Abstract
Watershed acidification and poor water quality can deleteriously affect amphibian populations. Between 1990 and 2008, we sampled 333 small, permanent (inundated year round) waterbodies that drain forested areas in the Algoma, Muskoka and Sudbury regions of central Ontario, Canada to determine whether water chemistry parameters, fish presence, and waterbody area and depth predict amphibian presence or diversity. Amphibians were present in some low-pH waterbodies, contrasting earlier studies, and generally water chemistry was not a strong indicator of amphibian presence or diversity in central Ontario. We suspect that other biotic and abiotic factors have a stronger effect on amphibian presence, and that the relationships between chemical and physical attributes and amphibian presence are complex. Future research should focus on long-term habitat change in central Ontario waterbodies to determine how watershed degradation has affected amphibians.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Widespread concern for amphibian populations has grown recently due to large scale, deleterious anthropogenic impacts on the environment (e.g., development, pollution, climate change), and concomitant amphibian population declines (Beebee and Griffiths 2005; Guderyahn et al. 2016). Although habitat loss, degradation and fragmentation are significant causes of amphibian declines (Ficetola and Bernardi 2004; Beebee and Griffiths 2005), other factors also negatively impact amphibian populations (e.g., disease and pathogens; Battaglin et al. 2016).
Parts of North America and Europe have been subject to dramatic alterations to soils and aquatic systems associated with acidic precipitation (Likens et al. 1996; Jeziorski et al. 2008), and consequently the influence of lake and pond acidification on amphibian distribution and survival has received considerable attention (Bradford et al. 1994). Studies have focused on identifying species and habitats most vulnerable to acidic deposition by characterizing relationships between the acidification of habitats and biological changes, and developing monitoring programs that can track biological responses as acidifying emissions are reduced (e.g., Freda 1986; McNicol et al. 1995a, b). However, the relationships between amphibian occurrence, and chemical and physical characteristics of lakes and wetlands (e.g., water quality, hydrology, morphometry) are complex (Beebee and Griffiths 2005; Battaglin et al. 2016), and the potential for deleterious synergistic impacts of anthropogenic pollution on aquatic food webs, habitat structure and composition, as well as on amphibian reproduction and physiology, suggests that further investigation of these relationships is warranted.
Lakes and wetlands of eastern Canada are of special concern due to the predominance of thin, poorly buffered soils in many watersheds and a history of high levels of acidic deposition (Hecnar and M’Closkey 1996), which have had adverse effects on macroinvertebrates, fish and aquatic birds (McNicol et al. 1995a, b; Jeffries et al. 2003). Among the amphibian species present in Canada, about 50% of their ranges have been historically exposed to acid precipitation (Clark 1992; Longcore et al. 1993). Watershed acidification can influence amphibian populations through death of eggs and larvae, changes to embryonic development patterns and malformation of larvae, altered ionic and water exchange across skin and gill membranes, and reduced growth and swimming performance (Freda et al. 1991; Jung and Jagoe 1995). Despite large reductions in the levels of acidic deposition in eastern Canada and the improvement of acid status for some lakes (e.g., Mallory et al. 1998; Keller et al. 2018), the majority of monitored waterbodies have shown limited chemical recovery (Jeffries et al. 2003; Keller et al. 2018). Collectively, the suite of environmental stressors that continue to affect water quality in eastern Canadian watersheds may have reduced the suitability of many aquatic habitats for amphibian breeding.
As part of a federal program to evaluate and monitor the effects of acidic precipitation on biota in eastern Canada (McNicol et al. 1995a, b), we sampled larval amphibian presence in permanent waterbodies (inundated year round) of central Ontario between 1990 and 2008. Previous studies suggested that chemical changes in water quality due to acid inputs [e.g., reduced pH or alkalinity, increased aluminum (Al)] had a negative impact on amphibian occurrence and numbers in lakes and wetlands (Freda 1986; Freda et al. 1991), including research located in our study area (Glooschenko et al. 1992). Given that many waterbodies in our study region had low natural buffering capacity and thus were acid-sensitive with high Al (Mallory et al. 1998), our overall hypothesis was that water chemistry, and particularly parameters influenced by local and long-range transport of acidifying pollutants, would limit the suitability of many permanent waterbodies for amphibian habitation. Thus, we predicted that amphibian occurrence in our study sites would be reduced in low pH waterbodies. We assessed whether other chemical and physical waterbody attributes influenced amphibian occurrence or species richness. We expected that amphibians would more likely be captured in larger waterbodies with higher nutrient concentrations due to increased food resources.
Materials and Methods
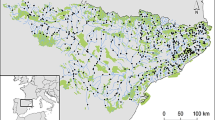
Our study was carried out in central Ontario, Canada, in three ~ 100 km × 100 km study regions: Algoma (45 waterbodies; centre 47° 01′N, 83° 55′W), Muskoka (163 waterbodies; centre 45° 30′N, 79° 06′W), and Sudbury (125 waterbodies; centre 46° 54′N, 80° 41′W; Fig. 1). In general, waterbodies in each region cover a similar range of physical and chemical characteristics (McNicol et al. 1995a). We sampled a variety of waterbodies in this study: bogs, fens, marshes, small lakes and ponds, and a few larger, downstream lakes. Most waterbodies drained small, forested watersheds (in Sudbury, the average watershed covered 84 ha; calculated from Mallory et al. 1998), were remote from human activities (e.g., urban, agriculture, roads) other than logging, and could only be accessed by walking, canoeing or helicopter. Water samples were collected 1−8 times from 333 waterbodies across central Ontario between 1990 and 2008 (Table S1 in Supplementary Material); we used mean values from all sampling periods in our analyses. Most waterbodies were relatively small and shallow with short flushing times (Mallory et al. 1998), so we collected water samples from mid-lake in early October. We assumed that waters were well mixed in autumn due to winds and turnover, and thus depicted typical water chemistry for all sites (acknowledging that water chemistry can vary spatially within lakes, and that pH may be lower earlier in the year following spring runoff; Wetzel 1983). Waterbody areas were measured by geographic information system data while maximum depths were measured using a portable fish finder (Mallory et al. 1998).
Thirteen amphibian species may occur in this area (Cook 1984). Our study was focused on permanent waterbodies, somewhat larger than those in Glooschenko et al. (1992), and thus species that principally use quite shallow or ephemeral ponds were not well sampled in our work. Amphibians (principally newts and tadpoles, but rarely adult frogs) were captured in standard wire minnow traps (30 cm × 70 cm, 6 cm opening, 0.5 cm × 0.5 cm mesh) baited with ~ 125 mL generic dry dog food, and totally submerged for 24 h near shore at depths < 2 m (McNicol et al. 1995a). These traps were set principally to capture fish, but also targeted larval amphibians (hence confirming successful breeding in these waterbodies). Six traps were set (occasionally fewer for ponds < 1 ha) in June or July around the perimeter of each waterbody. When traps were retrieved, all contents were immediately placed into a jar of MS-222 to be euthanized (as per approved Animal Care protocols and permits), and then transferred to jars with formalin. Back at the laboratory, collections were sorted and amphibians removed. In early years we were confident of newt identifications but not confident of the identifications of some larval frogs (notably bullfrog Rana catesbeiana and green frog R. clamitans tadpoles). Thus, we excluded early year identifications in the analyses for bullfrog and green frog tadpoles, but included early year identifications in the analyses for newts, which resulted in different sample sizes (n = 216 vs. n = 333 respectively). After the early years of sampling, tadpoles were identified by an expert herpetologist against Canadian national reference collections.
Fish presence/absence was assigned for each waterbody by scoring fish as “present” if at least one fish was caught in the site in any year of sampling. If we never caught fish in a waterbody across all years of sampling, fish were deemed “absent” for analyses. Few waterbodies sampled in this study supported larger gamefish. Instead, fish assemblages (if present) were dominated by small, gape-limited minnows, and some waterbodies supported yellow perch (Perca flavescens), white sucker (Catostomus commersoni) and brook trout (Salvelinus fontinalis; McNicol et al. 1995a).
Twenty chemical parameters were determined for filtered water samples from each waterbody in most years: pH, conductivity (µS/cm), alkalinity (analogous to acid-neutralizing capacity, µeq/L), calcium (Ca, mg/L), magnesium (Mg, mg/L), sodium (Na, mg/L), potassium (K, mg/L), sulphate (SO4, mg/L), silica dioxide (SiO2, mg/L), chloride (Cl, mg/L), total nitrogen (TN, mg/L), total phosphorus (TP, µg/L), dissolved organic carbon (DOC, mg/L), total inorganic carbon (TIC, mg/L), total aluminum (Al, µg/L), total iron (Fe, µg/L), total manganese (Mn, µg/L), total zinc (Zn, µg/L), total nickel (Ni, µg/L) and total copper (Cu, µg/L). Chemical analysis procedures are described in Mallory et al. (1998).
We examined whether amphibian presence was predicted by acidity, waterbody area and depth, and water column nutrient levels, organic content and solute concentrations. We produced Pearson correlations among all continuous variables to identify collinearity (i.e., high correlations at |r| > 0.50) among potential explanatory variables. Calcium, Mg and Na concentrations were highly correlated with alkalinity and conductivity concentrations (r > 0.66), and TN concentrations were highly correlated with TP concentrations (r > 0.70). Log-alkalinity was highly correlated with pH levels (r > 0.90). Moreover, Al concentrations were highly correlated with pH levels, and Ca, Mg and Na concentrations (Mallory et al. 1998). Thus, to minimize collinearity, we did not include Al, Ca, Cl, Mg, Na, TN and alkalinity as explanatory variables in our generalized linear models. We used conductivity as a surrogate for solute concentrations, TP as a surrogate for nutrient status, pH to measure acidity, DOC as a surrogate for organic content, and SiO2 as a surrogate for the degree of weathering inputs from the watershed, and ran models with these chemicals as predictors to assess their biological relevance.
We compared characteristics of the waterbodies with and without each amphibian species using t tests to assess which variables influence the presence of amphibians. We also summed how many amphibian species were found in each waterbody then grouped waterbodies by species richness into four categories based on the number of amphibian species that we found. Then, we compared characteristics of waterbodies among the four categories using MANOVA to assess amphibian species richness. If significant effects were found, we explored where that variation came from by conducting univariate ANOVA tests for each parameter.
Subsequently, we created generalized linear models (GLM) with binomial distributions, and we chose the best-fitted models using an information-theoretic approach with R software (RStudio version 0.99.491; as shown in Burnham and Anderson 2002, Symonds and Moussalli 2011). We created separate GLMs for the presence of individual species that had adequate presence rates (> 10% of the sites). These models used the mean values of water chemistry and morphometric measurements collected from each of the waterbodies between 1990 and 2008 to determine whether they could explain amphibian occurrence. If amphibians were collected at any time in a waterbody between 1990 and 2008, amphibian use was considered as present. Because there were several well-supported models for each response variable, we used model averaging to identify the strongest explanatory variables, and obtain model average estimates and parameters for competing GLMs (Burnham and Anderson 2002). We defined the global model to include waterbody surface area and maximum depth, and the linear forms of a single chemical parameter chosen to represent acidity, nutrients, organic content, solute concentrations and the degree of weathering inputs. Global models were standardized using the package “arm” in R software. We conducted model averaging using the ‘top model set’ approach (Burnham and Anderson 2002). We calculated models at ∆AICc < 2 (Burnham and Anderson 2002), and their secondary Akaike Information Criterion (AICc) values, delta AICc (∆AICc) values, AICc weights (Wi), number of fitted parameters (K), R2 values, and unconditional model average estimates (with their 95% confidence intervals) were calculated and reported using packages “MuMIn”, “AICcmodavg” and “piecewiseSEM” (Symonds et al. 2011). We considered explanatory variables as “significant” when confidence limits did not include 0.
Results and Discussion
Amphibians were captured in 76% of the permanent waterbodies in central Ontario (n = 333) between 1990 and 2008 (Table S1). We captured the following amphibian species: American toad (Bufo americanus), bullfrog, eastern newt (Notophthalmus viridescens), gray tree frogs (Hyla versicolor), green frog, mink frog (R. septentrionalis), mole salamander (Ambystoma sp.), northern leopard frog (R. pipiens), spring peeper (Pseudacris crucifer), and wood frog (R. sylvatica). American toads, gray tree frogs, northern leopard frogs, spring peepers, and mole salamanders were present in < 2% of the study sites, and may not have been captured well by our methodology (e.g., younger, small amphibians may have moved through the traps) or in these type of waterbodies (see Glooschenko et al. 1992). Thus, we did not further examine characteristics of waterbodies where they occurred, but still included them in the species richness calculations. Wood frogs were found in ~ 5% of the waterbodies. Although we compared waterbody characteristics where wood frogs did and did not occur, data were insufficient to produce reliable models. Green frogs, bullfrogs, mink frogs and eastern newts were present in > 10% of the waterbodies we sampled in central Ontario, and GLMs were created for their presence.
Waterbodies in central Ontario varied considerably in their chemical and physical attributes (Table 1), which have been described previously for Algoma, Muskoka and Sudbury (McNicol et al. 1987, 1995a, b; Mallory et al. 1998), and had pH measurements low enough to negatively affect amphibian breeding (Dale et al. 1985; Glooschenko et al. 1992). They were typically small (71% < 10 ha) and shallow (74% < 10 m maximum depth), and many waterbodies (16%) were highly acidified (pH < 5.1). Most conductivity measurements (95%) were < 50 µS/cm, although some waterbodies had relatively higher levels (up to 149 µS/cm). Most waterbodies (72%) were oligotrophic (TP < 10 µg/L), with the remainder mesotrophic (17%; 10−20 µg/L TP), and meso-eutrophic and eutrophic (11%; >20 µg/L TP; principally waterbodies in the Muskoka region; Canadian Council of Ministers of the Environment 2004).
Minimum pH levels for waterbodies where bullfrogs, green frogs, mink frogs, wood frogs and eastern newts were captured, respectively, were fairly acidic (generally pH < 5). In general, larval amphibians were found in a wide range of waterbodies with varying chemical conditions and physical attributes which in some cases differed from waterbodies where these species were absent (Table 2). Waterbodies where we captured bullfrogs were larger, deeper, and had higher pH levels than those where bullfrogs were absent, whereas green frogs were captured in smaller waterbodies with lower pH and lower conductivity levels than those where they were absent. There were no significant differences in pH levels between waterbodies where mink frogs, wood frogs and eastern newts were present or absent.
The minimum pH level where fish were caught (pH = 4.44) was comparable to minimum pH levels where amphibians were caught; however, fish were captured in only 5 of 44 (11%) waterbodies with pH < 5.0 whereas amphibians were captured in a significantly higher proportion of low-pH waterbodies (30 of 44, 68%; Fisher Exact test, p < 0.001). We caught amphibians in a higher proportion of permanent waterbodies lacking fish (123 of 156; 79%) compared to those where fish were captured (122 of 177; 69% Fisher Exact test, p = 0.046). Thus, amphibians were more commonly found in low pH waterbodies than small fish (also see Lacoul et al. 2011).
All eastern newt and mink frog models were inadequate due to low R2 values (< 0.10) and/or underdispersion, and were not considered further. For green frogs, pH was not a significant predictor of green frog presence in our models. Instead, various combinations of chemical and physical parameters were suitable predictors for their presence in the best-fitted models (∆AICc < 2), but variation explained was low overall (Tables S2, S3). Although pH was a significant predictor in the bullfrog models, pH was not a dominant predictor of bullfrog presence (counter to some of the earlier work on amphibian presence in this landscape; e.g., Clark 1992), and other explanatory variables often had a strong influence (e.g., conductivity; Tables S4, S5).
We grouped the waterbodies into four categories based on the number of amphibian species that we found: none (n = 81), one (n = 93), two (n = 24), three or more (n = 18), and compared overall chemical and physical waterbody characteristics by category, which suggested a significant difference among richness categories (MANOVA; Wilk’s Lambda test criterion 0.8, F1,24 = 1.7, p = 0.02). However, the only parameter that strongly influenced variation among richness groups was DOC (F3,212 = 3.08, p = 0.029); all other ANOVA univariate comparisons were non-significant (all F3,212 ≤ 1.88, all p ≥ 0.13). Amphibian species richness was not well discriminated by waterbody area, depth or chemical characteristics except that richness differed in a non-linear manner among waterbodies with different DOC concentrations (Fig. S1), somewhat counter to Glooschenko et al. (1992) who showed reduced amphibian occurrence with higher DOC.
Overall, our regional results suggest that pH and alkalinity may not be the most important factors affecting larval amphibian presence in permanent waterbodies and that some amphibian species can tolerate acidic conditions, which is consistent with work from other areas (Dale et al. 1985; Hecnar and M’Closkey 1996; Lacoul et al. 2011), and consistent among models across and within regions (i.e., Algoma, Muskoka, Sudbury). We cannot discount the possibility that there are synergistic, negative effects of low-pH waterbodies that negatively influence amphibians, however, such as reduced cations entering watersheds (Jeziorski et al. 2008) and increased concentrations of toxic elements like Al (Glooschenko et al. 1992; Jung and Jagoe 1995). Our data support the assertion that the relationship between the presence of amphibians and water chemistry is complex, and that amphibian presence and species richness cannot be predicted on habitat water quality alone due to an array of other biotic and abiotic factors that can directly or indirectly affect habitat requirements (Beebee and Griffiths 2005; Guderyahn et al. 2016). In particular, habitat alteration, degradation and fragmentation, and watershed land-use practices can disrupt aquatic and terrestrial habitat and connectivities for amphibians (e.g., Popescu et al. 2012), and can interfere with critical stages in the amphibian life cycle (Werner et al. 1995; Guderyahn et al. 2016). The watersheds around most of the waterbodies in our study have been subject to some change from forest harvesting, especially around Sudbury where forests were cleared to fuel early metal smelting operations (e.g., Gunn 2012). We also note that the most commonly captured amphibians in the waterbodies we sampled in central Ontario were green frogs and bullfrogs. Both species are habitat generalists, and not highly sensitive to landscape and habitat disturbance (Werner et al. 1995; Jancowski and Orchard 2013), which could allow them to outcompete more sensitive species in our study area, but might also make modelling habitat preferences more difficult.
Our methods employed for capturing amphibians and recording their presence have limitations, including our protocol of using only one trapping technique which was not augmented with other approaches such as calling surveys (Parris 1999). Consequently, additional types of sampling might improve the representation of amphibian occurrence (Beebee and Griffiths 2005) and increase our somewhat low proportion of occurrence (76% of waterbodies). Furthermore, our analyses focused on whether (principally) larval amphibians were captured in the permanent waterbodies (i.e., present or absent); we did not test whether amphibians inhabited the surrounding habitat and attempted to reproduce in the sampled waterbodies. The latter question could be addressed by a survey for egg masses (e.g., Parris 1999; Glooschenko et al. 1992), and a subsequent comparison of where masses were observed and whether larvae hatched and survived. However, we undertook considerable ecological research on most of these waterbodies between 1984 and 2008 (Mallory et al. 1994; McNicol et al. 1995a, b, c), which included focal collections and observations along shorelines. During that research, we did not surmise that we were missing amphibian detections based on observations of egg masses, larvae or adults, nor from listening to amphibian calls. Nonetheless, we recommend additional research to address finer scale issues of possible relationships between environmentally-relevant chemistry and breeding success, as a means of interpreting our results.
Early studies of the central Ontario region clearly linked the presence of various plankton, aquatic macroinvertebrates and fish to changes in waterbody chemistry and acidification (Longcore et al. 1993; McNicol et al. 1987, 1995a, b; Likens et al. 1996), and suggested the same for amphibians (Clark 1992; Glooschenko et al. 1992). However, the relationships between amphibian occurrence, and chemical and physical waterbody attributes may be more multifaceted than for other aquatic biota because most amphibians can cross terrestrial regions and exploit a variety of habitats. In this sense, drawing linkages between changes in habitat characteristics and amphibian presence is challenging, as it is for waterfowl (McNicol et al. 1995c). Our analyses suggest that amphibian distribution among permanent waterbodies in central Ontario is not predominantly influenced directly by acidity, as pH was not a strong predictor of species occurrence, and factors other than water chemistry likely have a stronger effect. Even if our sampling techniques provided some false negatives (i.e., failed to capture amphibians in lakes where they were present), the distribution of larval amphibians that we did capture were such that most species were found in waterbodies with low buffering capacity (low pH and alkalinity), suggesting that earlier studies in central Ontario overstated the negative relationship between lake acidity and amphibian occurrence. The federal acid rain monitoring program ended in the early 2000s, and no one has returned to resurvey our study sites to date. A current effort is underway to conduct such a survey for common loons (Gavia immer) because they are known to be negatively influenced by acid precipitation (McNicol et al. 1995d). Regardless, a new survey for amphibians would help to better understand factors that strongly influence amphibian presence in central Ontario. Future research should focus on long-term habitat change (e.g., resource development, road construction) and connectivity in the watersheds around these waterbodies, as we suspect that these factors may have a stronger influence on amphibian presence or recovery of amphibian populations than water chemistry.
References
Battaglin WA, Smalling KL, Anderson C, Calhoun D, Chestnut T, Muths E (2016) Potential interactions among disease, pesticides, water quality and adjacent land cover in amphibian habitats in the United States. Sci Total Environ 566–567:320–332
Beebee TJC, Griffiths RA (2005) The amphibian decline crisis: a watershed for conservation biology? Biol Conserv 125:271–285
Bradford DF, Gordon MS, Johnson DF, Andrews RD, Jennings WB (1994) Acidic deposition as an unlikely cause for amphibian population declines in the Sierra Nevada, California. Biol Conserv 69:155–161
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Canadian Council of Ministers of the Environment (2004) Phosphorus: Canadian guidance framework for the management of freshwater systems. Environment Canada. http://ceqg-rcqe.ccme.ca/download/en/205
Clark KL (1992) Monitoring the effects of acidic deposition on amphibian populations in Canada. Canadian Wildlife Service Occasional Paper Number 76:44–56
Cook FR (1984) Introduction to Canadian amphibians and reptiles. National Museums of Canada, Ottawa, p 200
Dale JM, Freedman B, Kerekes J (1985) Acidity and associated water chemistry of amphibian habitats in Nova Scotia. Can J Zool 63:97–105
Ficetola GF, De Bernardi F (2004) Amphibians in a human-dominated landscape: the community structure is related to habitat features and isolation. Biol Conserv 119:219–230
Freda J (1986) The influence of acidic pond water on amphibians: a review. Water Air Soil Pollut 30:439–450
Freda J, Sadinski WJ, Dunson WA (1991) Long term monitoring of amphibian populations with respect to the effects of acidic deposition. Water Air Soil Pollut 55:445–462
Glooschenko V, Weller WF, Smith PGR, Alvo R, Archbold JHG (1992) Amphibian distribution with respect to pond water chemistry near Sudbury, Ontario. Can J Fish Aquat Sci 49:114–121
Guderyahn LB, Smithers AP, Mims MC (2016) Assessing habitat requirements of pond-breeding amphibians in a highly urbanized landscape: implications for management. Urban Ecosyst 19:1801–1821
Gunn JM (ed) (2012) Restoration and recovery of an industrial region: progress in restoring the smelter-damaged landscape near Sudbury, Canada. Springer Science & Business Media
Hecnar S, M’Closkey R (1996) Amphibian species richness and distribution in relation to pond water chemistry in South-Western Ontario, Canada. Freshw Biol 36:7–15
Jancowski K, Orchard S (2013) Stomach contents from invasive American bullfrogs Rana catesbeiana (= Lithobates catesbeianus) on southern Vancouver Island, British Columbia, Canada. NeoBiota 16:17–37
Jeffries DS, Clair TA, Couture S, Dillon PJ, Dupont J, Keller W, McNicol DK, Turner MA, Vet R, Weeber R (2003) Assessing the recovery of lakes in southeastern Canada from the effects of acidic deposition. Ambio 32:176–182
Jeziorski A, Yan ND, Paterson AM, DeSellas AM, Turner MA, Jeffries DS, Keller B, Weeber RC, McNicol DK, Palmer ME, McIver K (2008) The widespread threat of calcium decline in fresh waters. Science 322:1374–1377
Jung RE, Jagoe CH (1995) Effects of low pH and aluminum on body size, swimming performance, and susceptibility to predation of green frog (Hyla cinerea) tadpoles. Can J Zool 73:2171–2183
Keller W, Heneberry JH, Edwards BA (2018) Recovery of acidified Sudbury, Ontario Canada, lakes: a multi-decade synthesis and update. Environ Rev 27:1–16
Lacoul P, Freedman B, Clair T (2011) Effects of acidification on aquatic biota in Atlantic Canada. Environ Rev 19:429–460
Likens GE, Driscoll CT, Buso DC (1996) Long-term effects of acid rain: response and recovery of a forest ecosystem. Science 272:244
Longcore JR, Boyd H, Brooks RT, Haramis GM, McNicol DK, Newman JR, Smith KA, Stearns F (1993) Acidic depositions: effects on wildlife and habitats. Wildlife Soc Tech Rev 93–1:42
Mallory ML, Blancher PJ, Weatherhead PJ, McNicol DK (1994) Presence or absence of fish as a cue to macroinvertebrate abundance in boreal wetlands. Hydrobiologia 279/280:345–351
Mallory ML, McNicol DK, Cluis DA, Laberge C (1998) Chemical trends and status of small lakes near Sudbury, Ontario, 1983–1995: evidence of continued chemical recovery. Can J Fish Aquat Sci 55:63–75
McNicol DK, Bendell BE, Ross RK (1987) Studies of the effects of acidification on aquatic wildlife in Canada: waterfowl and trophic relationships in small lakes in northeastern Ontario. Canadian Wildlife Service Occasional Paper Number 62, p 76
McNicol DK, Bendell BE, Mallory ML (1995a) Evaluating macroinvertebrate responses to recovery from acidification in small lakes in Ontario, Canada. Water Air Soil Pollut 85:451–456
McNicol DK, Mallory ML, Wedeles CHR (1995b) Assessing biological recovery of acid-sensitive lakes in Ontario, Canada. Water Air Soil Pollut 85:457–462
McNicol DK, Ross RK, Mallory ML, Brisebois LA (1995c) Trends in waterfowl populations —evidence of recovery from acidification. Ch. 16. In: Gunn J (ed) Restoration and recovery of an industrial region. Springer, New York, pp 205–217
McNicol DK, Mallory ML, Vogel HS (1995d) Using volunteers to monitor the effects of acid precipitation on common loons (Gavia immer) in Canada: the Canadian Lakes Loon Survey. Water Air Soil Pollut 85:463–468
Parris KM (1999) Amphibian surveys in forests and woodlands. Contemp Herpetol 1:1–14
Popescu VD, Patrick DA, Hunter ML, Calhoun AJK (2012) The role of forest harvesting and subsequent vegetative regrowth in determining patterns of amphibian habitat use. Forest Ecol Manage 270:163–174
Symonds MRE, Moussalli A (2011) A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav Ecol Sociobiol 65:13–21
Werner EE, Wellborn GA, McPeek MA (1995) Diet composition in postmetamorphic bullfrogs and green frogs: implications for interspecific predation and competition. J Herpetol 29:600
Wetzel RG (1983) Limnology, 2nd edn. Saunders College Publishing, Toronto
Acknowledgements
We thank Dr. Francis Cook and Ross MacCulloch for amphibian species identifications, Environment Canada for water analyses, Don McNicol and numerous field assistants for assistance with the field work, and Elyse Howat for the map. Financial support was provided by Environment Canada and Acadia University. We thank anonymous referees and the BECT Editorial Board for insightful comments on an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Loder, A.L., Weeber, R., Wong, S.N.P. et al. Correlates of Waterbody Characteristics and the Occurrence or Diversity of Larval Amphibians in Central Ontario, Canada. Bull Environ Contam Toxicol 103, 571–578 (2019). https://doi.org/10.1007/s00128-019-02698-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-019-02698-8