Abstract
The current study was performed to explore potential toxic effect of nickel oxide nanoparticles (NiO NPs) on muscle tissue of catfish, Heteropneustes fossilis. Fishes were exposed to different concentrations of NiO NPs (12 mg/L, 24 mg/L, 36 mg/L and 48 mg/L) for a period of 14 days. Results revealed that NiO NPs caused significant increase in Ni accumulation, metallothionein content, lipid peroxidation and activity of different antioxidant enzymes (catalase, glutathione s transferase and glutathione reductase) while decrease in activity of superoxide dismutase (p < 0.05). Data also reported induction of Na+/K+ ATPase activity initially and then its decrease in concentration dependent manner. Fourier transform infrared spectroscopy revealed shift and changes in spectra of muscle of NiO NPs treated fishes. Fluctuations in activity of aspartate amino transferase, alanine amino transferase and alkaline phosphatase were also noticed. Nutritional contents like protein, lipid, and moisture significantly reduced while glucose and ash percent increased.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Fish has widely been remained a rich source of proteins having essential amino acids and other nutrients like omega-3 highly unsaturated fatty acids (HUFA) i.e., eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (Mesías et al. 2015). It has many health benefits and prevents many diseases like cancer, blood pressure, inflammatory disease etc. (Balami et al. 2019). Fish muscle is the major edible part and an important source of protein for human beings.
Nowadays, environmental health problems of the aquatic ecosystem have received major concern due to health hazards caused by different toxicants like heavy metals (Vaseem 2019), endocrine disruptors (Akhbarizadeh et al. 2021) and nanoparticles (Kakakhel et al. 2021). Among these toxicants, information related to toxic impact caused by nanoparticles on living organisms especially aquatic animals is very scanty. Also very few studies have been performed showing harmful effect of NPs on fishes. Nanoparticles (NPs) are the nano sized particles having at least one dimension with 1–100 nm in size.
Among different nanoparticles, nickel oxide nanoparticles (NiO NPs) have been given major attention because of their wide uses in many sectors e.g., battery electrodes, sensor magnetic materials, electrochemical films, printing inks, catalyst and diesel fuel additives (Aitken et al. 2006; Brody 2006).
Adverse effect of nanoparticles have been demonstrated by many studies in different tissues of various fishes: liver of Pangasius hypophthalmus treated with selenium nanoparticles (Kumar et al. 2018); gills of Oreochromis niloticus treated with titanium dioxide nanoparticles (Firat and Bozat 2019); serum of Pangasianodon hypophthalmus treated with zinc nanoparticles (Kumar et al. 2020). While studies related to their impacts on muscle tissue is very less (Mani et al. 2020; Mahboob et al. 2017; Shahzad et al. 2019).
In case of NiO NPs, no data is reported till now regarding its toxicity to fish muscle tissues. There are only few studies that reported its toxicity to fishes like changes in haematology, biochemical parameters and enzymatic activities in Heteropneustes fossilis (Samim and Vaseem 2021), alterations in antioxidant enzymes in Labeo rohita (Aziz et al. 2021) and toxicity to different stages of embryonic development in zebra fish (Kovrižnych et al. 2013).
Till now, no study has been conducted to examine impact of NPs on nutrient content of fishes. Such study will be very useful to monitor the quality of NPs exposed fishes in terms of nutrient content. Heteropneustes fossilis, an economically important fish was selected for this study due to its hardness and capacity to survive in several harsh conditions.
Therefore, this study was designed to manifest harmful impact caused by NiO NPs on fish by analyzing bioaccumulation of Ni, oxidative stress, metabolic enzymes and nutrient content of muscle tissue.
Materials and Methods
NiO NPs were procured from St. Louis, Missouri, United States, with an average particle size < 50 nm and trace metal basis 99.8% (CAS. 1313-99; MW: 74.69 gm/mol; d: 6.67 gm/ml at 25oC). Detailed characterization of NiO NPs has been discussed in our previous published paper (https://doi.org/10.1007/s11356-021-14451-y).
Fish (H. fossilis) were obtained from native fish market, Aligarh, India and acclimatized in laboratory condition for 4 weeks in water having temperature, pH, salinity, dissolve oxygen, total alkalinity, total hardness in the range of 26 ± 3oC, 7.2 ± 0.4, 0.4 ± 0.08 pg/l, 6.8 ± 0.04 mg/l, 20.3 ± 6.1 mg/l and 16 ± 0.04 mg/l respectively. Fish were given ad libitum as food at regular one day interval.
The 96-h lethal concentration (LC50) value of NiO NPs for H. fossilis was determined and was found to be 240 mg/l. After that fishes were exposed to sub-lethal concentrations such as 5% of LC50 i.e., 12 mg/l, 10% of LC50 i.e., 24 mg/l, 15% of LC50 i.e., 36 mg/l and 20% of LC50 i.e., 48 mg/l for 14 days for the toxicity studies. Details regarding LC50 value have been given in our previous published paper (Samim et al. 2022).
The detailed experimental design and exposure procedure of fishes with different concentrations of NiO NPs (12 mg/l, 24 mg/l, 36 mg/l and 48 mg/l) have already been explained in our previous published paper (Samim and Vaseem 2021) (https://doi.org/10.1007/s11356-021-14451-y). Irrespective of sex, ten fishes of almost similar length and weight (18 ± 2.5 cm and 25 ± 3 g) were sacrificed from each group and muscles were collected from control and all exposed groups after 14 days of exposure. 0.6% normal saline was used to wash tissues during collection to get rid of any impurity and blood. After that, tissues were kept at − 80oC.
For analysis of NiO NPs, collected muscles tissues were dried in hot air oven. Thereafter, 1 gm of tissue from each exposed fish was dried and digested in two acids i.e., HNO3 and HClO4 in 2:1 ratio at 100oC. Samples were filtered after the mixing of digested samples with distilled water. The concentration (mg/kg) of Ni in muscle tissue was measured using a flame atomic absorption spectrophotometer (Perkin Elmer Model 2380, Inc., walk, CT, USA) (Samim et al. 2022). Quality control measures were used to detect contamination and to ensure data reliability. After every ten readings, the instrument was calibrated by running a blank and a nickel standard solution (catalog Number: 1.19792.0500, Merck). Detection limit for Ni was 0.01 mg/kg.
For enzymatic analysis, muscle tissues were homogenized in a pH 7.4 ice cold 50mM phosphate buffer containing 0.25 M sucrose at 4oC. Homogenates were then centrifuged at 2500 rpm for 10 min while keeping the temperature at 4oC. A sample of supernatant was used for lipid peroxidation and protein analysis. Further, to obtain post mitochondrial supernatant (PMS), the supernatant was centrifuged for 25 min at 12,000 rpm maintaining 4oC temperature. For additional enzyme analyses, the PMS was kept at − 20oC. Metallothionein content was analyzed by the method of Viarengo et al. (1997). Lipid peroxidation of muscle was estimated by Ohkawa et al. (1979) method. The super oxide dismutase (SOD) activity of muscle tissues was assayed by the method described by Das et al. (2000). The method of Aebi (1984) was used to analyze catalase (CAT) activity. Glutathione s transferase (GST) activity was estimated by Habig et al. (1974). Carlberg and Mannervik (1985) method was followed to measure glutathione reductase (GR) activity. Na+/K+ ATPase activity was estimated by Shiosaka et al. (1971). The functional groups in muscles associated with nanoparticle interaction were examined using Fourier Transform Infra-Red (FTIR) spectroscopy.
Reitman and Frankel (1957) method was used to measure aspartate amino transferase (AST) & alanine amino transferase (ALT) activities. Activity of alkaline phosphatase (ALP) was analyzed following the method suggested by Kind and King (1954).
Total protein, lipid and glucose level were estimated by Lowry et al. (1951), Folch et al. (1957) and Trinder (1969) method respectively. For moisture content, samples were first weighed (initial weight), and then dried in an electric oven for 24–30 h at 105°C to get a constant weight. The following formula was used to calculate moisture content: Moisture% = (initial weight – dry weight) × 100/ Initial weight. To determine ash, 1 g of sample was heated in a muffle furnace at 550oC until a constant weight was obtained. The following formula was used to calculate ash content: Ash% = Ash weight × 100/sample weight.
Data were shown as mean value with standard deviation (SD) (n = 3). One-way ANOVA was performed followed by Duncan’s Multiple Range Test (p < 0.05). SPSS software version 16.0. was used for statistical analyses. Furthermore, to evaluate the impact of NiO NPs in overall parameters of muscle target fish, principal component analysis (PCA) was done using Minitab Statistical Software (Version 19.1).
Results and Discussion
Owing to potential in addressing hunger problems, the importance of fish in terms of food and nutrition security is growing day by day (Thilsted et al. 2016). Therefore, many countries are identifying fisheries and aquaculture as critical resources for addressing food and nutrition security issues (NFNC 2011). However, pollution has been remained a great threat to aquatic organisms including fishes due to harmful effect of various toxicants on them. Along with other biological parameters, these toxicants also cause deterioration in the quality (nutrient content) of aquatic food especially fishes.
As muscle do not remain in direct contact with external environment, they are considered to be secondary target organ. Despite of being secondary target organ, muscle still remains in great threat of getting affected by toxicants. Moreover, muscles are also the main consumed part of the fish. Therefore, changes in the quality of fish muscle would be reflected in human health.
There are many studies which have reported effect of variety of NPs on fish muscle (Mani et al. 2020; Kakakhel et al. 2021) while best of our knowledge, no study has reported impact of NiO NPs on fish muscle.
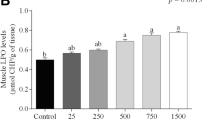
Fishes are capable of absorbing metals from their surroundings and can accumulate them in their tissues. These metals are neither excreted nor egested that in turn may cause deteriorating biological impact on physiology of fishes (Abdel-Khalek et al. 2015, 2018). Figure 1 showed accumulation of Ni (mg/kg) in muscle which was significantly elevated (p < 0.05) in all exposed fishes. Highest accumulation was observed in 48 mg/l NiO NPs exposed fishes while lowest was observed in 12 mg/l exposed ones. However, Ni accumulation in control group was found to be below detectable limit. Ni accumulation in muscles may lead to deteriorating effects on fish health. Accumulation of Ni might be responsible for generation of free radicals which led to oxidative stress and ultimately resulted in damaging alterations in various vital biomarkers. Consumption of Ni accumulated muscles may also cause serious health issues in human being as well as other organisms. In present study, alterations in different antioxidant & metabolic enzymes as well as nutritional contents in NiO NP–treated fish are the consequences of Ni accumulation in muscle. Kakakhel et al. (2021) showed similar kind of results who reported higher level of significant accumulation of silver nanoparticles in muscle of Cyprinus carpio.
Metallothioneins are very significant molecular biomarker, involved in detoxification of metals due to their chelating capacity and are being commonly used to evaluate the effect of toxicants (Vicari et al. 2018). The present study indicated significant increase in metallothionein concentrations in all exposed group compared to control one (Fig. 2). The increased metallothionein in muscle might be due to higher accumulation of nickel in exposed groups (Fig. 1). Further, muscle is the site of oxidative metabolism where production of free radicals might be higher because of greater accumulation of nickel that indicate higher binding affinity of metallothionein to nickel in order to detoxify, metabolize and maintenance of homeostasis in fish (Abdel-Tawwab and Wafeek 2014; Klaassen et al. 2009).
One of the known mechanism of toxicity of nanoparticles is to induce oxidative stress which is the result of many cellular responses (Shahzad et al. 2019). Oxidative stress is caused by reactive oxygen species which produce free radicals resulting into induction of cytotoxicity. Lipid peroxidation and different antioxidant enzymes like SOD, CAT, GST and GR play vital role in measuring oxidative stress.
In aquatic organism, polyunsaturated fatty acids are essential part of cell membrane and maintain its fluidity. Also there are greater chance of oxidation of these fatty acids in aquatic organism than terrestrial organisms (Monserrat et al. 2007). Therefore, LPO is used as key biomarker of membrane damage and stress caused by nanoparticles (Ma et al. 2010).
Regarding oxidative stress biomarkers, the present study found a significant increase in LPO in muscle tissue as a result of NiO NPs exposure (Fig. 3a). Increased lipid peroxidation in treated fishes might be attributed to rise in Ni accumulation (Fig. 1) in muscle which demonstrate higher affinity of Ni to lipids of plasma membrane, resulting in increased lipid damage.
Lipid peroxidation (a) and activity of different antioxidant enzymes (SOD, CAT, GST and GR) in muscle b, c, d & e of Heteropneustes fossilis after exposure of different concentrations of NiO NPs for a period of 14 days. The data are presented as mean± SD. The different superscripts indicate a statistically significant difference at a significant level of p < 0.05
In present study, SOD activity was significantly decreased (Fig. 3b) but CAT activity was significantly enhanced (Fig. 3c) in all the NiO NPs treated fishes. This could be due to SOD protein damage caused by reactive oxygen species overproduction. Increase in CAT activity might be to counteract against oxidative stress caused by NiO NPs. Similarly, decreased SOD activity was noticed in muscle of zebra fish treated with copper oxide nanoparticles (Mani et al. 2020). Decreased SOD activity and increase in CAT activity were also observed in Goodea atripinnis fish treated with Lake Yuriria water sample (Ortiz-Ordoñez et al. 2011). GST activity showed highly significant increase (Fig. 3d). This might be due to rapid adaptive response to neutralize harmful impact of NiO NPs. Similarly, it was observed that selenium nanoparticles could induce GST activity in fish tissues like gill, liver and brain of Pangasius hypophthalmus (Kumar et al. 2018). Similar to GST, GR activity (Fig. 3e) also increased in present study. Firat and Bozat (2019) also showed significantly higher level of GR and GST activity in gill of Oreochromis niloticus exposed to TiO2 NPs. Elevated GR activity in this study might be to protect the fish from the oxidative stress triggered by NiO NPs.
The membrane bound ATPase are significant parameter for determining noxious impact of toxicants in fishes. In teleost fishes, Na+/K+ gradient between intra and extracellular fluid across plasma membrane is maintained by Na+/K+ ATPase enzyme (McCormick 1993). In this study, activity of Na+/K+ ATPase initially increased and then decreased significantly (Fig. 4). This could de due to harmful impact of NiO NPs on ATPase function or accumulation of Ni content in tissue. It could also be due to changes in lipid peroxidation (Fig. 3a) leading to damage in plasma membrane. This is an agreement with the study of Agrahari and Gopal (2007) who showed decrease Na+/K+ ATPase activity in various tissues of fresh water fish Channa punctatus. Bao et al. (2020) also reported decreased Na+/K+ ATPase activity in liver of zebra fish exposed to AgNPs.
During accumulation of toxicants, numbers of detoxification process are involved to protect cells from their detrimental effects. In this process, various chemical modifications take place and as a result number of bonds are made and dissociated. This mechanism can be studied clearly by FTIR spectra of tissues exposed to NiO NPs. Therefore, FTIR spectra of fishes treated with NiO NPs were analyzed to know the interactions of different biomolecules with NiO NPs in muscle during exposure period. The FTIR spectra was assessed with NiO NPs treated fishes with that of spectra of control group.
In Fig. 5a–e it was evidently observed that there was shift and alterations in spectra of NiO NPs treated fishes in comparison to control one. This might be due to accumulations of NiO NPs in muscles which involved interactions of NiO NPs to different biomolecules or due to detoxification process in which number of bonds are made and dissociates. Shift in peaks in catfish, Clarias gariepinus exposed to TiO2 NPs was also observed (Matouke 2019). Similarly Punitha et al. (2014) also reported that ZnS nanoparticles could shift in peaks in liver tissue of fish Oreochromis niloticus.
a FTIR spectra of muscle in control group. b FTIR spectra of muscle exposed to 12mg/l of NiO NPs for a period of 14 days. c FTIR spectra of muscle exposed to 24mg/l of NiO NPs for a period of 14 days. d FTIR spectra of muscle exposed to 36mg/l of NiO NPs for a period of 14 days. e FTIR spectra of muscle exposed to 48mg/l of NiO NPs for a period of 14 days
The present study reported a significant rise in AST, ALT & ALP activity in most of the NiO NPs exposed fishes (Fig. 6). Elevation in AST & ALT activities might be due to increase in glucose production (Fig. 7b) during gluconeogenesis pathway to generate higher energy to withstand stress or to generate intermediates of tri-carboxylic acid (TCA) cycle by enhancing transamination pathway through deamination of amino acids (Kumar et al. 2014). Increased AST & ALT activities were observed by Kumar et al. (2020) in gills, liver, muscle and kidney of Pangasianodon hypophthalmus exposed to Zn NPs. Elevated ALP activity might be an indication of disruption in membrane permeability which is a direct evident of lipid peroxidation (Fig. 3a). Similar result was observed in muscle, liver and gills of Labeo rohita with exposure to silver nanoparticles (Rajkumar et al. 2016).
For nourishment and growth of the body, nutrients are required in proper amount. Among different nutrients, fish protein play very important role in muscle building, immunity and blood quality improvement. According to Mohanty et al. (2019), fish protein is involved in prevention of protein-calorie malnutrition.
The total protein content decreased significantly in muscle of treated fishes (Fig. 7a) showing deterioration of quality of fish flesh. Significantly lower level of protein content in muscle of Mystus gulio exposed to silver nanoparticles was also demonstrated by Abirami et al. (2017).
In present study, higher glucose content (Fig. 7b) might be produced in gluconeogenesis pathway by breaking stored glycogen in order to produce more energy to fight against stress caused by NiO NPs. Muscle of Chapalichthys pardalis treated with AgNPs (Valerio-García et al. 2017) also showed higher glucose level.
Nutritional contents (protein, glucose, lipid, moisture & ash) in muscle a, b, c, d & e of Heteropneustes fossilis after exposure of different concentrations of NiO NPs for a period of 14 days. The data are presented as mean± SD. The different superscripts indicate a statistically significant difference at a significant level of p < 0.05
In present study, significantly lower level of lipid content was obtained in muscle of NiO NPs treated fish (Fig. 7c). This could be attributed with overutilization of lipid like those of protein content to protect against stress caused by NiO NPs. Similar kind of results were noticed by Valerio-García et al. (2017) who reported decrease level of lipid content in muscle of Chapalichthys pardalis exposed to silver nanoparticles.
Alterations in moisture and ash content (percent) also indicated susceptibility of fish Heteropneustes fossilis to NiO NPs toxicity. Moisture content significantly decreased in all exposed fishes (Fig. 7d) indicating osmoregulatory disruption caused by accumulation of NiO NPs in muscle of fish while ash content was found to be significantly increased (Fig. 7e) which might be due to higher accumulation of nickel. Vaseem and Banerjee (2016) also observed similar results where they reported metals and other toxicants could decrease the moisture content and induce ash content in fish muscle of Labeo rohita.
To elucidate impact of NiO NPs on various parameters response, principal component analysis was applied to all data acquired from muscle tissue. The PCA plots summarize the comparison among samples exposed to NiO NPs. Figure 8a depicts the location of working treatments while Fig. 8b depicts the distribution of biomarkers in space defined by first and second PCA dimensions. The principal component analysis represented 97.3% (PC1 = 86.9% and PC2 = 10.4%) in muscle of total variance. The location of working treatments revealed a clear distinction between control and NiO NPs treated fish (Fig. 8a). Figure 8a clearly indicated correlation of biomarkers in response to NiO NPs concentration where it was observed that protein, lipid, moisture, SOD activity and Na+/K+ ATPase activity were negatively correlated with the concentrations of NiO NPs. However, Ni accumulation, lipid peroxidation, different antioxidant enzymes (CAT, GST & GR), glucose, ash, metabolic enzymes (AST, ALT & ALP) and metallothionein were found to be positively correlated with concentrations of NiO NPs. Therefore, this study depicted that NiO NPs are capable to decrease the quality of nutritional composition in Heteropneustes fossilis by causing oxidative stress.
References
Abdel-Khalek AA, Elhaddad E, Mamdouh S et al (2018) The chronic exposure to discharges of Sabal drain induces oxidative stress and histopathological alterations in Oreochromis niloticus. Bull Environ Contam Toxicol 101(1):92–98
Abdel-Khalek AA, Kadry MA, Badran SR et al (2015) Comparative toxicity of copper oxide bulk and nano particles in Nile tilapia; Oreochromis niloticus: biochemical and oxidative stress. J Basic Appl Zool 72:43–57
Abdel-Tawwab M, Wafeek M (2014) Influence of water temperature and waterborne cadmium toxicity on growth performance and metallothionein–cadmium distribution in different organs of Nile tilapia, Oreochromis niloticus (L). J Therm Biol 45:157–162
Abirami T, Jose AGR, Govindarajulu BAVANI et al (2017) Ecotoxicology of green synthesized silver nanoparticles on fresh water fish Mystus gulio. Int J Pharm Pharm Sci 9(11):192–198
Aebi H (1984) Catalase in vitro, In: SP Colowick, NO Kaplane (Eds). Methods Enzymol 105:121–126
Agrahari S, Pandey KC, Gopal K (2007) Biochemical alteration induced by monocrotophos in the blood plasma of fish, Channa punctatus (Bloch). Pestic Biochem Phys 88(3):268–272
Aitken RJ, Chaudhry MQ, Boxall ABA, Et al (2006) Manufacture and use of nanomaterials: current status in the UK and global trends. Occup Med 56:300–306
Akhbarizadeh R, Russo G, Rossi S et al (2021) Emerging endocrine disruptors in two edible fish from the Persian Gulf: occurrence, congener profile, and human health risk assessment. Mar Pollut Bull 166:112241
Aziz S, Abdullah S, Anwar H et al (2021) Effect of engineered nickel oxide nanoparticles on antioxidant enzymes in freshwater fish, Labeo rohita. Pak Vet J 41(3):424–428
Balami S, Sharma A, Karn R (2019) Significance of nutritional value of fish for human health. Malays J Halal Res 2(2):32–34
Bao S, Tang W, Fang T (2020) Sex-dependent and organ-specific toxicity of silver nanoparticles in livers and intestines of adult zebra fish. Chemosphere 249:126172
Brody AL (2006) Nano and food packaging technologies converge. Food Technol 60:92–94
Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113:484–490
Das K, Samanta L, Chainy GBN (2000) A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. Indian J Biochem Biophys 37:201–204
Fırat Ö, Bozat RC (2019) Assessment of biochemical and toxic responses induced by titanium dioxide nanoparticles in Nile tilapia Oreochromis niloticus. Hum Ecol Risk Assess: Int J 25(6):1438–1447
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J biol Chem 226(1):497–509
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139. https://doi.org/10.1016/S0021-9258(19)42083-8
Kakakhel MA, Wu F, Sajjad W et al (2021) Long-term exposure to high-concentration silver nanoparticles induced toxicity, fatality, bioaccumulation, and histological alteration in fish (Cyprinus carpio). Environl Sci Eur 33(1):1–11
Kind PR, King EG (1954) Colorimetric determination of alkaline phosphatase activity. J Clin Pathol 7:322
Klaassen CD, Liu J, Diwan BA (2009) Metallothionein protection of cadmium toxicity. Toxicol Appl Pharmacol 238(3):215–220
Kovrižnych JA, Sotníková R, Zeljenková D et al (2013) Acute toxicity of 31 different nanoparticles to zebrafish (Danio rerio) tested in adulthood and in early life stages–comparative study. Interdiscip Toxicol 6(2):67
Kumar N, Chandan NK, Wakchaure GC et al (2020) Synergistic effect of zinc nanoparticles and temperature on acute toxicity with response to biochemical markers and histopathological attributes in fish. Comp Biochem Phys Part C: Toxicol Pharmacol 229:108678. https://doi.org/10.1016/j.cbpc.2019.108678
Kumar N, Gupta S, Chandan NK et al (2014) Lipotropes protect against pathogen-aggravated stress and mortality in low dose pesticide-exposed fish. PLoS ONE 9(4):e93499. https://doi.org/10.1371/journal.pone.0093499
Kumar N, Krishnani KK, Singh NP (2018) Comparative study of selenium and selenium nanoparticles with reference to acute toxicity, biochemical attributes, and histopathological response in fish. Environ Sci Pollut Res 25(9):8914–8927
Lowry OH, Rosenbrough NJ, Ferry AL et al (1951) Protein measurement with folin-phenol reagent. J Bioll Chem 193:265–275
Ma X, Geiser-Lee J, Deng Y et al (2010) Interactions between engineered nanoparticles (ENPs) and plants: phytotoxicity, uptake and accumulation. Sci Total Environ 408(16):3053–3061
Mahboob S, Al-Ghanim KA, Al-Mulhim NM (2017) Fish exposure to sub-lethal toxicity of nano-titanium oxide and changes in muscular antioxidant enzymes and protective role of vitamins c and e in Clarias gariepinus. Int J Agric Biol 19:1505–1510. https://doi.org/10.17957/IJAB/15.0454
Mani R, Balasubramanian S, Raghunath A et al (2020) Chronic exposure to copper oxide nanoparticles causes muscle toxicity in adult zebrafish. Environ Sci Pollut Res 27(22):27358–27369
Matouke MM (2019) FTIR study of the binary effect of titanium dioxide nanoparticles (nTiO2) and copper (Cu2+) on the biochemical constituents of liver tissues of catfish (Clarias gariepinus). Toxicol Rep 6:1061–1070
McCormick SD (1993) Methods for nonlethal gill biopsy and measurement of Na+, K+-ATPase activity. Can J Fish Aquat Sci 50(3):656–658. https://doi.org/10.1139/f93-075
Mesías M, Holgado F, Sevenich R et al (2015) Fatty acids profile in canned tuna and sardine after retort sterilization and high pressure thermal sterilization treatment. J Food Nutr Res 54:171–178
Mohanty BP, Mahanty A, Ganguly S et al (2019) Nutritional composition of food fishes and their importance in providing food and nutritional security. Food Chem 293:561–570
Monserrat JM, Martínez PE, Geracitano LA et al (2007) Pollution biomarkers in estuarine animals: critical review and new perspectives. Comp Biochem Physiol Part C: Toxicol Pharmacol 146(1–2):221–234
National Food and Nutritional Commission of Zambia (NFNC) (2011) National Food and nutritional strategic plan 2011–2015
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358. https://doi.org/10.1016/0003-2697(79)90738-3
Ortiz-Ordoñez E, Uría-Galicia E, Ruiz-Picos RA et al (2011) Effect of Yerbimat herbicide on lipid peroxidation, catalase activity, and histological damage in gills and liver of the freshwater fish Goodea atripinnis. Arch Environ Contam Toxicol 61(3):443–452
Punitha P, Shoba V, Krishnapriya K et al (2014) Toxicity evaluation of mg doped ZnS nanoparticles and bougainvillae glabra flower extract on fresh water fish Oreochromis mossambicus. Int J Mod Res Rev 2:246–250
Rajkumar KS, Kanipandian N, Thirumurugan R (2016) Toxicity assessment on haemotology, biochemical and histopathological alterations of silver nanoparticles-exposed freshwater fish Labeo rohita. Appl Nanosci 6(1):19–29. https://doi.org/10.1007/s13204-015-0417-7
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28(1):56–63. https://doi.org/10.1093/ajcp/28.1.56
Samim AR, Singh VK, Vaseem H (2022) Assessment of hazardous impact of nickel oxide nanoparticles on biochemical and histological parameters of gills and liver tissues of Heteropneustes fossilis. J Trace Elem Med Biol 74:127059. https://doi.org/10.1016/j.jtemb.2022.127059
Samim AR, Vaseem H (2021) Assessment of the potential threat of nickel (II) oxide nanoparticles to fish Heteropneustes fossilis associated with the changes in haematological, biochemical and enzymological parameters. Environ Sci Pollut Res 28(39):54630–54646
Shahzad K, Khan MN, Jabeen F et al (2019) Toxicity of zinc oxide nanoparticles (ZnO-NPs) in tilapia (Oreochromis mossambicus): tissue accumulation, oxidative stress, histopathology and genotoxicity. Int J Environ Sci Technol 16(4):1973–1984
Shiosaka T, Okuda H, Fujii S (1971) Mechanism of the phosphorylation of thymidine by the culture filtrate of Clostridium perfringens and rat liver extract. Biochim Biophys Acta (BBA) 246(2):171–183
Thilsted SH, Thorne-Lyman A, Webb P et al (2016) Sustaining healthy diets: the role of capture fisheries and aquaculture for improving nutrition in the post-2015 era. Food Policy 61:126–131
Trinder P (1969) Enzymatic colorimetric method of glucose. Ann Clin Biochem 6:24–27
Valerio-García RC, Carbajal-Hernández AL, Martínez-Ruíz EB et al (2017) Exposure to silver nanoparticles produces oxidative stress and affects macromolecular and metabolic biomarkers in the goodeid fish Chapalichthys pardalis. Sci Total Environ 583:308–318
Vaseem H (2019) Analysis of heavy metal concentrations and haematological parameters of two fishes, Channa punctatus and Heteropneustes fossilis from a fish market, Aligarh, India. EC Pharmacol Toxicol 7:523–530
Vaseem H, Banerjee TK (2016) Evaluation of pollution of Ganga River water using fish as bioindicator. Environ Monit Assess 188(8):1–9
Viarengo A, Ponzano E, Dondero F et al (1997) A simple spectrophotometric method for metallothionein evaluation in marine organisms: an application to Mediterranean and Antarctic molluscs. Mar Environ Res 44:69–84
Vicari T, Dagostim AC, Klingelfus T et al (2018) Co-exposure to titanium dioxide nanoparticles (NpTiO2) and lead at environmentally relevant concentrations in the neotropical fish species Hoplias intermedius. Toxicol Rep 5:1032–1043
Acknowledgements
This work was funded by University Grant Commission (UGC), by the STARTUP Grant Number F.30–409/ 2018(BSR)), UGC, Government of India, New Delhi, India.
Author information
Authors and Affiliations
Contributions
Both ARS & HV contributed to conceptualization, formal analyses, data curation, methodology, investigation and validation. HV supervised this work and provided necessary resources to ARS to conduct this research. ARS wrote the original manuscript which was further edited by HV. Both authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Samim, A.R., Vaseem, H. Exposure to Nickel Oxide Nanoparticles Induces Alterations in Antioxidant System, Metabolic Enzymes and Nutritional Composition in Muscles of Heteropneustes fossilis. Bull Environ Contam Toxicol 110, 79 (2023). https://doi.org/10.1007/s00128-023-03714-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00128-023-03714-8