Abstract
The present study has been conducted to evaluate the potential threat of NiO nanoparticles (NiO NPs) on an edible fish Heteropneustes fossilis. Fishes selected for the study were exposed to four concentrations of NiO NPs (12, 24, 36 and 48 mg/l) for the period of 14 days, and various haematological, biochemical and enzymological changes in the exposed fishes were examined. Results revealed that maximum fluctuations were seen in 48-mg/l-exposed fishes when compared with the control in terms of the haematological parameters (RBC count, WBC count, Hb content, Ht% and O2 carrying capacity of blood), enzymatic activities (AST, ALP, ALT and LDH) and biochemical parameters (level of cholesterol, triglycerides, glucose, total protein, albumin, globulin, bilirubin and creatinine). However, 12 mg/l treatment to the fishes showed its least impact on aforesaid parameters. Furthermore, Ni accumulation and changes in cortisol level in the blood were also noticed in all the treated fishes. Structural changes, such as membrane and nuclear disintegration, micronucleus, deformed and vacuolated cells, and enucleation were also observed in RBCs of NiO NP–treated fishes. Conclusively, our study provides useful information and insight for the possible ecotoxicity of NiO NPs on aquatic organisms and emphasizes upon the importance of treatment of effluents containing nanoparticles before their release into the aquatic system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, there has been extensive use of nanoparticles in several fields, and their demand has also been increased many folds in various sectors like electronics, textiles, optics, cosmetics, medical devices, food packaging, water treatment technology, biosensor, catalysts and agents for environmental remediation (Aitken et al. 2006; Brody 2006; Freitas 2005; Karnik et al. 2005; Lanone and Boczkowski 2006; National Science and Technology Council 2004; Roco 2003). Nanoparticles are the nanosize materials with one dimension of approximately 1–100 nm or less in size. Their small size and high surface/charge ratio make them highly reactive with enhanced properties that attract the special attention of many industries. However, these properties of nanoparticles have also become one of the reasons for their toxicity to the environment and turned them into one of the major pollutants. They may leak and enter into the water bodies during their different stages of production, storage, transportation, consumption, disposal or reproduction and may cause adverse effects on the environment as well as human health.
Nowadays, nickel oxide nanoparticles (NiO NPs) have drawn particular interest because of their widespread application in various fields such as battery electrodes, printing inks, electrochromic films, catalyst, sensor magnetic materials and diesel-fuel additives (Salimi et al. 2007; Rao and Sunandana 2008; Schrand et al. 2010). Despite of increasingly large application of NiO NPs in different sectors, there is limited information available regarding their potential toxic impact on living organisms especially upon aquatic animals. Release of NiO NPs into the aquatic environment may increase their exposure to humans and other terrestrial animals as they consume the water as well as aquatic animals according to their needs and thereby come into the contact of NiO NPs. Therefore, it is a matter of urgent need to assess the potential health hazards caused by these nanoparticles so that their ecological risks can be monitored.
There are very few studies which have reported different physiological anomalies in fishes caused by various types of nanoparticles like reproductive toxicity (Wang et al. 2011) and developmental toxicity in Danio rerio (Cui et al. 2016; George et al. 2014), oxidative stress mediated apoptosis in carp larva (Cyprinus carpio) (Naeemi et al. 2020) and respiratory stress in Eurasian perch (Perca fluviatilis) (Bilberg et al. 2010). In addition, nanoparticles also cause histological changes (Khan et al. 2017c) and alteration in genetic material of Labeo rohita (Khan et al. 2017a). They are also known to exhibit haematological alteration in Labeo rohita (Remya et al. 2015; Rajkumar et al. 2016; Zutshi et al. 2010), Tilapia mossambica (Sheeba and Noorjahan 2018) and Oncorhynchus mykiss (Imani et al. 2015).
Other studies have reported NiO NP toxicity in bacteria and microalgae (Baek and An 2011; Gong et al. 2011), acute toxicity in Zebra fish (Kovrižnych et al. 2013) and their accumulation in Gracilaria lemaneiformis (Han et al. 2012). Horie et al. (2011) also reported oxidative stress related lung injury due to NiO NPs. Similarly, Adeel et al. (2019) reported NiO NPs induced ultrastructural changes in earthworms such as abnormalities in epithelium layer, microvilli and mitochondria as well as adverse effects on the gut barrier. NiO NPs also induced oxidative stress leading to damage in DNA of earth worm. Biochemical as well as histological changes have also been observed in Wistar rats due to NiO NP toxicity (Dumala et al. 2019).
Since there are very limited studies which show impact of NiO NPs on aquatic organisms, it is important to have detailed knowledge of their toxic effect on these organisms, especially upon fishes.
Recently, fish haematology has been given importance in assessing the physiological response of fishes to the nanoparticles (Kaviani et al. 2020; Canli et al. 2017 & 2018) because any alteration in the physiology of fish caused by toxicants is finally getting reflected in the haematological parameters.
The intensively studied haematological markers are red blood cell (RBC) count, haemoglobin (Hb), haematocrit (Ht %) and white blood cell (WBC) count. The red blood cell is the important component of blood which provides oxygen to the tissues of entire body through circulatory system. Likewise, haemoglobin is the component of blood which delivers desire amount of oxygen to the body under a variety of circumstances (Voet and Voet 1990). It has been reported that the determination of haemoglobin concentration is an important indicator of anaemic conditions in fish (Blaxhall and Daisley 1973). The haematocrit refers to the measurement of plasma and corpuscles and is used in determining the oxygen carrying capacity of blood (Larsson et al. 1985). It is also reported that haematocrit is a good indicator of health status of fish under stress condition (Munkittrick and Leatherland 1983). The calculated haematological indices like mean corpuscular volume (MCV), mean corpuscular Hb (MCH) and mean corpuscular Hb concentration (MCHC) are the relevant indicators in diagnosis the anaemia in most of the animals (Coles 1986).
The white blood cells or leucocytes are the important part of immune system which is associated with the protection of the body against the stress or pathological condition (Vaseem and Banerjee 2012; Fazio 2019; Shah et al. 2020).
The serum biochemical parameters are also often used as important and powerful tool to elucidate the toxic effects of metals and other toxicants (Jorgensen 2010; Wood et al. 2012a, 2012b). Serum parameters supply actual data for metabolism as well as the health status of vertebrates, including fish. In addition, it also provides the meaningful data to determine the damages in organ, tissue and cell concerns (Bernet et al. 2000; Grosell et al. 2004; Oner et al. 2008; Atli et al. 2015; Canli and Canli 2015; Canli et al. 2017). Therefore, it can also be very useful in determining the potential impact of NiO NPs on fish health.
The cholesterol, triglyceride, glucose, protein, albumin and globulin are the important serum biomarkers to examine the health status of fish (Atli et al. 2015; Canli and Canli 2015). Likewise, creatinine, bilirubin and cortisol are also relevant stress biomarkers that are used to indicate health condition of fish (Canli et al. 2018).
Damage or malfunctioning in liver can be examined by measuring the activities of some enzymes like aspartate amino transferase (AST), alanine amino transferase (ALT) and alkaline phosphatase (ALP) as they are produced in the liver and leaked out into the blood in stress condition. Lactate dehydrogenase (LDH) is also a good stress biomarker and can be used in assessing NiO NP toxicity to the fish.
In the present study, HeteKovriznychropneustes fossilis, an economically important, air breathing catfish, has been used to evaluate NiO NP impact. This fish is very hardy, capable of adapting to various habitats and can survive in unfavourable condition. Therefore, it may be a good representative of aquatic animals to evaluate the impacts of polluting toxicants.
Keeping all these points in consideration, it was decided to determine the potential toxic impact of NiO NPs on the H. fossilis by analysing different haematological, enzymological and biochemical parameters.
Materials and methods
Characterization of NiO NPs
NiO NPs were purchased from St. Louis, MO, USA, with an average particle size <50 nm and 99.8% trace metal basis (CAS. 1313-99; MW 74.69 g/mol; d 6.67 g/ml at 25 °C). The morphology and individual diameter of NiO NPs were characterized by transmission electron microscopy (TEM) at an accelerating voltage of 200 kV. For TEM analysis, a drop of NiO NP stock solution was placed onto a mesh copper grid treated with a carbon layer and dried at room temperature (27 °C). The electron micrographs were obtained in a JEOL (JEM-2100) microscope by means of the software Scandium (Olympus Soft Imaging Solutions GmbH). The surface topography of NiO NP aggregates were observed using scanning electron microscopy (SEM) operated at 15 kV. The elemental compositions of NiO NPs were analysed by energy-dispersive X-ray (EDX).
Experimental setup and acclimatization of fishes
Experimental fish, H. fossilis, belonging to the Heteropneustidae was obtained from local fish market, Aligarh, India. All fish were transported to the laboratory in plastic containers and were maintained (temperature 25 ± 2 °C) for 4 weeks in plastic tubs with 50 l dechlorinated tap water (pH 7.1 ± 0.3; salinity 0.3 ± 0.07 pg/l; dissolve oxygen 6.7 ± 0.05 mg/l; total alkalinity 20.0 ± 6.0 mg/l and total hardness 15 ± 0.05 mg/l). Photoperiod was 12 h light and 12 h dark. During acclimatization, the fish were fed ad libitum daily with 1-day interval. The water was changed daily after every 24 h, and fish showing any unusual activities were excluded.
Preparation of NiO NP suspension
A series of exposure suspensions (12, 24, 36 and 48 mg/l) was prepared by weighing dry NiO NP powder into the ultrapure water. The suspending solutions containing NiO NPs were then dispersed by a digital ultrasonic cleaner Cd-4820 (ICS-CITIZEN) (ultrasonic power 100 W, frequency 50 Hz, continuous/pulse 180 s) for 30 min. The prepared suspending solutions were used to expose the fish.
Experimental design
A series of ten fishes of approximately same weight and length (weighing 25 ± 3 g and with a length of 18 ± 2.5 cm) was randomly allocated in aquaria in triplicate groups containing 20 l dechlorinated tap water. Fishes were then exposed to different concentrations of NiO NPs, i.e. 12 mg/l, 24 mg/l, 36 mg/l and 48 mg/l separately in triplicate for a period of 14 days. Water was replaced daily. Parallel series of control fishes (ten fishes in each series) were exposed to tap water without NiO NPs under similar laboratory conditions. The experimental conditions were kept like those of acclimatization period, and water was examined every day for temperature, pH and dissolve oxygen. Food was not given to the fishes 24 h prior to the experiment to minimise the absorption of NPs on food or faecal matters. However, they were fed during experiment once in every 3 days to avoid any negative effect related to hunger. Feeding was done just 1 h prior to water change. In each feeding, food was consumed rapidly and no leftover food was obtained before water change. After 14 days of exposure, all randomly selected fishes from control as well as exposed groups were sacrificed for haematological as well as biochemical analyses.
Blood and serum collection
Blood (1–1.5 ml) from control as well as exposed fishes was collected from the caudal vein using plastic disposable syringe fitted with a 23-gauge needle and collected in separate potassium EDTA anticoagulant tubes and immediately kept in ice box. For serum collection, aliquots of blood in Eppendorf vials having no anticoagulant were kept for 5 h at 4 °C. After clotting of blood, samples were centrifuged at 2000 rpm for 10 min, and serum was collected and stored in −20 °C for biochemical analyses.
Bioaccumulation of NiO NPs
Blood samples of exposed as well as control fishes were subjected to NiO NP accumulation. Samples were digested with diacid HClO4 and HNO3 in 1:2 ratio at 100 °C until all the materials were dissolved. After cooling of digested samples, distilled water was added into them and then they were filtered. The concentration of NiO NPs in serum was analysed using flame atomic absorption spectrophotometer (Model 2380, Perkin Elmer, Inc., Norwalk, CT, USA) and expressed as milligram per litre of blood.
Haematological studies
Blood samples from the control as well as exposed groups were analysed for the haematological studies. RBC count and WBC count were analysed with Neubauer’s improved haemocytometer using Hayem’s and Tuerk’s solution as diluting fluid, respectively (Samuel 1986). Haemoglobin and haematocrit content were measured by Sahli’s haemoglobinometer and Wintrobe’s method, respectively. Haemoglobin content was multiplied by 1.25 oxygen combining power of Hb/g to calculate the oxygen carrying capacity of blood (Johansen 1970). The calculated indices like MCV, MCH and MCHC were calculated by the standard formulae of Dacie and Lewis (1991).
For determination of the differential leucocytes, blood smears were made on glass slides and then air dried. After air dried, slides were fixed in methanol and stained with Giemsa stain. The percentage of different types of leucocytes in an individual fish was found out by counting 100 cells from the best three of its smears, and a mean of three was taken. The cells were counted from the same areas on each slide, and the extreme edges were avoided. The cells observed were categorized as lymphocytes, monocytes and three types of granulocytes (neutrophils, eosinophils and basophils) (Saunders 1967).
Structural changes in erythrocytes
Blood smears were made on glass slides and then air dried. After that, slides were fixed using methanol and stained with Giemsa stain. Subsequently, erythrocytes were observed under microscope to see their structural changes following the method of Ghiasi et al. (2010).
Biochemical analysis
Serum samples were analysed for biochemical parameters using enzymatic-colorimetric methods. The cholesterol, triglyceride and glucose level in serum were measured according to the method suggested by Trinder (1969). Serum cortisol level was measured using commercial ELISA kit (Diametra: DK0001-96T) based on the manufacturer instructions. The total protein in serum was estimated by Lowry et al. (1951) while serum albumin content was measured according to the method described by Corcoran and Durnan (1977). Globulin concentration was determined as the difference between total protein and albumin following the method suggested by Bayunova et al. (2002). For the determination of the albumin:globulin ratio, the value of albumin was divided by the value of globulin. Serum creatinine level was estimated by the method of Bowers and Edward (1980). Bilirubin level was determined according to the method of Jendrassik and Grof (1938).
AST and ALT activities in serum were analysed according to the method suggested by Reitman and Frankel (1957) while ALP activity was assessed according to Kind and King’s (1954) method. Serum LDH activity was analysed following the method described by Weishaar et al. (1975).
Statistical analysis
Kolmogorov-Smirnov test was done to examine the normality of data. One-way analysis of variance (ANOVA) followed by Duncan test was used to determine significant difference among treated groups at a probability level of P < 0.05. All the obtained data are given as mean ± standard deviation (SD). Statistical analysis was done using SPSS Software version 16.0.
Results
Characterization of nickel(II) oxide nanoparticles
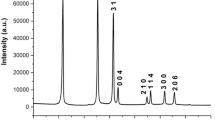
Figure 1 A shows transmission electron microscopy images of NiO NPs which revealed their dimension. The dimension of NiO NPs was found to be less than 50 nm. The surface topography and morphology of NiO NPs have been characterized using scanning electron microscopy and shown in Fig. 1B. The morphology of nanoparticles was found to be amorphous. The energy-dispersive X-ray was done (Fig. 2) to detect the elemental composition of NiO NPs, and it was obtained that atomic percentage of oxygen and nickel was 73.74 and 26.26, respectively. The weight percentage of oxygen was 43.35 and nickel was 56.65, and in total, it was 100% of NiO NPs.
Bioaccumulation of Ni in the blood of NiO NP–exposed fishes
The concentration (mg/l) of nickel(II) in the blood of fishes exposed to different concentrations of NiO NPs is presented in Fig. 3. The accumulation of Ni in control group was below detectable limit. However, the accumulation of Ni was found to be significantly (P < 0.05) increased in all the treated groups in concentration-dependent manner. The pattern of Ni accumulation (mg/l) in serum was as follows: control (bdl) <12 mg/l (0.128 ± 0.021) <24 mg/l (0.643 ± 0.023) = 36 mg/l (0.702 ± 0.037) <48 mg/l (1.333 ± 0.057).
Effect of different concentrations of NiO NPs on haematological parameters of fishes
RBC count, WBC count, haemoglobin and haematocrit
Effect of different concentrations (12, 24, 36 and 48 mg/l) of NiO NPs on the haematological parameters of Heteropneustes fossilis is shown in Fig. 4. Figure 4 shows that a number of RBCs (million/mm3) decreased significantly (P < 0.05) in the fishes exposed to all the concentrations of NiO NPs (3.06 ± 0.20 (12 mg/l), 2.33 ± 0.24 (24 mg/l), 3.20 ± 0.26 (36 mg/l) and 2.84 ± 0.11 (48 mg/l)) when compared with the control group (3.75 ± 0.51). The lowest value of RBCs was recorded in the treatment of 24 mg/l of NiO NPs. No significant difference in the number of RBCs (P < 0.05) was found between 12- and 36-mg/l-exposed groups.
Likewise, the total number of WBCs (thousand/mm3) also decreased significantly (P < 0.05) in all the treated fishes when compared with the control group (36.98 ± 1.42). Their values (thousand/mm3) in 12-mg/l-, 24-mg/l-, 36-mg/l- and 48-mg/l-treated groups were 20.83 ± 0.20, 24.8 ± 1.44, 31.23 ± 0.54 and 31.45 ± 0.79, respectively. The lowest number of WBCs was found in 12-mg/l-treated group. An increasing trend in WBC count was noticed in concentration-dependent manner from 12 to 48 mg/l treatment. However, no significant difference (P < 0.05) was observed between 36- and 48-mg/l-treated groups.
Exposure of H. fossilis to NiO NPs also caused significant alteration (P < 0.05) in Hb content (g/dl) (8.57 ± 0.60 (control), 7.6 ± 0.17 (12 mg/l), 9.5 ± 0.5 (24 mg/l), 9.17 ± 0.76 (36 mg/l) and 6.7 ± 0.17 (48 mg/l)) and Ht (%) (26.13 ± 1.29 (control), 21.93 ± 1.45 (12 mg/l), 27.7 ± 0.44 (24 mg/l), 28.4 ± 1.82 (36 mg/l) and 19.25 ± 1.09 (48 mg/l)). Significant decrease in Hb and Ht values was observed in treatments of 12 mg/l and 48 mg/l when compared with their respective control groups. However, no significant alterations (P < 0.05) in these parameters were recorded between 24 and 36 mg/l treatments.
Differential leucocyte count (DLC) like neutrophils, eosinophils, basophils, lymphocytes and monocytes also showed alterations in their mean percentage values after exposure to NiO NPs (Fig. 5). The mean percentage values of neutrophils, eosinophils and basophils were found to be significantly higher in all the treated groups than in the control (Fig. 5). In case of basophils, they were not detected in control as well as 12 mg/l of treated fishes (Fig 5). However, the mean percentage of lymphocytes was significantly lower in all the treated groups (except 48-mg/l-treated group) than in the control group (Fig. 5). A significantly lower mean percentage value of monocytes was also observed in all the treated groups when compared with the control group (Fig. 5).
MCV, MCH, MCHC and OCC (calculated indices)
The effect of NiO NPs on the calculated indices is shown in Table 1. From Table 1, it can clearly be seen that values of MCV and MCH have shown significant increase (P < 0.05) in 24-mg/l-treated group when compared with the control. A significant increase in MCV was also observed in 36-mg/l-treated group. However, no significant variations (P < 0.05) in these parameters were noticed in other treated groups, i.e. 12 mg/l and 48 mg/l. The different concentrations of NiO NPs did not show any remarkable effects on MCHC. The significant decrease in oxygen carrying capacity was observed in 12-mg/l- and 48-mg/l-treated groups when compared with the control.
Effect of NiO NPs on the structure of erythrocytes of NiO NP–exposed fishes
The results of the alteration in the morphology of erythrocytes in H. fossilis after exposure to NiO NPs for a period of 14 days are shown in Fig. 6. Alteration in the structure of erythrocytes, due to NiO NP toxicity, could clearly be seen in all the treated groups while intact and healthy cells were viewed in the control group. Initiation of membrane and disintegration of erythrocytes as well as dividing nucleus were observed in 12 mg/l treatment while more membrane disintegrated erythrocytes were found in 24 mg/l treatment. Nuclear disintegration and deformed erythrocytes were also observed in 24-mg/l-treated group. Many vacuolated cells as well as enucleation were noticed in 36-mg/l-treated group while more micronucleus and deformed erythrocytes were viewed in 48-mg/l-treated fishes.
Effect of different concentrations of NiO NPs on biochemical parameters of fish serum
Cholesterol, triglyceride, glucose and cortisol levels
Alteration in the concentration of different serum biochemical parameters due to exposure of different concentrations of NiO nanoparticles is shown in Table 2. In case of serum cholesterol level (mg/dl), some fluctuations were observed. It showed significantly higher values (P < 0.05) in 24 mg/l and 36 mg/l of NiO NPs while it decreased significantly in 12 mg/l treatment when compared with the control. However, treatment with 48 mg/l did not show any significant variations (P < 0.05) in its level.
Triglyceride level (mg/dl) also showed significant variation by decreasing in all the treated groups when compared with the control. However, no significant changes (P < 0.05) were observed in its level among 12-mg/l-, 24-mg/l- and 36-mg/l-treated groups.
Furthermore, the serum analysis showed that different concentrations of NiO NPs caused a significant increase (P < 0.05) in the glucose level (mg/dl) in all the exposed groups compared with the control.
Cortisol (ng/ml) level also showed significant increase in all the treated groups when compared with the control. While no significant difference was observed in its level between 24-mg/l- and 36-mg/l-treated groups.
Total protein, albumin, globulin and albumin: globulin
The serum analysis revealed that all the treated groups showed significant decrease (P < 0.05) in the total protein concentration (mg/dl) when compared with the control with the highest decrease in 48 mg/l treatment. Likewise, serum albumin (mg/dl) and globulin (mg/dl) levels also decreased significantly (P < 0.05) in all the treated groups (except globulin level in 12 mg/l treatment) compared with the control while total serum protein, albumin and globulin levels did not show any significant difference (P < 0.05) between 24 and 36 mg/l treatments. Concerning to albumin globulin ratio, there was a significant decrease (P < 0.05) in their ratio in all the treated groups when compared with the control. However, no significant difference (P < 0.05) was recorded in their ratio between 24 and 36 mg/l treatments.
Creatinine and bilirubin
Serum creatinine level (mg/dl) showed significant increase (P < 0.05) in all the treated groups except in 12 mg/l treatment when compared with the control group. Interestingly, it showed significantly increased (P < 0.05) level in concentration-dependent manner after 12 mg/l concentration treatment.
Regarding bilirubin level (mg/dl), it also showed significant increase (P < 0.05) in all the treated groups (except in 12-mg/l-treated group) when compared with the control group. After 12 mg/l treatment, the bilirubin level was also found to be significantly (P < 0.05) increased in concentration-dependent manner. Simultaneously, the highest level (P < 0.05) of bilirubin was observed in the 48-mg/l-treated group.
Effect of NiO NPs on the serum enzymatic activities (ALT, ALP, AST and LDH) of NiO NP–exposed fishes
Table 3 represents the effects of different concentrations of NiO NPs on liver function in terms of different enzymatic activities in the serum of H. fossilis after 14 days of NiO NP exposure. Interestingly, the highest activities of ALT (IU/l), ALP (KA/100 ml) and AST (IU/l) were observed in 48-mg/l-treated groups. ALT activity did not show any significant difference (P < 0.05) in the treated groups (except in 48 mg/l treatment) from the control. In case of ALP, all the treated groups showed significantly lower (P < 0.05) activity (except in 48-mg/l-treated group) than the control group. Significantly (P < 0.05) higher activity of AST was observed in 24-mg/l-treated group while it showed decrease in 12-mg/l- and 36-mg/l-treated groups when compared with the control group. In case of serum lactate dehydrogenase activity (LDH), it showed a general trend of significant (P < 0.05) increase in concentration-dependent manner in all the treated groups when compared with the control fishes.
Discussion
Nowadays, pollution has become one of the major reasons for most of the environmental problems and reflects a major threat to the global ecosystem and welfare of billions of people (Brand 2001). Aquatic organisms are also greatly affected by the pollution as aquatic system is one of the major receivers of variety of pollutants and wastes. Currently, fishes are being increasingly used in the monitoring of water pollution (Luoma and Rainbow 2008; Monteiro et al. 2010). They have many incredible qualities such as greater sensitivity towards changes in the quality of aquatic environment and ability to metabolize, concentrate and accumulate different types of toxicants which make them a good bioindicator of aquatic ecosystem. In addition, their physiological, biochemical or histological responses are also used as biomarker to measure water pollution and toxic effect of different toxicants (Ogunola 2017).
Nanoparticles, the new emerged pollutant, may cause toxicity to the organism by binding with specific receptors localized on the cell surface or membrane of cell organelles that lead to the cellular changes at structural as well as functional levels resulting impairment of normal functioning of cell.
Blood parameters are regarded as patho-physiological indicator of the whole body. Therefore, they are being used as very useful tool in detecting the structural and functional status of fishes exposed to different toxicants (Adhikari and Sarkar 2004; Maheswaran et al. 2008). Currently, they are being used as an important tool in toxicological as well as pharmacological research for monitoring and evaluating the health status of organisms (Lasheen et al. 2012). Hence, in the present study, we used different haematological and biochemical parameters of blood to assess the toxic effect of NiO NPs on fish health.
Ni accumulation in blood
Significant accumulation of Ni in the blood elucidates toxic effect of NiO NPs on fish (Fig. 3). Ni accumulation might have caused production of ROS, i.e. oxidative stress, which resulted in damaging alteration in various vital parameters. In present study, changes in haematological and biochemical parameters and structural changes in RBCs in NiO NP–exposed fish are the results of the accumulation of Ni in blood.
RBC count, WBC count, DLC, Hb and Ht
The current study elucidated a significant decrease in RBCs number in all the treated groups (12 mg/l, 24 mg/l, 36 mg/l and 48 mg/l) of H. fossilis exposed to NiO NPs in comparison to the control while Ht and Hb did not significantly differ among control, 24 mg/l and 36 mg/l concentrations (Fig. 4). In the present study, significant decrease in RBC number in the treated groups might be due to accumulation of NiO NPs in the RBCs that has caused changes in their structure and function and resulted in deleterious effects on them (Ale et al. 2018). Accumulation of Ni nanoparticles in the blood and structural deformities are also evident in present study and are shown in Figs. 2 and 6, respectively.
The observed results of haemoglobin and haematocrit content might be because of changes in numbers and structure of RBCs (Fig. 4) due to NiO NP accumulation (Fig. 2). The observed increase in haemoglobin and haematocrit content in 24 mg/l and 36 mg/l of NiO NP–exposed fishes in comparison to the control reflects increased demand for oxygen (Fig. 4) due to NiO NP toxicity (Anand et al. 2015). From the correlation matrix (Fig. 7), it is clearly seen that both Ht and Hb have shown a strong positive correlation with OCC (Ht vs OCC (r) = 0.954; Hb vs OCC (r) = 1) that led to the increased demand for oxygen (Anand et al. 2015) in stress condition caused by NiO nanoparticles.
In previous studies, it has been reported that Labeo rohita treated with the lethal concentration (LC50) of AgNPs showed a significant decrease in RBC number, Hb and Ht values (Rajkumar et al. 2016). Hajirezaee et al. (2019) also reported a significant decrease in RBCs number with no significant changes in Hb or Ht values in common carp treated with TiO2 NPs.
Likewise, Shaluei et al. (2013) found significant decrease in RBCs and Ht in silver carp exposed to AgNP. A significant decrease in % Ht level in rainbow trout after 8 days of AgNP exposure was also reported by Imani et al. (2015).
Consequently, the harmful effects of NiO NPs on haematology include the disintegration of RBCs which might be due to peroxidation of fatty acids in plasma membranes by Ni nanoparticles (as nanoparticles are known to cause production of ROS) (Massarsky et al. 2014) as well as due to changes in the morphology of RBCs and the presence of micronucleus (Sayed et al. 2018).
WBCs or leucocytes can be considered as an essential part of the immune system involved in defending the body from both infectious diseases and foreign materials (Vaseem and Banerjee 2012). They can be used as a good biomarker of NiO NP toxicity to the fish. In the present study, initially, the WBC counts decreased and eventually increased in concentration-dependent manner in 14 days of NiO NP exposure. But they remained significantly lower than the control fishes (Fig. 4).
In previous studies also, a significant decrease in WBC count was observed in fishes like Labeo rohita exposed to Fe2O3 NPs (Remya et al. 2015) and Oreochromis niloticus treated with AgNP (Thummabancha et al. 2016) when compared with their respective control groups. Cheraghi et al. (2013) also noted decreased WBC count in rats administered to different concentrations of silver nanoparticles.
In the present study, decreased number of WBCs indicates decreased nonspecific immunity of fish due to NiO NP stress. Accumulation of nanoparticles in the cells might also be the reason for the disruption of cellular function (Bystrzejewska-Piotrowska et al. 2009) as significant amount of Ni is reported in the presented study. Alterations in DLC in treated groups also attributed to the detrimental impact of NiO NPs on fish health (Fig. 5).
Calculated haematological indices
Alterations in RBC, Hb and Ht might have caused changes in the calculated indices such as MCV, MCH and MCHC (Table 1). In the present study, different concentrations of NiO NPs had no significant effect on MCHC and MCH values (except in 24-mg/l-exposed fishes for MCH) while it showed a significant impact on MCV in 24 and 36 mg/l.
A previous study reported no significant difference in MCV, MCH and MCHC of Caspian trout (Salmo trutta caspius) exposed to CuO NP (Kaviani et al. 2019) which is in agreement with our findings. Our study is also in agreement with the findings of Ali Alkaladi et al. (2015) who observed no significant difference in MCV, MCH and MCHC values in Oreochromis niloticus exposed to ZnO NPs. No significant effect on MCH and MCV was also observed in AgNPs exposed common carp by Sara et al. (2020).
In current study, the increased MCV and MCH in 24-mg/l-treated group indicate macrocytic anaemia (Saravanan et al. 2011) which is also in agreement with the other similar toxicological researches in common carp exposed to diazinon (Svoboda et al. 2001) and imidan, and dichlorvos (Svobodova 1971). A strong correlation between MCV and MCH confirms macrocytic anaemia in blood cells of the treated groups caused by NiO NPs (Fig. 7).
Structural changes in erythrocytes
Nowadays, morphological studies of erythrocytes are being used as one of the most specific and sensitive indicators in toxicological research to investigate the potential impacts of different toxicants (Jindal and Kaur 2014; Alkaladi et al. 2015). To assess the potential effect of NiO NPs on the physiology of the fish, it is necessary to take into account the morphological changes occurring in the erythrocytes because deformities in the structure of erythrocytes are one of the markers of inconsistency in the physiology of respiration.
In our study, all the treated groups showed deformation in the morphology of erythrocytes when compared with control group (Fig. 6). In the control group, all the cells were intact and elliptical in shape. However, membrane disintegration of erythrocytes was observed in 12-mg/l- and 24-mg/l-exposed fishes with higher membrane damage in 24-mg/l-exposed fishes than the 12-mg/l-exposed one. The membrane disintegration could be the result of NiO NP toxicity that led to breakage in erythrocytes causing changes in the binding affinity of haemoglobin. Nuclear disintegration in 12-mg/l and 24-mg/l-exposed groups revealed improper cell division due to deleterious effects of NiO NPs on erythrocytes. Vacuolated cytoplasm and enucleation in erythrocytes were also noticed in the treatment of 36 mg/l concentration of NiO NPs. Furthermore, deformed erythrocytes and the micronucleus in many cells were also observed in the treated group of 48 mg/l concentration. The formation of micronucleus might be due to defects in spindle fibre during the segregation process of anaphase. Notably, a recent study in adult zebrafish reported peripheral erythrocytes abnormalities such as erythrocyte micronucleus, lobed, notched and blebbed nuclei and vacuolated cytoplasm after exposure to TiO2 NPs and Ag-doped TiO2NPs (Mahjoubian et al. 2021). Similarly, Vidya and Chitra (2018) demonstrated the formation of micronucleus in erythrocytes as well as genetic damage in Oreochromis mossambicus after exposure of some selected nanoparticles. Micronucleus was also found to be induced in blood cells of fish after exposure of cadmium chloride and copper sulphate in the study carried out by Cavas et al. (2005). An earlier literature of Anbumani and Mohankumar (2012) also showed higher frequency of micronucleus in Catla catla after irradiating with gamma radiation.
Cholesterol, triglyceride, glucose and cortisol levels
Cholesterol is also an important biomarker to determine the stress condition in toxicological research which is produced in response to different toxicants. In the present study, the elevated level of cholesterol was observed in most of the treated groups and was found to be significantly different from control, while only 12-mg/l-treated group showed a significant decrease in serum cholesterol level (Table 2). The increased cholesterol level after exposure of NiO NPs indicates liver dysfunction which ultimately caused the release of cholesterol into the blood. In the present study, liver dysfunction has also been observed in terms of the changes in the activities of different enzymes like AST, ALT and ALP (Table 3). The elevated levels of cholesterol have also been reported in the serum of Cirrhinus mrigala (Kumar et al. 2005), Labeo rohita (Vaseem and Banerjee 2012) and Channa punctatus (Kaur and Kaur 2006) exposed to lead, sea nodule effluent and nickel-chrome electroplating effluents, respectively.
Triglyceride level also showed variations and significantly decreased in all the treated groups of NiO NPs as compared to the control group (Table 2). This might also have occurred due to liver damage and its dysfunction. Our results are also supported by the findings of Said et al. (2019), who observed decrease in triglyceride level and liver damage in African catfish Clarias gariepinus after exposure to copper nanoparticles. The decrease level of triglyceride was also noticed in Wistar rats after ingestion of silver nanoparticles (Razavian and Masaimanesh 2015).
Glucose is one of the most sensitive parameters for assessing the stressed condition of an organism. Its high concentration in the blood indicates that the organism is in stress and using its energy reserves to curb the stress level (Vosyliene 1999; Javed and Usmani 2013). In the present study, an increment in glucose level was observed in all the treated groups when compared with the control (Table 2). The elevated glucose level might be due to the breakdown of stored glycogen or the high rate of gluconeogenesis in order to supplement the demand of additional energy during the stress condition caused by NiO NPs. A strong correlation (r = 0.988) between glucose and Ni accumulation (Fig. 7) suggests the impact of NiO NPs on glucose content.
The decreased protein level in all the exposed fish also confirms its utilization in glucose production to combat the stress. A strong negative relation of protein with glucose (r = −0.983) also indicates increase in gluconeogenesis where protein has been utilized for energy production in the form of glucose (Fig. 7).
The observed findings in the present study also showed an agreement with Abdel-Khalek et al. (2015) and Firat and Kargin (2010) who exposed Nile tilapia to Zn NPs, and different concentrations of Zn, Cd and Zn + Cd, respectively, and reported increased serum glucose level.
Cortisol is a stress hormone that is released during stress condition. It causes increase in glucose production through glycogenolysis (Zhang et al. 2015) as well as mobilization of energy reserves (Sadoul and Vijayan 2016) to combat the stress caused by toxicants in organisms. A strong correlation (r = 0.988) between cortisol and glucose also indicates that their level increased in the body to combat the stress caused by NiO NPs (Fig. 7). In the present study, serum cortisol level increased significantly in all the exposed groups (Table 2), that might be due to weakened immune responses, immunosuppression and liver malfunction (Hontela et al. 1992; Tort 2011; Shaluei et al. 2013).
A previous study also showed a significant increase in both serum cortisol and glucose level in common carp exposed to Ag NPs, which is in agreement with our findings (Sara et al. 2020). Other literatures are also in agreement with our results (Clark et al. 2018; Canli et al. 2018; Ghafari et al. 2017; Hedayati et al. 2019).
Total protein, albumin, globulin and albumin/globulin ratio
Proteins, the important building blocks of all cells and tissues, are essential for the growth and development of the body. Likewise, albumin is also an important protein comprising more than half of the total serum protein and involved in the transport of vitamins, hormones, bilirubin, drugs and toxicants. Similarly, globulin is also a protein that plays an important key role in the immune system which is used for the transport of nutrients and fights against toxicants. These proteins are predominantly generated and entrusted in liver tissues. In the present study, total protein, albumin and globulin levels significantly decreased in all the treated groups (except globulin level in the 12-mg/l-treated group) when compared with the control (Table 2). The observed hypoalbuminaemia and hypoglobulinaemia in treated groups were due to hypoproteinaemia (Alkaladi et al. 2015). Reduction in protein level (albumin and globulin) in treated groups might be the result of gluconeogenesis where it is utilized for energy production in the form of glucose. In the present study, increase in glucose level in treated groups is evidence of gluconeogenesis and consequently reduction of proteins. The damages in the liver and kidneys caused by NiO NPs might have also caused reduction in the protein synthesis (Imani et al. 2015). Our findings are in agreement with the findings of Sara et al. (2020) and Alkaladi et al. (2015), who also noticed decrease in protein, albumin and globulin levels in the Cyprinus carpio exposed to AgNPs and O. niloticus exposed to ZnO NPs, respectively.
The albumin/globulin (A/G) ratio is also an important indicator of tissue damages and widely used in clinical research. In our study, albumin/globulin ratios significantly decreased in all the groups exposed to NiO NPs compared with the control (Table 2). Reduced A/G ratio indicates damage and injury in vital tissues like the liver and kidneys.
Creatinine and bilirubin
The serum creatinine level is being used as a rough index of glomerular filtration rate and kidney dysfunction (Maita et al. 1985). In the present study, serum creatinine level showed significant increase in all the treated groups (except in 12-mg/l-treated groups) from control (Table 2). The elevation of creatinine in serum might be an indicator of kidney dysfunction due to NiO NPs. A strong correlation (r = 0.948) between creatinine and Ni accumulation in the blood shows increase in creatinine level with the increase in Ni accumulation (Fig. 7).
Previously, a significant increase in the serum creatinine level has been reported in all the treated groups of Nile tilapia after exposure of CuO NPs (Abdel-Khalek et al. 2015). Our results also agree with the findings of Zaghloul et al. (2006) who studied the effects of copper toxicity in three fish C. gariepinus, O niloticus and T. zillii and obtained higher level of serum creatinine level when compared with the control.
Bilirubin is a waste product generated by the haemoglobin catabolism in the liver and is secreted via the liver to bile. It is an important indicator of liver injury. In our study, bilirubin level significantly increased in most of the treated groups in comparison to the control (Table 2). The increased level of bilirubin might be associated with the damage in liver caused by NiO NPs. Strong correlation (r = 0.989) between bilirubin and Ni accumulation also shows impact of NiO NPs on the level of bilirubin (Fig. 7). Increased level of bilirubin in serum has also been reported by Gupta and Guha (2006) in H. fossilis exposed to microcystin. Similar results were also noticed in the study of Carbis et al. (1996) in C. carpio intoxicated with microcystin. Earlier literature of Young et al. (1994) also agrees with the findings of our study.
AST, ALT, ALP and LDH activities
In aquatic ecotoxicology, Serum enzymes and biochemical indices have been used as very sensitive and suitable biomarkers as they reflect the main primary indicator of potential health hazards to aquatic organisms (Nel et al. 2009).
Nanoparticles may have capability to enter the body through respiratory and digestive system because of their very small diameter (<100 nm) (Handy et al. 2008; Shi et al. 2013) and can be dispersed through the circulation of blood and ultimately deposited into the liver (Evans et al. 1993; Van der et al. 2003). AST and ALT are mainly found in the liver and are intracellular enzymes associated with gluconeogenesis and metabolism of amino acid. Due to NiO NP toxicity, the liver may get disrupted and causes leakage of ALT and AST into the blood (Banaee et al. 2011; Sookoian and Pirola 2012). The other important membrane-associated enzyme is the ALP, and any change in the membrane of liver cells could change its activity in serum (Molina et al. 2005). In earlier literatures, the mechanism of action of NiO NPs has not been explored well and studies related to NiO NP toxicity are also very few. Therefore, in our perspectives, the smaller size of NiO NPs might have caused its infiltration into the cell membrane through diffusion and endocytosis process and produced reactive oxygen species, which in turn damaged the proteins, DNAs, RNAs, mitochondria, etc. Therefore, the core mechanism of NiO NP toxicity might be the production of reactive oxygen species (ROS) and oxidative damage to the cells.
In the present study, elevation in the activities of ALT, AST and ALP in most of the treated groups (Table 3) might be the result of injury in liver and excoriation in hepatopancreases due to NiO NP toxicity which ultimately ascertain the potential damage in hepatocytes, damage in parenchymal cells, etc. (Farkas et al. 2004; Kandeel 2004). A strong correlation among these enzymes shows their dependency on one another (Fig. 7).
In earlier literature, activities of ALT, AST and ALP in serum were also found to be significantly increased in O. niloticus after exposure of Zn in comparison to the control group (Abdel-Khalek et al. 2015). Similar results were also observed by Zaghloul et al. (2006) after studying the copper toxicity on three fish species: O. niloticus, T. zillii and C. gariepinus.
The present study also showed an agreement with the findings of Abdel-Khalek et al. (2015) who exposed O. niloticus to 1/10 and 1/20 LC50/96 h of CuO (BPs and NPs) for 30 days and observed a significant increase in ALT, AST and ALP activities in serum when compared with the control group.
Lactate dehydrogenase is one of the main enzymes of carbohydrate metabolism involved in glycolysis to catalyse the oxidation of lactate and reduction of pyruvate to ultimately generate energy from sugars. This enzyme is found almost in every cell of the body including the muscles, brain, blood, kidney, pancreas and liver. In the present investigation, a significant increase was noticed in the activity of LDH in all the NiO NP–treated groups than the control (Table 3). The increase level of LDH in serum might be due to catabolism of sugars to generate energy to overcome the stress condition caused by NiO NPs. In the present study, the higher level of LDH in treated groups is the evidence of injury in the liver, kidney and other tissues which lead to release of LDH in the blood and increase in its level. Therefore, LDH might be an important indicator of damage to the body’s tissues. A strong correlation of LDH with glucose (r = 0.982) as well as with Ni accumulation (r = 0.988) also indicates increased production of glucose to provide energy to overcome the stress caused by NiO NPs (Fig. 7).
Our findings are also supported by the results of Imani et al. (2015) who observed significantly higher level of LDH after exposing rainbow trout, Oncorhynchus mykiss, to silver nanoparticles. Among others, recent studies also demonstrated that LDH level increased in Caspian Roach (Rutilus rutilus caspius) exposed to ZnO NP exposure (Khosravi-Katuli et al. 2018) and, in Caspian Trout and Salmo trutta caspius exposed to Copper oxide nanoparticles (Kaviani et al. 2019).
Conclusion
The present study illustrates that NiO NPs caused significant toxic impact on H. fossilis as demonstrated by Ni accumulation in the blood, alteration in the haematological and biochemical parameters and changes in the activity of different enzymes in the blood. It was found that lower dose of NiO NPs was less toxic to the fishes while higher dose exhibited more deteriorating effect. Significant accumulation of Ni was noticed in the blood of NiO NP–exposed fishes that induced substantial toxicity and changes in the haematological parameters (RBC count, WBC count, Hb content and Ht%) as well as calculated haematological indices (MCV, MCH, MCHC and oxygen carrying capacity). Changes in the morphology of erythrocytes also demonstrate toxicological effects of NiO NPs. Furthermore, it also induced significant changes in the mean percentage of differential leucocytes like neutrophils, eosinophils, basophils, lymphocytes and monocytes. Significant alterations in the biochemical profiles viz. cholesterol, triglyceride, glucose, protein, albumin, globulin, albumin-globulin ratio, creatinine, bilirubin and cortisol and serum enzyme activity like AST, ALT, ALP and LDH are also an indication of toxic effect exhibited by NiO NPs. In conclusion, the present study suggests to assess the toxic effect of NiO NPs in the aquatic organisms so that their hazards risk can be monitored and organisms could be protected from their detrimental impact.
References
Abdel-Khalek AA, Kadry MAM, Badran S, Marie MAS (2015) Comparative toxicity of copper oxide bulk and nano particles in Nile Tilapia; Oreochromis niloticus: Biochemical and oxidative stress. J Basic Appl Zool 72:43–57
Adeel M, Ma C, Ullah S, Rizwan M, Hao Y, Chen C, Jilani G, Shakoor N, Li M, Wang L, Tsang DCW, Rinklebe J, Rui Y, Xing B (2019) Exposure to nickel oxide nanoparticles insinuates physiological, ultrastructural and oxidative damage: a life cycle study on Eisenia fetida. Environ Pollut 254(2019):113032
Adhikari S, Sarkar B (2004) Effects of cypermethrin and carbofuran on certain haematological parameters and prediction of their recovery in fresh water teleost, Labeo rohita (Ham). Ecotoxicol Environ Saf 58:220–226
Aitken RJ, Chaudhry MQ, Boxall ABA, Hull M (2006) Manufacture and use of nanomaterials: current status in the UK and global trends. Occup Med 56:300–306
Ale A, Bacchetta C, Rossi AS, Galdopórpora J, Desimone MF, de la Torre FR, Gervasio S, Cazenave J (2018) Nano silver toxicity in gills of a neotropical fish: metal accumulation, oxidative stress, histopathology and other physiological effects. Ecotoxicol Environ Saf 148:976–984. https://doi.org/10.1016/j.ecoenv.2017.11.072
Alkaladi A, El-Deen N, Afifi M, Abu Zinadah O (2015) Hematological and biochemical investigations on the effect of vitamin E and C on Oreochromis niloticus exposed to zinc oxide nanoparticles. Saudi J Biol Sci 22:556–563
Anand SR, Mathan R, Manoharan S, Rama KP, Subramanian B, Devaraj N (2015) Iron oxide nanoparticles to an Indian major carp, Labeo rohita: impacts on haematology, iono regulation and gill Na+/K+ ATPase activity. J King S Uni - Sci 27:151–160
Anbumani S, Mohankumar MN (2012) Gamma radiation induced micronuclei and erythrocyte cellular abnormalities in the fish Catla catla. Aquat Toxicol 122–123:125–132
Atli G, Ariyurek SY, Kanak EG, Canli M (2015) Alterations in the serum biomarkers belonging to different metabolic systems of fish (Oreochromis niloticus) after Cd and Pb exposures. Environ Toxicol Pharmacol 40(2):508–515
Baek YW, An YJ (2011) Microbial toxicity of metal oxide nanoparticles (CuO, NiO, ZnO, and Sb2O3) to Escherichia coli, Bacillus subtilis, and Streptococcus aureus. Sci Total Environ 409:1603–1608
Banaee M, Sureda A, Mirvaghefi AR, Ahmadi K (2011) Effects of diazinon on biochemical parameters of blood in rainbow trout (Oncorhynchus mykiss). Pestic Biochem Physiol 99:1–6. https://doi.org/10.1016/J.PESTBP.2010.09.001
Bayunova L, Barannikova I, Semenkova T (2002) Sturgeon stress reactions in aquaculture. J Appl Ichthyol 18:397–404. https://doi.org/10.1046/j.1439-0426.2002.00410.x
Bernet D, Schmidt H, Wahli T, Burkhardt-Holm P (2000) Effluent from a sewage treatment works causes changes in serum chemistry of brown trout (Salmo trutta L.). Ecotoxicol Environ Saf 48:140–147
Bilberg K, Malte H, Wang T, Baatrup E (2010) Silver nanoparticles and silver nitrate cause respiratory stress in Eurasian perch (Perca fluviatilis). Aquat Toxicol 96:159–165. https://doi.org/10.1016/j.aquatox.2009.10.019
Blaxhall PC, Daisley KW (1973) Routine haematological methods for use with fish blood. J Fish Biol 5:771–781
Bowers LD, Edward TW (1980) Kinetic serum creatinine assays. II. A critical evaluation and review. Clin Chem 26(5):555–561
Brand ME (2001) Bioaccumulation of metals in Labeo congoro from the Olifants River (Mpumalanga) and the effect of nickel on the haematology of fish (M.Sc. Sci. thesis). Rand Afrikaans Univ
Brody AL (2006) Nano and food packaging technologies converge. Food Technol 60:92–94
Bystrzejewska-Piotrowska G, Golimowski J, Urban PL (2009) Nanoparticles: their potential toxicity, waste and environmental management. Waste Manag 29(9):2587–2595. https://doi.org/10.1016/j.wasman.2009.04.001
Canli EG, Canli M (2015) Low water conductivity increases the effects of copper on the serum parameters in fish (Oreochromis niloticus). Environ Toxicol Pharmacol 39(2):606–613
Canli EG, Atli G, Canli M (2017) Response of the antioxidant enzymes of the erythrocyte and alterations in the serum biomarkers in rats following oral administration of nanoparticles. Environ Toxicol Pharmacol 50:145–150
Canli EG, Dogan A, Canli M (2018) Serum biomarker levels alter following nanoparticle (Al2O3, CuO, TiO2) exposures in freshwater fish (Oreochromis niloticus). Environ Toxicol Pharmacol 62:181–187. https://doi.org/10.1016/j.etap.2018.07.009
Carbis CR, Mitchell GF, Anderson JW, McCauley I (1996) The effects of microcystins on the serum biochemistry of carp, Cyprinus carpio L., when the toxins are administered by gavage, immersion and intraperitoneal routes. J Fish Dis 19:151–159
Cavas T, Garanko NN, Arkhipchuk VV (2005) Induction of micronuclei and binuclei in blood, gill and liver cells of fishes chronically exposed to cadmium chloride and copper sulphate. Food Chem Toxicol 43:569–574
Cheraghi J, Hosseini E, Hoshmandfar R, Sahraei R (2013) Hematologic parameters study of male and female rats administrated with different concentrations of silver nanoparticles. Int J Agric and J Crop Sci 5:789–796
Clark NJ, Shaw BJ, Handy RD (2018) Low hazard of silver nanoparticles and silver nitrate to the haematopoietic system of rainbow trout. Ecotoxicol Environ Saf 152:121–131. https://doi.org/10.1016/j.ecoenv.2018.01.030
Coles EH (1986) Veterinary clinical pathology (4th Ed); W. B. Saunders Co, Philadelphia
Corcoran RM, Durnan SM (1977) Albumin determination by a modified bromocresol green method. Clin Chem 23(4):765–766
Cui B, Ren L, Xu QH, Yin LY, Zhou XY, Liu JX (2016) Silver nanoparticles inhibited erythrogenesis during zebrafish embryogenesis. Aquat Toxicol 177:295–305. https://doi.org/10.1016/j.aquatox.2016.06.005
Dacie JA, Lewis SM (1991) Practical haematology A, 7th edn. Churchill Livingstone, London
Dumala N, Mangalampalli B, Kalyan Kamal SS, Grover P (2019) Repeated oral dose toxicity study of nickel oxide nanoparticles in Wistar rats: a histological and biochemical perspective. J Appl Toxicol 1–18:1012–1029. https://doi.org/10.1002/jat.3790
Evans DW, Dodoo DK, Hanson PJ (1993) Trace element concentrations in fish livers: implications of variations with fish size in pollution monitoring. Mar Pollut Bull 26:329–334. https://doi.org/10.1016/0025-326X(93)90576-6
Firat Ö, Kargin F (2010) Individual and combined effects of heavy metals on serum biochemistry of Nile Tilapia, Oreochromis niloticus. ArchEnviron Contam Toxicol 58:151–157
Farkas J, Farkas P, Hyde D (2004) Liver and gastroenterology tests. In: Lee M 3rd (ed) Basic skills in interpreting laboratory data. American Society of Health-System Pharmacists, Bethesda, pp 330–336
Fazio F (2019) Fish hematology analysis as an important tool of aquaculture: a review. Aquaculture 500:237–242. https://doi.org/10.1016/j.aquaculture.2018.10.030
Freitas RA (2005) What is nanomedicine? Nanomedicine 1:2–9
George S, Gardner H, Seng EK, Chang H, Wang C, Yu Fang CH, Richards M, Valiyaveettil S, Chan WK (2014) Differential effect of solar light in increasing the toxicity of silver and titanium dioxide nanoparticles to a fish cell line and zebrafish embryos. Environ Sci Technol 48:6374–6382. https://doi.org/10.1021/es405768n
Ghafari FH, Binde DH, Jamali H, Hasanpour S, Mehdipour N, Rashidiyan G (2017) The protective role of vitamin E on Oreochromis niloticus exposed to ZnONP. Ecotoxicol Environ Saf. https://doi.org/10.1016/j.ecoenv.2017.07.005
Ghiasi F, Mirzargar SS, Badakhshan H, Shamsi S (2010) Effects of low concentration of cadmium on the level of lysozyme in serum, leukocyte count and phagocytic index in Cyprinus carpio under the wintering conditions. J Fish Aquat Sci 5:113–119. https://doi.org/10.3923/jfas.2010.113.119
Gong N, Shao K, Feng W, Lin Z, Liang C, Sun Y (2011) Biotoxicity of nickel oxide nanoparticles and bio-remediation by microalgae Chlorella vulgaris. Chemosphere 83:510–516
Grosell M, McDonald MD, Wood CM, Walsh PJ (2004) Effects of prolonged copper exposure in the marine gulf toadfish (Opsanus beta) I: hydromineral balance and plasma nitrogenous waste products. Aquat Toxicol 68:249–262
Gupta US, Guha S (2006) Microcystin toxicity in a freshwater fish, Heteropneustes fossilis (Bloch). Curr Sci 91:9–10
Hajirezaee S, Mohammadi G, Naserabad SS (2019) The protective effects of vitamin C on common carp (Cyprinus carpio) exposed to titanium oxide nanoparticles (TiO2- NPs). Aquaculture 518:734734. https://doi.org/10.1016/j.aquaculture.2019.734734
Han ZX, Zhang M, Xia LC (2012) Bioaccumulation and toxicity of NiO nanoparticles in Gracilaria lemaneiformis. Adv Mater Res 518–523:942–945
Handy RD, Kammer FVD, Lead JR, Hassellöv M, Owen R, Crane M (2008) The ecotoxicity and chemistry of manufactured nanoparticles. Ecotoxicology 17:287–314
Hedayati SA, Farsani HG, Naserabad SS, Hoseinifar SH, Van Doan H (2019) Protective effect of dietary vitamin E on immunological and biochemical induction through silver nanoparticles (AgNPs) inclusion in diet and silver salt (AgNO3) exposure on Zebrafish (Danio rerio). Comp Biochem Physiol C Toxicol Pharmacol 222:100–107. https://doi.org/10.1016/j.cbpc.2019.04.004
Hontela A, Rasmussen JB, Audet C, Chevalier G (1992) Impaired cortisol stress response in fish from environments polluted by PAHs, PCBs, and mercury. Arch Environ Contam Toxicol 22:278–283. https://doi.org/10.1007/BF00212086
Horie M, Fukui H, Nishoi K, Endoh S, Kato H, Fujita K, Miyauchi A, Nakamura SM, Ishida N, Kinugas S, Morimoto Y, Niki N, Yoshida Y, Iwahashi H (2011) Evaluation of acute oxidative stress induced by NiO nanoparticles in vivo and in vitro. J Occup Health 53:64–74. https://doi.org/10.7897/2230-8407.0910238
Imani M, Halimi M, Khara H (2015) Effects of silver nanoparticles (AgNP) on haematological parameters of rainbow trout, Oncorhynchus mykiss. Comp Clin Pathol 24(3):491–495. https://doi.org/10.1007/s00580-014-1927-5
Javed M, Usmani N (2013) Investigation on accumulation of toxicants and health status of freshwater fish Channa punctatus, exposed to sugar mill effluent. Int J Zool 3(1):43–48
Jendrassik L, Grof P (1938) Colorimetric method of determination of bilirubin. Biochem Z 297:81–82
Jindal R, Kaur M (2014) Phenotypic alterations in erythrocytes of Ctenopharyngodon idellus (Cuvier & Valenciennes) induced by chlorpyrifos: SEM Study. Int J of Fish and Aqua Sci 4(1):23–30
Johansen K (1970) Air-breathing fishes. In: Hoar WS, Randall DT (eds) Fish physiology, vol 4. Academic, New York, pp 361–411
Jorgensen SW (2010) A derivative of encyclopaedia of ecology. Ecotoxicology. Academic Press, London, p 390
Kandeel NMS (2004) Toxicological and metabolic studies of some molluscicides on harmful terrestrial snails (M.Sc. thesis). Zoology Dep., Faculty of Science, Cairo University
Karnik BS, Davies SH, Baumann MJ, Masten SJ (2005) Fabrication of catalytic membranes for the treatment of drinking water using combined ozonation and ultrafiltration. Environ Sci Technol 39:7656–7661
Kaur A, Kaur K (2006) Impact of nickel-chrome electroplating effluent on the protein and cholesterol contents of blood plasma of Channa punctatus (Bl.) during different phases of the reproductive cycle. J Environ Biol 27(2):241–245
Kaviani EF, Naeemi AS, Salehzadeh A (2019) Influence of copper oxide nanoparticle on haematology and plasma biochemistry of Caspian trout (Salmo trutta caspius), following acute and chronic exposure. Pollution 5(1):225–234. https://doi.org/10.22059/poll.2018.251034.383
Kaviani EF, Naeemi AS, Salehzadeh A (2020) Acute toxicity and effects of titanium dioxide nanoparticles (TiO2 NPs) on some metabolic enzymes and hematological indices of the endangered Caspian trout juveniles (Salmo trutta caspius Kessler, 1877). Iran J Fish Sci 19(3):1253–1267
Khan MS, Qureshi NA, Jabeen F (2017a) Assessment of toxicity in fresh water fish Labeo rohita treated with silver nanoparticles. Appl Nanosci. https://doi.org/10.1007/s13204-017-0559-x
Khan MS, Qureshi NA, Jabeen F, Shakeel M, Asghar MS (2017c) Assessment of waterborne amine-coated silver nanoparticle (Ag-NP)-induced toxicity in Labeo rohita by histological and hematological profiles. Biol Trace Elem Res 182:1–10. https://doi.org/10.1007/s12011-017-1080-5
Khosravi-Katuli K, Lofrano G, Pak Nezhad H, Giorgio A, Guida M, Aliberti F, Siciliano A, Carotenuto M, Galdiero E, Rahimi E, Libralato G (2018) Effects of ZnO nanoparticles in the Caspian roach (Rutilus rutilus caspicus). Sci Total Environ 626:30–41
Kind PR, King EG (1954) Colorimetric determination of alkaline phosphatase activity. J Clin Pathol 7:322–326
Kovrižnych JA, Sotníková R, Zeljenková D, Rollerová E, Szabová E, Wimmerová S (2013) Acute toxicity of 31 different nanoparticles to zebra fish (Danio rerio) tested in adulthood and in early life stages – comparative study. Interdiscip Toxicol 6:67–73
Kumar PYG, Gupta VT, Shakti T, Singh A (2005) Haematological and biochemical abnormalities in Cirrhinus mrigala (Ham.) induced by lead. J Ecophysiol Occupl Hlth 5:213–216
Kumar SM, KP, Ramesh M (2011) Haematological and biochemical responses of fresh water teleost fish; Cyprinus carpio (Actinopterygii: Cyprinusformes) during acute and chronic sublethal exposure to lindane. Pest Biochem Physiol 100: 206-211
Lanone S, Boczkowski J (2006) Biomedical applications and potential health risks of nanomaterials: Molecular mechanisms. Curr Mol Med 6:651–663
Larsson A, Haux C, Sjobeck M (1985) Fish physiology and metal pollution. Results and experiences from laboratory and field studies. Ecotoxicol Environ Saf 9:25–281
Lasheen MR, AbdelGawad FK, Alaneny AA, Abdelbary HMH (2012) Fish as Bio indicators in aquatic environmental pollution assessment: a case study in Abu-Rawash Area, Egypt. World Appl Sci J 19:265–275
Lowry OH, Rosenbrough NJ, Ferry AL, Randall RJ (1951) Protein measurement with Folin-phenol reagent. J Biol Chem 193:265–275
Luoma SN, Rainbow PS (2008) Sources and cycles of trace metals. In: Metal contamination in aquatic environments: science and lateral management. Cambridge University Press, Cambridge, pp 47–66
Maheswaran R, Devapaul A, Velmurugan B, Ignacimuthu S (2008) Haematological studies of freshwater fish, Clarias batrachus (L.) exposed to mercuric chloride. Int J Integr Biol 2(1):49–54
Mahjoubian M, Naeemi AS, Sheykhan M (2021) Toxicological effects of Ag2O and Ag2CO3 doped TiO2 nanoparticles and pure TiO2 particles on zebrafish (Danio rerio). Chemos. 263:128182
Maita M, Shiomitsu L, Ikeda Y (1985) Health assessment by the climogram of hemochemical constituents in cultured yellowtail. Bull Jpn Soc 51: 205–211
Massarsky A, Abraham A, Nguyen KC, Rippstein P, Tayabali AF, Trudeau VL, Moon TW (2014) Nanosilver cytotoxicity in rainbow trout (Oncorhynchus mykiss) erythrocytes and hepatocytes. Comp Biochem Physiol Part C: Toxicol Pharmacol 159:10–21. https://doi.org/10.1016/j.cbpc.2013.09.008
Molina R, Moreno I, Pichardo S, Jos A, Moyano R, Monterde JG, Cameán A (2005) Acid and alkaline phosphatase activities and pathological changes induced in Tilapia fish (Oreochromis sp.) exposed sub-chronically to microcystins from toxic cyanobacterial blooms under laboratory conditions. Toxicon. https://doi.org/10.1016/j.toxicon.2005.07.012
Monteiro D, Rantin F, Kalinin A (2010) Inorganic mercury exposure: toxicological effects, oxidative stress biomarkers and bioaccumulation in the tropical freshwater fish matrinxã, Brycon amazonicus (Spix and Agassiz, 1829). Ecotoxicology 19:105–123
Munkittrick KR, Leatherland JF (1983) Haematocrit values in feral goldfish, Carassius auratus L., as indicators of the health of the population. J. Fish Biol 23:153–161
Naeemi AS, Elmi F, Vaezi G, Ghorbankhah M (2020) Copper oxide nanoparticles induce oxidative stress mediated apoptosis in carp (Cyprinus carpio) larva. Gene Repor 19:100676. https://doi.org/10.1016/j.genrep.2020.100676
National Science and Technology Council (2004) National Nanotechnology Initiative Strategic Plan; Executive Office of the President of the United States: Washington, DC, US
Nel AE, Mädler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, Thompson M (2009) Understanding bio physicochemical interactions at the nano-bio interface. Nat Mater 8(7):543–557
Ogunola OS (2017) Physiological, immunological, genotoxic and histopathological biomarker responses of molluscs to heavy metal and water-quality parameter exposures: a critical review. J Oceanogr Mar Res 5:158. https://doi.org/10.4172/2572-3103.1000158
Oner M, Atli G, Canli M (2008) Changes in serum biochemical parameters of freshwater fish Oreochromis niloticus following prolonged metal, (Ag, Cd, Cr, Cu, Zn) exposures. Environ Toxicol Chem 27:360–366
Rajkumar KS, Kanipandian N, Thirumurugan R (2016) Toxicity assessment on haematology, biochemical and histopathological alterations of silver nanoparticles-exposed freshwater fish Labeo rohita. Appl Nanosci 6(1):19–29
Rao KV, Sunandana CS (2008) Effect of fuel to oxidizer ratio on the structure, micro structure and EPR of combustion synthesized NiO nanoparticles. J Nanosci Nanotechnol 8:4247–4253
Razavian MH, Masaimanesh M (2015) Ingestion of silver nanoparticles leads to changes in blood parameters. Nanomed J 1(5):339–345
Reitman S, Frankel SA (1957) Colorimetric method for the determination of serum glutamic oxaloacetic and glutamic-pyruvic transaminase. Am J Clin Pathol 28–56
Remya AS, Ramesh M, Saravanan M, Poopal RK, Bharathi S, Nataraj D (2015) Iron oxide nanoparticles to an Indian major carp, Labeo rohita: impacts on hematology, iono regulation and gill Na+/K+ ATPase activity. Journal of King Saud University – Science 27(2):151–160
Roco MC (2003) Nanotechnology convergence with modern biology and medicine. Curr Opin Biotechnol 14:337–346
Sadoul B, Vijayan MM (2016) 5 - stress and growth. In: Schreck LT, Farrell AP, Colin J, Brauner Carl B (eds) Fish physiology, Biology of Stress in Fish. Academic Press, pp 167–205
Said REM, Hasieb HE, Moustafa MA, Soliman SME, Klaos W, Osman GM (2019) Haematological, serological and genotoxic findings in the African catfish Clarias gariepinus after the administration of copper nanoparticles and penconazole. EC Veterinary Science 4(10):01–14
Salimi A, Sharifi E, Noorbakhsh A, Soltanian S (2007) Direct electrochemistry and electrocatalytic activity of catalase immobilized onto electrodeposited nano-scale islands of nickel oxide. Biophys Chem 125:540–548
Samuel RM (1986) Haematology. In: Notes on clinical lab techniques, 4th edn. Tailor, Madras
Sara V, Ghasem M, Kamran RT, Fatemeh M, Saeid SN (2020) The effects of silver nanoparticles (Ag-NPs) sublethal concentrations on common carp (Cyprinus carpio): bioaccumulation, haematology, serum biochemistry and immunology, antioxidant enzymes, and skin mucosal responses. Ecotoxi Environ Safety 194:110353. https://doi.org/10.1016/j.ecoenv.2020.110353
Saravanan M, Kumar, KP, Ramesh M (2011) Haematological and biochemical responses of fresh water teleost fish; Cyprinus carpio (Actinopterygii: Cyprinusformes) during acute and chronic sublethal exposure to lindane. Pest Biochem Physiol 100: 206–211
Saunders DC (1967) Differential blood cell counts of 121 species of marine fishes of Puerto Rico. Trans Am Microsc Soc 85:427–449
Sayed AEDH, Kataoka C, Oda S, Kashiwada S, Mitani H (2018) Sensitivity of medaka (Oryzias latipes) to 4-nonylphenol subacute exposure; erythrocyte alterations and apoptosis. Environ Toxicol Pharmacol 58:98–104. https://doi.org/10.1016/j.etap.2017.12.023
Schrand AM, Rahman MF, Hussain SM, Schlager JJ, Smith DA, Syed AF (2010) Metal-based nanoparticles and their toxicity assessment. Wiley Interdisciplinary Reviews Nanomed Nanobiotech 2:544–568
Shah N, Khisroon M, Ali Shah SS (2020) Assessment of copper, chromium, and lead toxicity in fish (Ctenopharyngodon idella Valenciennes, 1844) through hematological biomarkers. Environ Sci Pollut Res 27:33259–33269
Shaluei F, Hedayati A, Jahanbakhshi A, Kolangi H, Fotovat M (2013) Effect of subacute exposure to silver nanoparticle on some hematological and plasma biochemical indices in silver carp (Hypophthalmichthys molitrix). Human Exp Toxicol 32:1270–1277.
Sheeba AS, Noorjahan CM (2018) Toxicity of copper nanoparticle on haematology and biochemistry of fish, Tilapia mossambica. Int Res J Pharm 9(10):121–124
Shi H, Magaye R, Castranova V, Zhao J (2013) Titanium dioxide nanoparticles: a review of current toxicological data. Part Fibre Toxicol 10:15. https://doi.org/10.1186/1743-8977-10-15
Sookoian S, Pirola CJ (2012) Alanine and aspartate aminotransferase and glutamine cycling pathway: their roles in pathogenesis of metabolic syndrome. World J Gastroenterol 18:3775–3781. https://doi.org/10.3748/wjg.v18.i29.3775
Svoboda M, Luskova V, Drastichova J, Iabek V (2001) The effect of diazinon on hematological indices of common carp (Cyprinus carpio L.). Acta Vet (Brno) 70:457–465
Svobodova Z (1971) Some hematological and metabolic changes in fish occurring after pesticide intoxication. Bull VUR Vodany 7:29–36
Thummabancha K, Onparn N, Srisapoome P (2016) Molecular characterization and expression analyses of cDNAs encoding the thioredoxin-interact-ing protein and selenoprotein P genes and histological changes in Nile tilapia (Oreochromis niloticus) in response to silver nanoparticle exposure. Gene. 577:161–173
Tort L (2011) Stress and immune modulation in fish. Dev Comp Immunol 35:1366–1375. https://doi.org/10.1016/j.dci.2011.07.002
Trinder P (1969) Enzymatic colorimetric method of glucose. Ann Clin Biochem 6:24–27
Van der OR, Beyer J, Vermeulen NPEE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149. https://doi.org/10.1016/S1382-6689(02)00126-6
Vaseem H, Banerjee TK (2012) Toxicity analysis of effluent released during recovery of metals from polymetallic sea nodules using fish haematological parameters. In: Ali M (ed) The functioning of ecosystem. Intech, Croatia, pp 249–260
Vidya PV, Chitra KC (2018) Evaluation of genetic damage in Oreochromis mossambicus exposed to selected nanoparticles by using micronucleus and comet bioassays. Croatian Journal of Fisheries 76:115–124. https://doi.org/10.2478/cjf-2018-0015
Voet F, Voet JG (1990) Biochemistry. John Wiley and Sons, New York, USA, pp 425–457
Vosyliene MZ (1999) The effect of heavy metal mixture on haematological parameters of rainbow trout. Heavy metals in environment. An integrated approach Ed. Lovejy DA. 295-298
Wang J, Zhu X, Zhang X, Zhao Z, Liu H, George R, Wilson-Rawls J, Chang Y, Chen Y (2011) Disruption of zebrafish (Danio rerio) reproduction upon chronic exposure to TiO2 nanoparticles. Chemos. 83(4):461–467
Weishaar D, Gossrou E, Faderl B (1975) Ranges of alpha-HBDH, LDH, AP and LAP as measured with substrate optimated test charges. Med Welt 26:387–392
Wood CM, Farrel AP, Brauner CJ (2012a) Homeostasis and toxicology of essential metals. Fish Physiol. 31A Academic Press, London, pp. 497
Wood CM, Farrel AP, Brauner CJ (2012b) Homeostasis and toxicology of non-essential metals. Fish Physiol. 31B Academic Press, London, pp. 507
Young G, Brown CL, Nishioka RS, Folmar LC, Andrews M, Cashman JR, Bern HA (1994) Histopathology, blood chemistry and physiological status of normal and moribund striped bass (Morone saxatilis) involved in summer mortality (‘die-off’) in the Sacramento-San Joaquin Delta of California. J Fish Biol 44:491–512
Zaghloul KH, Omar WA, Abdo-Hegab S (2006) Toxicity specificity of copper in some freshwater fishes. Egypt J Zool 47:383–400
Zhang Y, Zhu L, Zhou Y, Chen J (2015) Accumulation and elimination of iron oxide nanomaterials in zebrafish (Danio rerio) upon chronic aqueous exposure. J Environ Sci 30:223–230
Zutshi BSG, Prasad R, Nagaraja R (2010) Alteration in hematology of Labeo rohita under stress of pollution from Lakes of Bangalore, Karnataka, India. Environ Monit Assess 168(1-4):11–19
Acknowledgements
The authors duly acknowledge Analytical Discipline and Centralized Instrument Facility of Aligarh Muslim University for providing instrumental facilities. The authors also acknowledge Prof. Iqbal Parvez for providing microscope facility of Department of Zoology, Aligarh Muslim University.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding
This study was financially supported by University Grant Commission (UGC), by the grant of STARTUP (No. F.30-409/2018(BSR)), UGC, Government of India, New Delhi, India.
Author information
Authors and Affiliations
Contributions
A.R Samim has performed experiments, analysed data and prepared manuscript. H. Vaseem has designed and coordinated the experiments, interpreted the results and improved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Bruno Nunes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Samim, A.R., Vaseem, H. Assessment of the potential threat of nickel(II) oxide nanoparticles to fish Heteropneustes fossilis associated with the changes in haematological, biochemical and enzymological parameters. Environ Sci Pollut Res 28, 54630–54646 (2021). https://doi.org/10.1007/s11356-021-14451-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-14451-y