Abstract
Nanotechnology is an advancing and emerging field of all environmental, medical, and industrial applications. Magnesium oxide nanoparticles have been widely used in medicine, consumer products, industrial products, textiles, ceramics, alleviation of heartburn, stomach ulcers, and bone regeneration. In the present study, acute toxicity (LC50) of MgO nanoparticles and hematological and histopathological changes in Cirrhinus mrigala was analyzed. The lethal concentration for 50% of MgO nanoparticles was 4.2321 mg/L. Hematological parameters such as white blood cells, red blood cells, hematocrit, hemoglobin, platelets, mean corpuscular volume, mean corpuscular hemoglobin, and mean corpuscular hemoglobin concentration, as well as histopathological abnormalities in gills, muscle, and liver were observed on the 7th and 14th days of exposure. The WBC, RBC, HCT, Hb, and platelets count increased on the 14th day of exposure compared to the control and 7th day of exposure. The MCV, MCH, and MCHC levels decreased on the 7th day of exposure compared to the control and increased on the 14th day. Histopathological changes of MgO nanoparticles in gills, muscle, and liver highly damaged in the quantity of 3.6 mg/L compared to 12 mg/L on 7th and 14th days of exposure. This study finds the level of exposure in hematology and histopathological changes in tissues in relation to the exposure of MgO NPs.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The field of nanotoxicology focuses on the living organisms that are adversely affected by nanomaterials or nanoparticles engineered for use in nanotoxicology. The use of engineered nanoparticles in biomedical fields has raised serious concerns about their safety for human beings [1]. In biotechnological and pharmaceutical applications, nanoparticles are important tools because their large surfaces dominate the contributions made by the small bulk of the material, along with their quantum effects [2]. As nanomedicines, drug carriers, nanoparticles are widely regarded for their small sizes, unique morphologies, surface functional groups, and dose-dependent properties, which also contribute to the toxicity of healthy human cells, tissues, and organs [1]. A nanoparticle can cross the cell membrane and interact easily with intracellular metabolism [3]. Nanotoxicity is also quantified in terms of severity and frequency in relation to organism exposure. A wide variety of products, including consumer goods, industrial products, and healthcare technology, contain metal nanoparticles. These nanoparticles may enter the environment [4]. These materials can have different physical and chemical properties to bulk form. The use of nanomaterials is prevalent in a wide range of industries, consumer products, and aquaculture. It is perceived that the size range that gives the best advantage is under 100 nm; nonetheless, there are yet many applications for which bigger particles can give properties of extraordinary interest [5]. It can be nanoscale in one dimension, two dimensions, or three dimensions and can exist in single, intertwined, accumulated, or agglomerated structures with circular, cylindrical, and unpredictable shapes. Compared to larger molecules, nanomaterials are more bioavailable [6]. Nano-magnesium oxide (MgO) products have gained considerable worldwide attention in recent decades. Magnesium oxide nanoparticles are widely used in sunscreens, cosmetics, food products, paints, and ceramics. Medical applications for magnesium oxide nanoparticles include biosensors, antibiotics, antacids, detoxifying agents, bone regeneration, and drug delivery [7]. MgO nanoparticles have found fabulous applications in biomolecular detection, diagnostics, and microelectronics. Histopathological changes like those caused by other xenobiotics were observed in fish with chronic toxicity [8]. A nanoparticle absorbed into plasma can penetrate cells and be deposited in organs such as the lungs, stomach, brain, liver, kidney, heart, and testicles [9]. Stress is closely related to histological biomarkers, as many pollutants, toxic or nontoxic, require metabolic activation to culminate in cellular changes in affected organisms [10]. This present study investigates the toxic effect of MgO nanoparticles in different quantities of exposure in mrigal during the 7th and 14th days of the experiment.
Materials and Methods
MgO Nanoparticles
MgO nanoparticles (< 100 nm) were synthesized by co-precipitation method. The suspensions of nanosized MgO nanoparticles were prepared with single distilled water, dispersed with an ultrasonicator (20–100 kHz) 1 h for every time before the exposure of fish.
Characterization of MgO Nanoparticles
In this MgO nanoparticles, absorption level and functional groups are characterized by UV visible spectroscopy (GENESYS 1XX UV-VIS Double beam DUV 1100), FTIR, and Fourier transform infrared spectroscopy (JASCO 6200 spectra).
UV-Visible Spectroscopy (UV-Vis)
UV-visible spectroscopy (ultraviolet spectroscopy) is a kind of imbibing spectroscopy based on the penetration of electromagnetic radiation with the wavelength ranges of 200–400 nm. Ultraviolet-visible spectroscopy is used for characterizing MgO, exceptionally to tap the bandgap energy and the generation of MgO NPs. Optical spectacles were probed by UV- spectroscopy. Identifying, studying nanomaterial, and characterizing UV-visible are valued tools.
Fourier Transform Infrared Spectroscopy (FITR)
FTIR is a very versatile tool for surface characterization of nanoparticles and provides a specific setup attached to the spectrometer. Under specific conditions, the chemical composition of the nanoparticle surface determined and the surface reactive sites responsible for the surface reactivity can be identified.
Animal experiment
Fingerlings of mrigal Cirrhinus mrigala (1.53 ± 0.3 g) were collected from K.V.K fish farm, Palani, Dindigul, Tamilnadu, India. The selection of fish and experiments was framed according to guidelines [11]: 7 fish, 15 L volume of water induced per tank.
The MgO nanoparticle suspension was prepared and dispersed by ultrasonicator under standard laboratory conditions to avoid environmental influence. The fish were acclimated for 15 days in natural light and dark conditions (12 h light/12 h night); temperature and pH were both maintained at 24 °C and 7.4, respectively.
Acute Toxicity Test
This experiment was followed by Organization for Economic and Corporation and development guidelines (OECD). Healthy fingerlings (1.53 ± 0.3 g) were selected under static conditions. Five different concentrations (0, 3, 6, 12, and 24 mg/L) were established in median lethal concentration (LC50) for 96 h. Treatment was carried out in triplicate using the factorial design method. Water quality parameters were maintained in the same conditions during the experiment. Control was maintained without nanoparticles. Feed to the fish was stopped before 48 h of the experiment and 96 h LC50 was calculated by using probit analysis.
Hematological Parameters
The blood samples were collected from each exposure and the hematological parameters such as complete blood count (CBC), hemoglobin (gm/dL), red blood cells (RBC) (millions/cmm), hematocrit (Hct) (%), white blood cells (WBC) (cell/cumm), polymorph, neutrophils (%), lymphocytes (%), eosinophils (%), mean corpuscular hemoglobin (MCH) (pg), mean corpuscular hemoglobin concentration (MCHC) (%), and platelets (lakhs/cmm) were estimated after the 7th and 14th days. The WBC and RBC were counted by using the hemocytometer method [12], and hemoglobin (Hb) was analyzed by the cyanmethemoglobin method [13].
Histopathology of Gill, Muscle, and Liver
Seven fish were exposed to sub-lethal concentrations and blood was taken on the 7th and 14th days for hematological parameters. The gills, muscles, and liver of the fish were dissected and maintained in 10% formalin for 48 h. Ethanol was used for dehydration, and xylene was used to clean the tissue; after the xylene, the tissues were embedded in paraffin wax. A 5-µm-thick sagittal section was cut and mounted in slides. After deparaffinization and hydration in ethanol, the slides were stained with hematoxylin and eosin for structural analysis of tissue alternations, examined, and micrographs were taken in a fluorescence microscope (Olympus CX33) [14].
Statistical Analysis
In this study, data were processed using SPSS (16.0) to determine mean ± SD when comparing groups. ANOVA was used to determine the level of variation among groups. Statistical significance was set at P < 0.05.
Result and Discussion
Ultraviolet–Visible Spectroscopy Analysis
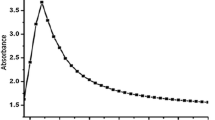
The UV–visible absorption spectroscopy is a widely used technique to examine the optical properties of the nanosized particle. A range of wavelengths 200–1100 was used to measure the absorbance spectra of magnesium oxide nanoparticles. It exhibits the strong absorption band at 240 nm, as shown in Fig. 1A. The UV–Vis spectroscopy technique is a reliable and helpful tool for identifying synthesized nanomaterials at a primary level. A range of wavelengths 200–1100 was used to measure the absorbance spectra of magnesium oxide nanoparticles. It exhibits the strong absorption band at 240 nm [15]. reported that the MgO nanoparticles are prepared via Amaranthus tricolor leaf extract and the optical absorbances of those nanosized particles are observed in the UV region at 320 nm. The UV–visible spectra of the prepared MgO nanopowder in the absorbance mode and in the wavelength range between 200 and 900 nm to determine the absorbance of MgO NPs obtained a distinctive absorption band of MgO up to 800 nm [16]. The UV–visible spectroscopy was adjusted to distinguish the age of magnesium oxide nanoparticles [17].
FTIR Analysis
The FT-IR measurement was carried out for identifying the possible chemical responsible for the reduction and capping of MgO synthesized. The FT-IR spectrum of magnesium oxide nanoparticles was analyzed at the wavenumber range of 400–4000 cm−1. The spectra show bands at 3450, 2922, 1639, 1383, 1250, 810, and 674 cm−1 functional group such as alcohol, phenol, alkaline, and ketone. The peaks observed at 674 cm−1 indicate the presence of magnesium oxide shown on Fig. 1B. FT-IR spectrum of magnesium oxide nanoparticles was analyzed at the wavenumber range of 400–4000 cm−1. The FT-IR spectrum of magnesium oxide nanoparticles shows bands at 3450, 2922, 1639, 1383, 1250, 810, and 674 cm−1 correspond to functional groups such as alcohol, phenol, alkaline, and ketone. The peaks observed at 674 cm−1 indicate the presence of magnesium oxide [18]. reported that MgO NPs using the FTIR spectroscopy and found the vibration mode in the range 487–677 cm−1 wavenumber, indicating the Mg–O–Mg bonds [19] reported that the peaks observed below 850 cm−1 confirmed the magnesium oxide. The MgO NPs using the FTIR and found the peak ~ 3702 cm−1 revealing the presence of hydroxyl group and the broad band at 473 cm.−1 indicating the Mg–O vibration [20].
Acute Toxicity Test
During the experiment, to avoid contamination, dead fish were immediately removed after 24 h of the experiment, and the concentration of 3 mg/L with no mortality in mrigal, control and 24 mg/L of MgO nanoparticles caused 100% mortality in 96 h of LC50 at the 95% confidence level as 4.2321 mg/L [21]. reported the MgO nanoparticles to have a 95% mortality in 12 and 24 mg/L, and 3 mg/L no mortality. The 96-h LC50 for ZnO nanoparticles in zebrafish was 4.92 mg/L, which is related to the physical and chemical characteristics of ZnO nanoparticles, and they were reported to cause toxic effects. The dose and time-dependent increase in fish mortality in lethal concentrations of AgNPs and AgNO3 led to the highest mortality rates at 48 h and 0.5 mg L1 (in both forms), while the lowest mortality rate was observed in control [22]. AgNPs synthesized chemically showed dose-dependent activity and mortality increased with concentrations of AgNPs. At concentrations of 500 and 1000 mgkg−1, a 100% mortality rate was observed, while no mortality was observed at concentrations of 25 and 50 mgkg−1. In the tests, 50% of the fish died at 100 mgkg−1 concentration, while there was no mortality observed in the control fish [23, 24] also reported that no mortality in common carp was caused by ZnO nanoparticles at a concentration of 1 mg/L, whereas the concentration of 16 mg/L caused 100% mortality, resulting in a 96-h LC50 of 4.897 mg/L at 95% certainty.
Hematological Parameters
Hematological parameters of mrigal are presented in Table 1. RBC and Hb counts are decreased on the 7th and 14th days of exposure. Hct counts were increased in T1 and T2 on the 7th day, and the T3 decreased compared to the control. In this study, WBC count gradually increased in control and significantly decreased on the 14th day of control, and exposure is (P < 0.05) shown in Table 1. MCH, MCV, and MCHC also increased on both the 7th and 14th days (Fig. 1C). At the highest dose (1000 mg/kg), red blood cells, platelets, and HCT were significantly increased, as well as a marginally significant increase in white blood cells. It might be because of NP interference with the process of erythropoiesis that RBC, HCT, and HB were reduced. The increase in WBC count indicated that the body’s innate defense system was active [25]. Different concentrations of nanoparticles on the hematological profile of C. gariepinus. indicate no significant differences (P < 0.05) in all the parameters tested when compared with control values [26, 27]. The toxicity assessment of standard hematological parameters, including determination of hematocrit (HCT), red blood cells (RBC), platelet count (PLT), white blood cells (WBC), hemoglobin (Hb) levels, mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and mean corpuscular volume (MCV), was observed that white blood cells significantly increased in MgO nanoparticle doses of 250 and 500 µg/mL, respectively (P < 0.05), compared to the control. Hectorite and lymphocyte counts in subjects exposed to 0.0026 mg/L decreased significantly (P < 0.05) after 10, 20, and 30 days. In comparison to control fish, the Caspian brown trout showed significant reductions in RBC and Hb and increases in neutrophil and leukocyte counts after 20 and 30 days of exposure [21].
Histopathology of Gill, Muscle, and Liver
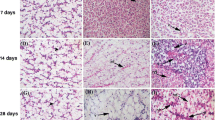
Histopathology analysis of the gill, muscle, and liver of Cirrhinus mrigala is presented in Fig. 1D. The lamellae, secondary lamellae, epithelial cells, water cannel, and numerous cells were damaged in the 3 and 6 mg/L of the 14th day of exposure, as shown in Fig. 1B. In 12 and 24 mg/L of exposure, both primary and secondary gills were damaged on both the 7th and 14th days of exposure. As a result of the 10% exposure group, there was cellular swelling and blood congestion; as a result of the 20% exposure group, there was hyperplasia of epithelial cells, lamellar fusion, aneurism, lamellar disorganization, and curling of the lamellae [28] observed that the primary lamella of control fish (PL) and secondary lamella of exposed fish (SL) had normal structure. However, gill epithelium hyperplasia (GE), a fusion of secondary lamellae (LF), dilated marginal channels (MC), epithelial lifting (EL), and epithelial rupture were observed in Ni NP-exposed fish [15]. Concentrations of 12 and 24 mg/L cause severe liver damage and changes in the liver bile ducts. Magnesium dioxide nanoparticles caused inflammation of the liver tissues and exposed apoptotic cells. Histological changes in the liver include proliferating bile ductules and some congestion in the liver sinusoids [29] reported that the inflammation of liver tissues was induced by magnesium oxide nanoparticles. Compared to the control, fish exposed to Ni NPs showed a few alterations, including the presence of irregularly shaped nuclei, nuclear hypertrophy (NH), cytoplasmic vacuolation (CV), nuclear degeneration (ND), and pyknotic nuclei (PN) [15, 29]. It is an abundance of bile ductules that are the cause of liver histopathology changes. In some liver sinusoids, congestion was observed, which indicated that MgO nanoparticles caused inflammation. A study of fish exposed to ZnO nanoparticles over 21 days revealed varying degrees of histopathological alteration, including hypertrophied hepatocytes in the liver [24].
Vertebrate musculature is present in the lateral muscles of the fishes. In control, 3 mg/L, and 6 mg/L MgO NP–expose mrigal, muscle fibers were arranged normally, and the myofibrils were arranged normally during the exposure of the 7th day and 14th day. In 12 and 14 mg/L MgO NP–exposed mrigal, muscle fibers arrange up normally, and the myofibrils also thicken and break the connective tissues [30] reported the low Ag NP levels in edible muscle tissue to indicate that Ag ended up in the body even after the recovery period [31] reported the MgO nanoparticle toxicity and histological analysis of liver and kidney tissue. Cells treated with MgO nanoparticles showed no signs of degeneration after being treated at different doses of exposure [27, 32]. In Ni NPs exposed to O. mossambicus, there was degeneration in muscle bundles (DM), focal areas of necrosis (NC), vacuolar degeneration in muscle bundles (VD), and edema between muscle bundles (ED) [15].
Conclusion
The concentration and exposure period of MgO nanoparticles and the changes were considered the biomarker of the toxicity induced. MgO nanoparticles to Cirrhinus mrigala were determined at 96 h LC50, and the hematological changes of WBC, RBC, Hb, HCT, Platelets, MCV, MCH, MCHC, and histopathological changes in the gills, liver, and muscle of Cirrhinus mrigala and the toxicity is based upon the size and the quantity of MgO NPs exposure in treatment.
Data Availability
Data availability upon request.
Abbreviations
- CBC:
-
Complete blood count
- Hb:
-
Hemoglobin
- RBC:
-
Red blood cells
- Hct:
-
Hematocrit
- WBC:
-
White blood cells
- MCH:
-
Mean corpuscular hemoglobin
- MCHC:
-
Mean corpuscular hemoglobin concentration
- MCV:
-
Mean corpuscular volume
- PL:
-
Primary lamella
- SL:
-
Secondary lamella of exposed fish (SL)
- GE:
-
Gill epithelium hyperplasia
- LF:
-
A fusion of secondary lamellae
- Mc:
-
Dilated marginal channels
- EL:
-
Epithelial lifting (EL)
- Er:
-
Epithelial rupture
- NH:
-
Nuclear hypertrophy
- Cv:
-
Cytoplasmic vacuolation
- ND:
-
Nuclear degeneration
- PN:
-
Pyknotic nuclei
- DM:
-
Degeneration in muscle bundles
- NC:
-
Focal areas of necrosis
- VD:
-
Vacuolar degeneration
- ED:
-
Epithelial degeneration
References
Egbuna C et al (2021) Toxicity of nanoparticles in biomedical application: nanotoxicology. J Toxicol 2021. https://doi.org/10.1155/2021/9954443
Saman S, Moradhaseli S, Shokouhian A, Ghorbani M (2013) Histopathological effects of ZnO nanoparticles on liver and heart tissues in Wistar rats. Adv Biores 4(2):83–88
Kumar V, Sharma N, Maitra SS (2017) In vitro and in vivo toxicity assessment of nanoparticles. Int Nano Lett 7(4):243–256. https://doi.org/10.1007/s40089-017-0221-3
Rajkumar KS, Kanipandian N, Thirumurugan R (2016) Toxicity assessment on haemotology, biochemical and histopathological alterations of silver nanoparticles-exposed freshwater fish Labeo rohita. Appl Nanosci (Switzerland) 6(1):19–29. https://doi.org/10.1007/s13204-015-0417-7
Pitkethly MJ (2004) Nanomaterials
Albrecht R et al (2006) The Tim21 binding domain connects the preprotein translocases of both mitochondrial membranes. EMBO Rep 7(12):1233–1238. https://doi.org/10.1038/sj.embor.7400828
Mangalampalli B, Dumala N, Grover P (2019) Toxicity assessment of magnesium oxide nano and microparticles on cancer and non-cancer cell lines. Nucleus (India) 62(3):227–241. https://doi.org/10.1007/s13237-019-00298-9
Ostaszewska T, Chojnacki M, Kamaszewski M, Sawosz-Chwalibóg E (2016) Histopathological effects of silver and copper nanoparticles on the epidermis, gills, and liver of Siberian sturgeon. Environ Sci Pollut Res 23(2):1621–1633. https://doi.org/10.1007/s11356-015-5391-9
Ajdari M, Ghahnavieh MZ (2014) Histopathology effects of nickel nanoparticles on lungs, liver, and spleen tissues in male mice. Int Nano Lett 4(3). https://doi.org/10.1007/s40089-014-0113-8
Suganthi KS, Manikandan S, Anusha N, Rajan KS (2015) Cerium oxide–ethylene glycol nanofluids with improved transport properties: preparation and elucidation of mechanism. J Taiwan Inst Chem Eng 49:183-191.Adel
OECD (2019) Test No. 203: Fish, acute toxicity test. 203:24. https://doi.org/10.1787/9789264069961-en
Stevens W (n.d.) Network Working Group
Lee RE, Costanzo JP (1998) Biological ice nucleation and ice distribution in cold-hardy ectothermic animals. Annu Rev Physiol 60:55–72. https://doi.org/10.1146/annurev.physiol.60.1.55
Ochei JO, Kolhatkar AA (n.d.) Medical laboratory science, theory and practice
Jayaseelan C et al (2014) Effect of sub-acute exposure to nickel nanoparticles on oxidative stress and histopathological changes in Mozambique tilapia, Oreochromis mossambicus. Ecotoxicol Environ Saf 107:220–228. https://doi.org/10.1016/j.ecoenv.2014.06.012
Almontasser A, Parveen A, Azam A (2019) “Synthesis, characterization and antibacterial activity of magnesium oxide (MgO) nanoparticles.” IOP Conf Ser: Mater Sci Eng, IOP Publishing Ltd. https://doi.org/10.1088/1757-899X/577/1/012051
Patil RB, Chougale AD (2021) “Analytical methods for the identification and characterization of silver nanoparticles: a brief review”. Mater Today: Proceed, Elsevier Ltd, 5520–5532. https://doi.org/10.1016/j.matpr.2021.03.384
Balamurugan S, Ashna L, Parthiban P (2014) Synthesis of nanocrystalline MgO particles by combustion followed by annealing method using hexamine as a fuel. J Nanotechnol 2014. https://doi.org/10.1155/2014/841803
Imani MM, Safaei M (2019) Optimized synthesis of magnesium oxide nanoparticles as bactericidal agents. J Nanotechnol 2019. https://doi.org/10.1155/2019/6063832.
Selvam NCS, Kumar RT, Kennedy LJ, Vijaya JJ (2011) Comparative study of microwave and conventional methods for the preparation and optical properties of novel MgO-micro and nano-structures. J Alloys Compd 509(41):9809–9815. https://doi.org/10.1016/j.jallcom.2011.08.032
Adel M, Oliveri Conti G, Dadar M, Mahjoub M, Copat C, Ferrante M (2016) Heavy metal concentrations in edible muscle of whitecheek shark, Carcharhinus dussumieri (elasmobranchii, chondrichthyes) from the Persian Gulf: A food safety issue. Food Chem Toxicol 97:135–140. https://doi.org/10.1016/j.fct.2016.09.002
Khosravi-Katuli K et al (2018) Effects of ZnO nanoparticles in the Caspian roach (Rutilus rutilus caspicus). Sci Total Environ 626:30–41. https://doi.org/10.1016/j.scitotenv.2018.01.085
Rajkumar M, Pandey PK, Aravind R, Vennila A, Bharti V, Purushothaman CS (2016) Effect of different biofloc system on water quality, biofloc composition and growth performance in Litopenaeus vannamei (Boone, 1931). Aquac Res 47(11):3432–3444. https://doi.org/10.1111/are.12792
Subashkumar S, Selvanayagam M (2014) “Online) an open access”. [Online]. http://www.cibtech.org/cjz.htm
Mangalampalli B, Dumala N, Grover P (2017) Acute oral toxicity study of magnesium oxide nanoparticles and microparticles in female albino Wistar rats. Regul Toxicol Pharmacol 90:170–184. https://doi.org/10.1016/j.yrtph.2017.09.005
Adewale OB et al (2020) Biological synthesis of gold and silver nanoparticles using leaf extracts of Crassocephalum rubens and their comparative in vitro antioxidant activities. Heliyon 6(11):e05501. https://doi.org/10.1016/j.heliyon.2020.e05501
Mazaheri N, Naghsh N, Karimi A, Salavati H (2019) In vivo toxicity investigation of magnesium oxide nanoparticles in rat for environmental and biomedical applications. Iran J Biotechnol 17(1):1–9. https://doi.org/10.21859/ijb.1543
Carpio C (2014) Zinc oxide ( Zno ) nanoparticles induced histopathological changes in the liver of freshwater. 3(544906):74–77
Mazaheri M et al (2019) Genome-wide association analysis of stalk biomass and anatomical traits in maize. BMC Plant Biol 19(1). https://doi.org/10.1186/s12870-019-1653-x
Naguib M, Mahmoud UM, Mekkawy IA, Sayed AEDH (2020) Hepatotoxic effects of silver nanoparticles on Clarias gariepinus; biochemical, histopathological, and histochemical studies. Toxicol Rep 7:133–141. https://doi.org/10.1016/j.toxrep.2020.01.002
Gelli K, Porika M, Anreddy RNR (2015) Assessment of pulmonary toxicity of MgO nanoparticles in rats. Environ Toxicol 30(3):308–314. https://doi.org/10.1002/tox.21908
Ibrahim KE, Al-Mutary MG, Bakhiet AO, Khan HA (2018) Histopathology of the liver, kidney, and spleen of mice exposed to gold nanoparticles. Molecules 23(8). https://doi.org/10.3390/molecules23081848
Acknowledgements
Our sincere gratitude goes out to the Head of the Biology Department, GRI-DTBU, for providing the necessary experimental resources. We are grateful to the GRI General Library for supplying the Ithenticate and Grammarly access.
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: study conception and design: first author Shanmugam Sudhabose; data collection: first and second authors Shanmugam Sudhabose and Balakrishnan Sooryakanth; analysis and interpretation of results: first author Shanmugam Sudhabose, second author Balakrishnan Sooryakanth, and corresponding author Muthuswami Ruby Rajan; draft manuscript preparation: first author Shanmugam Sudhabose. All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical Approval
The experimental design and use of animals for research purposes were carried out as per the existing Animal Welfare Law of India. Treatment and handling of fish for this study were performed as per the supervision and guidance given by Institutional Ethical Committee for Research on Human and Animal Subjects (IECRHAS), The Gandhigram Rural Institute (deemed to be University), Gandhigram, Dindigul, Tamilnadu, India.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sudhabose, S., Sooryakanth, B. & Rajan, M.R. Acute Toxicity, Hematological Profile, and Histopathological Effects of MgO Nanoparticles on Gills, Muscle, Liver of Mrigal, Cirrhinus mrigala. Biol Trace Elem Res 202, 736–742 (2024). https://doi.org/10.1007/s12011-023-03704-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03704-1