiseptic used as count (percentage) and mean (standard deviation) or median (interquartile range) for qualitative and quantitative variables, respectively.
The association between each antiseptic used and the risk of catheter infection in the 3SITES cohort was explored in a multivariate Cox model with robust covariance matrix estimation to account for multiple catheters per patient while adjusting for potential confounders.
To account for the ICU effects that were stable overtime, we also analyzed the data according to a quasi-experimental design (see details in the Supplemental Appendix). The propensity score model included the ICU as a fixed effect, which has been shown to successfully reduce the bias due to the omission of a cluster-level confounder [13]. We performed two different methods, namely propensity score matching (PSM) and inverse probability weighting treatment (IPWT) [14, 15], to correct for baseline differences between the groups. First, we performed a one-to-one greedy, five-to-one digit technique to match catheters by antiseptic group on the basis on their propensity score. Baseline characteristics were compared between groups before and after the matching procedure by the use of the standardized difference, as recommended [16]. Second, we used inverse probability of weighted treatment to adjust Kaplan–Meier curves [17] and Cox models [18]. The probability of end point was modelled in a Cox model with robust covariance matrix estimation to account for the matched design (PSM) and multiple catheters per patient (PSM and IPWT).
The potential for a period effect for the risk of catheter infection was explored by using the IPWT method described above in the ICUs that used the same antiseptic during the trial (control group).
The proportionality assumption in all Cox models was tested and met. A hazard ratio (HR) below one indicated a decreased probability of end point in the alternative antiseptic group compared with the alcoholic 5 % povidone–iodine group. A p value less than 0.05 was considered significant; all p values were two-tailed. Statistical analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc, Cary, NC, USA).
Results
Cohort study
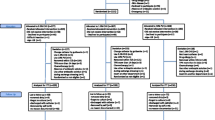
During the 3SITES study, 3471 catheters and 3027 patients were included in 10 ICUs: 1521 catheters (43.8 %) received 5 % PVI-a, 1116 (32.2 %) received 2 % CHX-a, 368 (10.6 %) received 10 % PVI, 357 (10.3 %) received <1 % CHX-a, and 109 (3.1 %) other or unknown antiseptics. All these catheters were included in the cohort analysis (Fig. 1).
The characteristics of the patients and catheters included in the cohort study are reported in Table S1 by antiseptic used. Compared with the most frequently used 5 % PVI-a antiseptic (4.5 per 1000 catheter-days), the use of 2 % CHX-a was independently associated with a decreased risk of CRI [2.0 per 1000 catheter-days, HR, 0.51; confidence interval (CI), (0.28–0.96); p = 0.037] in multivariate analysis while other antiseptics (PVI, 7.1; <2 % CHX-a, 3.8; other or unknown, 2.9 per 1000 catheter-days) were not (Table 1; Fig. S2). The choice of the antiseptic was not associated with CRBSI (Table 1) after adjusting for potential risk factors.
Quasi-experimental study
Four ICUs that were all using 5 % PVI-a agreed to switch to 2 % CHX-a (Supplemental Appendix Fig. S1), representing a total of 1592 central venous catheters inserted in 1368 patients, and these were included in the quasi-experimental analysis (Fig. 1).
Baseline characteristics
There were some difference in the two groups regarding co-morbidities. More catheterized patients with hypertension and with cancer were in the PVI-a group, and more patients with immunodeficiency were in the 2 % CHX-a group (Tables 2, 3). As a result of differences in the date an ICU switched to 2 % CHX-a during the 3SITES trial for catheter disinfection and care, the distribution of each product in each ICU was also different. As expected, the standardized difference between groups comparing baseline patient and catheter characteristics tended to be reduced and all were less than 10 % after matching on the propensity score (Fig. S3).
Catheter infection risk
The Kaplan–Meier curves of CRI and CRBSI by antiseptic group from IPWT and PSM analyses are shown in Fig. 2. Colonization results are shown in the Supplemental Appendix. Microorganisms identified on cultured catheter tips are presented by study group in the PSM analysis in the Supplemental Appendix Table S2.
Kaplan–Meier curves of time to catheter-related infection (left) and catheter-related bloodstream infection (right) by CHX-a and PVI-a groups in the quasi-experimental study. CVC central venous catheters, HR hazard ratio, CI confidence interval, IPWT inverse probability weighting treatment model, PSM propensity score matched
The results of the IPWT and PSM survival analyses are presented in Fig. 2.
The unadjusted incidence of CRI was higher in the PVI-a group compared with the 2 % CHX-a group [6.8 versus 2.0 per 1000 catheter-days, respectively; HR, 0.32; 95 % CI (0.16, 0.64), p = 0.001]. This difference remained significant after PSM [HR, 0.35; 95 % CI (0.15, 0.84), p = 0.02] and in the IPWT analyses [HR, 0.31; 95 % CI (0.14, 0.70), p = 0.005].
Regarding CRBSI, the unadjusted incidence was similar between the PVI-a group and the 2 % CHX-a group [2.4 versus 1.5 per 1000 catheter-days, respectively, HR, 0.67; 95 % CI (0.25, 1.76), p = 0.42]. The same was true after PSM [2.6 in the PVI-a group versus 2.0 per 1000 catheter-days in the 2 % CHX-a group; HR, 0.78; 95 % CI (0.24, 2.56); p = 0.68] and in the IPWT [HR, 0.55; 95 % CI (0.20, 1.55), p = 0.26] analyses.
When we replicated the IPWT analysis among the two ICUs #2 and #6 which started inclusion contemporaneously to the four ICUs analyzed in the quasi-experimental study and which did not change the antiseptic during the study (Supplemental Appendix Fig. S1), the risk of CRI was similar between the two periods [HR, 1.24; 95 % CI (0.25–6.19), p = 0.80].
Safety
There were no reports of severe contact dermatitis associated with the 2 % CHX-a or PVI-a during the follow-up. After dressing removal, mild erythema or superficial desquamation under the dressing was reported in three out of 1116 catheters in the 2 % CHX-a group (0.3 %) and none in the PVI-a group. These symptoms resolved within 24 h after discontinuation of 2 % CHX-a and without further intervention.
Discussion
The risk of CRI consistently decreased with the use of alcoholic 2 % chlorhexidine compared with the use of alcoholic 5 % povidone–iodine in the multivariate cohort study and the quasi-experimental study. The number of CRBSI was low and the risk of CRBSI was similar between antiseptic groups in all analyses.
There was no control over assignment of catheters to the antiseptic used. Therefore, internal validity needs to be discussed. Although the cohort study allowed us to compare several antiseptic products, we could not control for any center effect. For example, all the catheters which received <1 % CHX-a were from ICUs #6, #9, and #10. Moreover, the <1 % CHX group is a mixture of two different antiseptics, one of which contains a very low concentration of alcohol. This prompted us to develop the quasi-experimental study. In this subanalysis, selection bias is not likely because all consecutive catheters were included during the two periods of different antiseptic use within each ICU. Also, a seasonality effect would be limited by the fact that each ICU introduced alcoholic chlorhexidine at different periods of time (Supplemental Appendix Fig. S1), similar to a stepped wedge design [19]. In addition, our exploratory analysis among ICUs which did not modify their antiseptic product did not support the hypothesis of a decreased incidence during the second period. Of note, the site of insertion was randomized in each ICU and thus the distribution of this major risk factor for catheter infection [9] was similar among the antiseptic groups, by design. Nevertheless, some differences (i.e., standardized difference >10 %) regarding baseline characteristics between groups remained (Fig. S2), raising the concern of confounding. The use of multivariate models (cohort study) and propensity scores which mimics an experimental design by balancing risk factors for catheter infection (quasi-experimental study) may have contributed to limit this risk of bias. Noteworthy, the risk factors and outcomes used in this study were prospectively collected and monitored as a part of the 3SITES randomized trial, which targeted the risk of catheter infection as the primary outcome.
We are aware of only one randomized comparison between alcoholic 2 % chlorhexidine and alcoholic 5 % povidone–iodine for the prevention of CRI, which demonstrated the superiority of 2 % CHX-a over PVI-a for reducing CRBSI [8] in arterial, hemodialysis, and central venous catheters. In contrast with the CLEAN study [8], we studied only central venous catheters and therefore compared our study with the subsample of central venous catheters reported in the CLEAN study. Although the proportionality of the hazard ratio was met, examination of the Kaplan–Meier curves suggests the superiority of 2 % CHX-a after day 7 of catheterization (Fig. 2), a result consistent with the long-term activity of chlorhexidine also found by others using chlorhexidine [20]. Interestingly, the incidence of CRI was significantly lower in the 2 % CHX-a group compared with the PVI-a group, a result that was not found in CLEAN despite a large sample size, possibly owning to the low incidence rate of CRI in the PVI-a group. This finding reinforces the role of 2 % CHX-a in reducing risk of CRI within the subgroup of central venous catheters. Nevertheless, the incidence of CRBSI was similar in both groups.
The discrepancy between CRI and CRBSI could be explained by several factors. First, the number of CRBSI was low and therefore our study was probably not powered to demonstrate a significant difference for this end point. Second, the duration of catheterization was rather short (median of 5 days). Therefore, the subset of colonized catheters which qualified for our CRI definition could have been removed before the occurrence of a CRBSI as a result of the daily assessment of central venous catheterization and prompt removal of catheters when no longer needed. Third, patients on systemic antibiotics at the time of blood cultures for CRI that had activity against the organism isolated from the catheter tip could explain a positive colonization with negative concomitant blood cultures.
Although there are reports of severe cutaneous reactions to chlorhexidine [8, 21], there were no reports of severe contact dermatitis following administration of the 2 % CHX-a in this study. Severe skin reaction occurred in 27 patients randomized to the 2 % CHX-a in the CLEAN study [8], but only two patients had to discontinue 2 % CHX-a.
We are aware of limitations. First, this study was not randomized. Thus, residual confounding may persist despite the use of multivariate or propensity-adjusted analyses. Although we were able to compare 5 % PVI-a to other antiseptics used in the 3SITES cohort, including <1 % CHX concentrations, only patient and catheter characteristics could be controlled for but not ICU level characteristics. In addition, the sample size in the <1 % CHX group was three times lower than in the 2 % CHX-a group, limiting our ability to detect significant differences in the cohort study. This remains an area for future research with more robust design [22]. Third, the superiority of 2 % CHX-a was found for CRI but not CRBSI, which may limit its clinical relevance, in particular because sepsis resolution after catheter removal was not incorporated. Of note, CRBSI is highly correlated with catheter tip colonization [23] included in the CRI definition. On the other hand, the superiority of 5 % PVI-a over aqueous 10 % PVI has been demonstrated for CRI but not CRBSI [6] and had nonetheless influenced national guidelines [2, 24]. Fourth, the cutaneous tolerance of the antiseptic was not an outcome of the 3SITES study. Thus underreporting of side effects in the catheter follow-up cannot be excluded. Finally, compliance to antiseptics use was not monitored. Therefore our results are more likely to reflect effectiveness than efficacy.
To prevent CRBSI, optimization of a patient’s skin disinfection is only part of the central venous catheter care bundle [25]. Standardization of infection control practices and techniques [26] during multicomponent educational intervention such as those developed in the US Keystone ICU project are important and cost-effective [27].
In conclusion, our cohort and causal analyses from the 3SITES randomized controlled multicenter study strengthen the level of evidence of the superiority of one-step 2 % CHX-a compared with four-step 5 % PVI-a used for cutaneous disinfection before central venous catheterization and catheter care in reducing risk of catheter infection in ICU patients.
References
Mermel LA (2011) What is the predominant source of intravascular catheter infections? Clin Infect Dis 52:211–212
O’Grady NP, Alexander M, Burns LA et al (2011) Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control 39:S1–34
Mimoz O, Chopra V, Timsit J-F (2016) What’s new in catheter-related infection: skin cleansing and skin antisepsis. Intensive Care Med. doi:10.1007/s00134-016-4244-4
Maki DG, Ringer M, Alvarado CJ (1991) Prospective randomised trial of povidone–iodine, alcohol, and chlorhexidine for prevention of infection associated with central venous and arterial catheters. Lancet 338:339–343
Chaiyakunapruk N, Veenstra DL, Lipsky BA, Saint S (2002) Chlorhexidine compared with povidone–iodine solution for vascular catheter-site care: a meta-analysis. Ann Intern Med 136:792–801
Parienti J-J, du Cheyron D, Ramakers M et al (2004) Alcoholic povidone–iodine to prevent central venous catheter colonization: a randomized unit-crossover study. Crit Care Med 32:708–713
Maiwald M, Chan ESY (2012) The forgotten role of alcohol: a systematic review and meta-analysis of the clinical efficacy and perceived role of chlorhexidine in skin antisepsis. PLoS One 7:e44277
Mimoz O, Lucet J-C, Kerforne T et al (2015) Skin antisepsis with chlorhexidine-alcohol versus povidone–iodine-alcohol, with and without skin scrubbing, for prevention of intravascular-catheter-related infection (CLEAN): an open-label, multicentre, randomised, controlled, two-by-two factorial trial. Lancet 386:2069–2077
Parienti J-J, Mongardon N, Mégarbane B et al (2015) Intravascular complications of central venous catheterization by insertion site. N Engl J Med 373:1220–1229
Haute Autorité de Sante (2010) Check-list: pose d’un catheter veineux central (CVC) ou autre dispositif vasculaire (DV). http://www.has-sante.fr/portail/upload/docs/application/pdf/2011-01/11_01_check-list-cvc-dv.pdf. Accessed 8 June 2016
Brun-Buisson C, Abrouk F, Legrand P, Huet Y, Larabi S, Rapin M (1987) Diagnosis of central venous catheter-related sepsis. Critical level of quantitative tip cultures. Arch Intern Med 147:873–877
Raad I, Hanna HA, Alakech B, Chatzinikolaou I, Johnson MM, Tarrand J (2004) Differential time to positivity: a useful method for diagnosing catheter-related bloodstream infections. Ann Intern Med 140:18–25
Arpino B, Cannas M (2016) Propensity score matching with clustered data. An application to the estimation of the impact of caesarean section on the Apgar score. Stat Med 35:2074–2091
Austin PC, Grootendorst P, Anderson GM (2007) A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med 26:734–753
Robins JM, Hernán MA, Brumback B (2000) Marginal structural models and causal inference in epidemiology. Epidemiol Camb Mass 11:550–560
Austin PC (2009) Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 28:3083–3107
Cole SR, Hernán MA (2004) Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed 75:45–49
Bailly S, Pirracchio R, Timsit JF (2016) What’s new in the quantification of causal effects from longitudinal cohort studies: a brief introduction to marginal structural models for intensivists. Intensive Care Med 42:576–579
Hussey MA, Hughes JP (2007) Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials 28:182–191
Parienti J-J, Cattoir V (2013) Daily chlorhexidine bathing and hospital-acquired infection. N Engl J Med 368:2331
Timsit J-F, Schwebel C, Bouadma L et al (2009) Chlorhexidine-impregnated sponges and less frequent dressing changes for prevention of catheter-related infections in critically ill adults: a randomized controlled trial. JAMA 301:1231–1241
McCann M, Fitzpatrick F, Mellotte G, Clarke M (2016) Is 2% chlorhexidine gluconate in 70% isopropyl alcohol more effective at preventing central venous catheter-related infections than routinely used chlorhexidine gluconate solutions: a pilot multicenter randomized trial (ISRCTN2657745)? Am J Infect Control. doi:10.1016/j.ajic.2016.02.019
Rijnders BJA, Van Wijngaerden E, Peetermans WE (2002) Catheter-tip colonization as a surrogate end point in clinical studies on catheter-related bloodstream infection: how strong is the evidence? Clin Infect Dis 35:1053–1058
Société française d’anesthésie et de réanimation, Société de réanimation de langue française (2009) [Prevention of hospital-acquired sepsis in intensive care unit (except cross transmission and neonate)]. Ann Fr Anesth Reanim 28:912–920
Pronovost P, Needham D, Berenholtz S et al (2006) An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 355:2725–2732
Sherertz RJ, Ely EW, Westbrook DM et al (2000) Education of physicians-in-training can decrease the risk for vascular catheter infection. Ann Intern Med 132:641–648
Cooper K, Frampton G, Harris P et al (2014) Are educational interventions to prevent catheter-related bloodstream infections in intensive care unit cost-effective? J Hosp Infect 86:47–52
Acknowledgments
We thank the nurses, infecton control teams, clinical research assistants and physicians in the participating centers. We also thank Cynthia T. Crosby from the Department of Clinical Research, CareFusion (BD), San Diego for providing alcoholic chlorhexidine at no cost.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflicts of interest
JJP received research grants through his institution and personal fees from CareFusion. LAM received research grant support and personal fees from Marvao Medical and CareFusion, personal fees from Bard, 3M, and Fresenius. All other authors have no competing interests.
Additional information
Take-home message: Central venous catheter insertion site cutaneous antisepsis using one-step alcoholic 2 % chlorhexidine led to a greater reduction in catheter-related infection than four-step alcoholic 5 % povidone–iodine in ICU patients. Catheter-related bloodstream infection was not significantly different between antiseptics.
The members of the 3SITES Study Group are listed in the ESM.
Electronic supplementary material
Below is the link to the electronic supplementary material. Financial support: The 3SITES study was funded by the National Program for Clinical Research (PHRC-N 2010, 06-03) from the French Ministry of Health.
Rights and permissions
About this article
Cite this article
Pages, J., Hazera, P., Mégarbane, B. et al. Comparison of alcoholic chlorhexidine and povidone–iodine cutaneous antiseptics for the prevention of central venous catheter-related infection: a cohort and quasi-experimental multicenter study. Intensive Care Med 42, 1418–1426 (2016). https://doi.org/10.1007/s00134-016-4406-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-016-4406-4