Abstract
Anuran amphibians are highly dependent on aquatic ecosystems. Many amphibian species are exhibiting population declines primarily due to habitat destruction and water quality degradation as a result of urban expansion. The objective of this study was to examine combined effects of habitat degradation and water quality on amphibian assemblages in rivers affected by urban impacts. Twelve sites along three rivers were characterized in regard to urbanization and habitat condition using the calculation of a Habitat Model Affinity (HMA) score. Fifteen water quality parameters were assessed at each site. A Simplified Index of Water Quality (SIWQ) and a general Water Quality Index (WQI) were applied. Species richness and relative abundance of amphibians were estimated from visual encounter and calling surveys during summer season between 2009 and 2013. Species richness and abundance were negatively correlated with phosphate, nitrate concentrations and total coliforms, and positively correlated with HMA, electrical conductivity and dissolved oxygen. Species richness was also affected negatively by turbidity. Principal component analysis showed that sites with higher amphibian community metrics were also the ones with lower nutrient levels and better habitat conditions. This study identified important water quality parameters affecting amphibians in rivers with increasing urban impact; and provides information that can be used in the design of strategies to minimize the impacts of urbanization on aquatic biodiversity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urbanization is one of the major drivers causing the transformation of natural habitats (Calderon et al. 2014). Urban expansion is essentially the replacement of natural cover with impervious surfaces (Miller et al. 2014), resulting in habitat loss and fragmentation (McKinney 2008), as well as increasing soil compaction and runoff (Chen et al. 2017). Increased urban runoff causes major changes in the hydrological, chemical and physical characteristics of urban waterways (Walsh et al. 2005; Zhu et al. 2009), threatening the survival of many aquatic plant and animal species (Hamer and McDonnell 2008). Aquatic systems exposed to watershed stressors in the form of nonpoint source pollution, i.e. urban runoff produced by water flowing on the surface dissolving and washing away pollutants and soil sediments along its path, have shown lower water quality and have been linked to lower species diversity and changes in the ecosystem structure in general (Taebi and Droste 2004; Helms et al. 2005; Camargo et al. 2004; Fabricius et al. 2012; González et al. 2014; Calderon et al. 2017). Moreover, urban waterbodies are also exposed to point source of pollutants such as discharges from sewage and wastewater treatment plants (Taebi and Droste 2004).
Worldwide, amphibian species are declining more rapidly than any other group of animals (Garcia-Gonzalez and Garcia-Vazquez 2012). There are several contributing factors to the decline of amphibians including habitat degradation and alteration (Cushman 2006), disease and pathogens (Rollins-Smith 2009), global warming (Pounds et al. 2006), physiological effects due to the increase of chemical contaminants in water (Sowers et al. 2009), altered hydrology, and low water quality (Carey and Bryant 1995; Welsh and Ollivier 1998; McKibbin et al. 2008). Most anuran amphibians spend their first development stages in aquatic environments and therefore they are exposed to pollutants dissolved in water (Stebbins and Cohen 1997). Due to the complexity of their life cycle in both aquatic and terrestrial environments, and their permeable skin they can be susceptible to the decline of water quality (Boyer and Grue 1995) resulting from urbanization activities.
The effect of pollutants on amphibians has been widely documented: high values of electrical conductance, high concentrations of nitrates, nitrites, total phosphates, chloride, un-ionized ammonium, and trace metals, extreme values of pH, high concentrations of organochlorine and organophosphate pesticides, pharmaceuticals, high levels of organic matter, and low concentration of dissolved oxygen have been associated with low densities of egg masses, low hatching success, larvae survival, and development rate (Morin 1981, 1983; Laposata and Dunson 2000; De Solla et al. 2002a, b; Sowers et al. 2009; Dodd 2010; Babini et al. 2018). Therefore, the exposure to contaminants from urban runoff is a possible cause of population decline in amphibians (Castaneda 2014).
Amphibian species show specific microhabitat requirements (Stebbins and Cohen 1997) and human activities affect natural habitats in many ways. The alteration of the physical structure of habitats is one of the five factors affecting the structure and composition of resident biological communities (Karr 1991). Thus, the disturbance of physical habitats in aquatic environments influences amphibian communities as much as any other source of pollution and, often, inadequate habitat conditions can obscure the effects of pollutants (Barbour et al. 1999).
Urbanization has evident effects on amphibian anuran community structure. Species richness, density and diversity has been shown to have a negative relationship with increasing urban cover (Rubbo and Kiesecker 2005; Pillsbury and Miller 2008). Pillsbury and Miller (2008) found that anurans associated with short hydroperiods, early breeding activity, and substantial upland habitat use were the most affected by urbanization. Furthermore, Riley et al. (2005) found that streams in more developed watersheds showed a higher presence of exotic species and fewer native amphibians, effects that seemed particularly evident above 8% urban development.
In the last 25 years, the province of San Luis, Argentina has experienced an accelerated urban growth. According to the Instituto Nacional de Estadística y Censos (2010), the total populations of three of the most touristic localities, Potrero de los Funes, El Trapiche, and El Volcán, have risen from 410, 501 and 769 in 1991 to 1698, 1200 and 1775 respectively in 2010. The aforementioned localities are characterized by a high influx of tourists yearround, with the highest concentrations during summer months due to the presence of a relatively high-gradient river that flows through each town. Thus, these areas are perfect for the study of the effect of water quality and habitat degradation on amphibian communities in an urban context on a smaller scale.
The objectives of this study were: (i) to determine water quality and habitat condition in sites from three rivers affected by urban impacts in San Luis sierras, and (ii) to examine effects of habitat degradation and water quality on amphibian assemblages from these rivers. Since amphibian communities of urbanized aquatic environments are affected by water quality deficiency and impaired habitat conditions, we expect that sites in more impacted areas of the rivers will display a decrease in water quality and higher habitat degradation, resulting in a reduction of the richness and abundance of amphibians.
Methods
Study sites
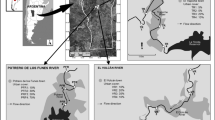
This studied was performed in San Luis province, Argentina. The province is located between the 31° and 36° parallels of South latitude and the 64° and 67° meridians of East longitude. Three rivers affected by human activity were selected: Trapiche River (TR), Volcán River (VR) and Potrero de los Funes River (PFR) (Fig. 1). The urbanization gradient was established at each river as a percent of urban land use (buildings, roads, and recreational infrastructure) in a radius of 150 m around each sampling plot. Four sampling sites were set along TR, PFR and VR and were arranged along a gradient of increasing urban land use (Table 1).
Assessment of stream reach physical habitat characteristics
A complete habitat assessment of the stream corridor condition was conducted at each site. The method used follows EPA’s Rapid Habitat Assessment protocol outlined by Barbour et al. (1999) for high gradient streams. The assessment characterizes the existence and severity of habitat degradation and identifies their sources and causes. Ten different habitat characteristics were assessed at each site and given a score on a scale of 0–20; 0 being poor and 20 being optimal. The characteristics included were epifaunal substrate/available cover, embeddedness, velocity/depth regime, sediment deposition, channel flow status, channel alteration, frequency of riffles, bank stability, vegetative protection, and riparian vegetative zone width. Interpretation of habitat assessment results were conducted through the calculation of Habitat Model Affinity (HMA) for high gradient streams (NYSDEC 2012). In this case, the HMA for high gradient streams was calculated based on comparison to a theoretical reference condition habitat model. The percentage of similarity between the HMA scores for the model condition and the HMA scores for each site classified the reaches into categorical assessments of habitat condition: natural (80–100%), altered (70–79%), moderate (60–69%), and severe (<60%).
Monitored physical–chemical parameters and analytical methods
Water samples were taken at the beginning, middle, and end of amphibians’ breeding season, between 2009 and 2013. The samples for the physical-chemical analysis were collected in glass containers of 2 L capacity, and for the analysis of dissolved oxygen, it was taken separately in a Winkler flask. Samples were collected at an equidistant depth between the bottom and the surface at the center of the river channel. For bacteriological analysis the water sample was taken aseptically with a sterilized 1 L glass bottles with screw cap, at a depth of about 15–30 cm from the surface. Water samples were transported within 2 h after collection and they were kept at ±5 °C until analysis within 6 h after collection. The preservation, transportation and analysis were performed following the standard protocols of the Standard Methods for the Examination of Water and Wastewater (APHA 2005) at the Instituto de Química San Luis (INQUISAL) lab of the Universidad Nacional de San Luis. The data set contained 15 parameters: temperature (T), pH, electrical conductivity (EC), dissolved oxygen (DO), turbidity (Tbd), total hardness (TH), biochemical oxygen demand (BOD), chemical oxygen demand (COD), chloride (Cl−), phosphate (PO43−), sodium (Na), nitrate (NO3−), total suspended solids (TSS), total coliforms (TC) and, fecal coliforms (FC). T, EC and pH were measured in situ using a portable waterproof meter, Oakton WD-35431-03. All the water quality parameters were expressed in milligram per liter, except pH, EC (μS cm−1), turbidity (Nephelometric Turbidity Units, NTU), TC and FC (MPN/100 mL).
A Simplified Index of Water Quality (SIWQ) was used to estimate water quality of each site (Queralt 1982). This index uses a simple algorithm that requires the assessment of five chemical parameters: T, COD, TSS, DO and EC (Broggi Colman and Bellagamba 2006). The final score was calculated from the formula: SIWQ = T (A + B + C + D). The variable A fluctuates as a function of chemical oxygen demand, assuming values between 0 and 30, and it is a measure of natural or artificial organic content, either biodegradable or not. B is a function of total suspended solids; it ranges from 0 to 25 and quantifies filterable particles, from organic, inorganic, industrial, and urban sources. The value C is a function of dissolved oxygen; it varies between 0 and 25 and is related to oxidability and to biodegradable organic matter content. D depends on electrical conductivity; it can take values between 0 and 20 and it is related to inorganic salts concentration, mainly chloride, sulfate, calcium, and sodium. The outcome of the equation is an overall water quality dimensionless value that can range from 0 to 100. SIWQ quantifies water quality for different uses, from the best to the poorest quality: all uses (100–85), swimming (85–75), fishing (75–60), navigation (60–45), crop irrigation (45–30) forestry irrigation (30–15), and no use allowed (15–0).
The Water Quality Index (WQI) (Mingo Magro 1981) was also calculated in order to obtain a value that not only integrates the physical-chemical characteristics of the water quality, but also the nutrient load levels and microbiological aspects. The WQI was calculated using twelve basic parameters for water quality characterization: DO, TSS, pH, EC, COD, BOD, Cl−, Na, TH, TC, PO43− and NO3−. The final score was calculated from the equation:
- Qi:
-
water score quality of parameter i. It is a non-dimensional value or quality level obtained through a mathematical equation or its corresponding graphic representation specific for each parameter.
- Wi:
-
weighting factor that reflects the importance of that parameter in the quality classification as follows:
- ai:
-
Coefficient that vary from 1 (very important parameter) to 4 (insignificant parameter) for the determination of water quality affected by natural or artificial contaminants. The values for ai for each parameter are described in Mingo Magro (1981).
The WQI is defined as the degree of contamination in the water of the sample expressed as a percentage of pure water. Thus, the WQI quantifies water quality as follows: Excellent (100–90), good (89–80), intermediate (79–70), acceptable (69–60) and unacceptable (59–0).
Amphibians surveys
Amphibian species richness was estimated from acoustic and visual encounter surveys. All sites were visited 6 to 12 times throughout the spring and summer seasons, which corresponds to the months of maximum amphibian activity and rainfall. Anuran calls were recorded for 5 min at each site. Two visual encounter surveys plots (100 m × 5 m) were established one in each side of the channel. After the calling registration and recording, observers searched the plots while walking at a standard pace using as much time as was needed to examine each area thoroughly. Data from the visual surveys was just used to contribute to the measure of species richness but were not included in statistical analysis including abundance measures.
In accordance with North American Amphibian Monitoring Program protocol, surveys were conducted at least 0.5 h after dusk and completed by 01:00 (Weir and Mossman 2005). The call index, proposed by Pillsbury and Miller (2008), was used as an indicator of relative abundance of amphibians, as follows: 0, no individuals of a given species heard; 1, one individual heard; 2, multiple individuals with no overlap in calls; 3, full chorus. Maximum relative abundance per survey and site was included in statistical comparisons.
Data analysis
The significance of differences in SIWQ, WQI, average amphibian relative abundance and richness values were analyzed using non-parametric Kruskal–Wallis test of differences in means as well as Dunn’s pairwise comparison test, using a significance level of α = 0.05. Spearman correlation analysis was performed to analyze the relations between habitat condition, physical-chemical water quality parameters and amphibian community metrics. Principal Component Analysis (PCA) was performed extracting significant PCs and to further reduce the contribution of variables with minor significance, in order to visualize the grouping of sites according to amphibian metrics and environmental variables (habitat and water quality) (Helena et al. 2000; Zhou et al. 2007; Bagur et al. 2009). Data were transformed before running this analysis and variables included were amphibian richness and abundance, WQI, HMA, nitrate and phosphate concentrations and electrical conductivity.
Results
HMA and physical-chemical water quality
Table 2 summarizes briefly the mean value and standard error of the 15 physical-chemical parameters assessed during the amphibian survey seasons. HMA values varied among sites along every category possible within the index, from natural to severely disturbed. SIWQ and WQI also varied among sites (Table 3).
SIWQ values were significantly different among rivers based on Kruskal–Wallis test (p < 0.01). Dunn’s pairwise comparison test revealed that SIWQ was significantly higher for TR compared with PFR (p < 0.05) and VR (p < 0.01). Significant differences were found among sites for TR and PFR, but no significant differences were found among sites at VR (p > 0.01) (Fig. 2a).
Boxplots depicting the mean, 25th and 75th quartiles (shaded boxes) and the largest and smallest values for the (a) Simplified Water Quality Index and (b) Water Quality Index at all sampling sites. Letters in bold indicate the differences among rivers. Boxes that share a letter do not differ significantly using Dunn’s tests and α = 0.05
WQI values were significantly different among rivers (p < 0.01). WQI was significantly higher for TR compared with VR (p < 0.01) and showed a tendency when compared with PFR (p < 0.1). Significant differences were found among sites for TR (p < 0.001) and for PFR (p < 0.01), but no significant differences were found among sites in VR (p > 0.01) (Fig. 2b).
Amphibian metrics
Five species of anuran amphibians were detected in the urbanized rivers studied: Rhinella arenarum, Odontophrynus occidentalis, Leptodactylus mystacinus, Boana cordobae and Boana pulchellus (Table 4).
Species richness values were significantly different among rivers. The mean value for richness was 0.839 (range 1.538–0.154) for TR, 0.667 (range 0.500–0.833) for PFR and 1.773 (range 1.429–2.200) VR. Dunn’s pairwise comparison test confirmed that amphibian species richness was higher in VR compared with TR and PFR (p < 0.001). No significant differences were identified between TR and PFR (p > 0.01). Significant differences in species richness were found among sites within TR (p < 0.01). TR4 was the site with the lowest richness compared with TR1 and TR2 (p < 0.01). No significant differences were identified between TR4 and TR3 (p > 0.01). No significant differences were found among sites in PFR and VR (p > 0.01) (Fig. 3a).
Boxplots depicting the mean, 25th and 75th quartiles (shaded boxes) and the largest and smallest values for (a) amphibians richness and (b) amphibians relative abundance at all sampling sites. Letters in bold indicate the differences among rivers. Boxes that share a letter do not differ significantly using Dunn’s tests and α = 0.05
Significant differences in amphibians average relative abundance were found among rivers (p < 0.01). Abundance mean values were 0.261 (range 0.058–0.481) for TR, 0.240 (range 0.125–0.292) for PFR and 1.11 (range 0.571–1.667) for VR. Dunn’s pairwise comparison test confirmed that VR had a higher amphibian abundance compared with TR and PFR (p < 0.001). No significant differences were found between TR and PFR (p > 0.01). No significant differences were found within TR, PFR and VR sites (p > 0.01) (Fig. 3b).
Relationships between amphibian community metrics, habitat model affinity, and physical-chemical water quality
Correlation analysis found that PO43−, NO3−, turbidity, TC, DO, HMA and EC were the only significant variables influencing amphibian community metrics. Species richness showed a negative correlation with PO43− (r = −0.409; p < 0.05) and NO3− (r = −0.572; p < 0.01) and a weaker, but significant correlation with turbidity (r = −0.386; p < 0.05) and TC (r = −0.373; p < 0.05). Amphibians richness was positively correlated with DO (r = 0.455; p < 0.01), HMA (0.382; p < 0.05) and EC (0.371; p < 0.01). Average abundance of amphibians was negatively correlated with PO43− (r = −0.389; p < 0.05) and NO3− (r = −0.373; p < 0.01) and TC (r = −0.330; p < 0.05) while it was positively correlated with EC (r = 0.508; p < 0.01) and DO (r = 0.397; p < 0.05). HMA negatively correlated with PO43− (r = −0.534; p < 0.01) and TC (r = −0.581; p < 0.01) and showed a positive correlation with DO (r = 0.412; p < 0.01), and WQI (r = 0.400; p < 0.01). Amphibians species richness and average abundance were positively correlated (r = 0.846; p < 0.01). No significant correlation was found among amphibians metrics and WQI, SIWQ and other water quality measurements (p > 0.05).
PCA analysis reduced the number of parameters (7) that explain most of the variance of the experimental data set. The two first principal components (PC1 and PC2) retained 78.51% of the variability of the system (rivers environmental quality data and amphibians metrics), according to the eigenvalue-one criterion (variances greater than 1) (Table 5). PC1, which explained 50.33% of the variance, had strong loadings (> 0.65) of amphibian richness, and phosphate and nitrate concentrations, so it is negatively driven by nutrient enrichment. PC2, explaining 28.18% of the variance, was mainly determined by water quality (Fig. 4).
Discussion
All the sites explored in this study showed some degree of impairment in regard to water quality and habitat. SIWQ and WQI indicated that water quality of the sites located in TR were better in comparison with the sites in PFR and VR. SIWQ and WQI showed a high positive correlation which indicates either of them could be used to express the water quality of an aquatic ecosystem. A significant correlation was expected since both indices are calculated based on many of the same parameters. The main difference between both indices is that WQI considers variables related with waste water inputs such as organic pollution, nutrient enrichment, and bacterial load. Therefore, WQI has the robustness of indicate water quality in a more comprehensive way (Chica-Olmo et al. 2005; Almeida et al. 2007). Yet, SIWQ constitutes a fair indicator of water quality with lower costs and equipment requirements. Water quality indices are used to encompass magnitudes of several parameters and transform the values to a non-dimensional number, allowing the comparison of the spatial and temporal variability of rivers and trends (Cude 2001). No significant correlation was found between water quality indices and amphibians metrics. This lack of relationship was not entirely unexpected since water quality indices often incur the loss of valuable information about individual variables and their interactions (Almeida et al. 2012), making it necessary to evaluate the effects of individual parameters on biota. Volcán River had lower values of WQI and SIWQ, and at the same time had higher values of species richness and abundance than the other rivers. However, when evaluating the relationship between amphibian metrics and individual water quality parameters, clearer results were obtained.
A negative relationship was found between the concentration of phosphate and amphibian richness and abundance. There is still no agreement regarding the effects of phosphate on amphibian populations. Literature suggests that, at environmentally relevant concentrations, phosphate effects on amphibians are likely indirect and related to nutrient enrichment and anoxia due to eutrophic conditions (De Solla et al. 2002b; Hamer et al. 2004; Earl and Whiteman 2009). Further research about the sensitivity of the local species to phosphate and the mechanisms involved is needed. The available information indicates that amphibian response to the concentration of phosphate in water depends on more aspects than the phosphate concentration itself.
As expected, species richness and abundance were negatively affected by nitrate, which was consistent with observations from other studies. Several negative responses have been related to high concentration of nitrate: lower densities of egg masses, lower hatching success, loss of tadpole body weight, variations of tadpoles feeding time, alteration of swimming patterns, restlessness, paralysis, morphological abnormalities, and lower larvae survival are some of the documented effects (Hatch and Blaustein 2000; Ortiz et al. 2004; Camargo et al. 2005; Smith et al. 2005; Krishnamurthy et al. 2006; Ortiz-Santaliestra et al. 2006; McKibbin et al. 2008; Earl and Whiteman 2009; Oromí et al. 2009). Marco et al. (1999) described that amphibian larvae exposed to different concentration of nitrates suffered a reduction of feeding activity, a decrease in the velocity of swimming, a disequilibrium and paralysis of their bodies, abnormalities and edemas, and eventually died with the increase of nitrate concentration. However, according to Smith et al. (2005), the sensitivity of amphibian larvae is species specific with no generalization in regard to the response of amphibians to nitrate concentration. Rouse et al. (1999) assessed the potential for nitrate to affect amphibian survival in several watersheds of North America. Nitrogen pollution, in this case, came from anthropogenic sources through agricultural runoff or percolation associated with nitrogen fertilization and effluents from industrial and human wastes. They reported that the concentration of nitrate at many sites was within the range of sublethal effects on amphibians (2.5–100 mg L−1). Concentrations detected at PFR were close and, in some sampling events, within the range where amphibians start suffering developmental abnormalities; with some sites directly receiving domestic waste discharges from pipes and septic tanks. It was evident to the authors that, in comparison with VR, amphibian activity was low in sites surveyed at PFR with no activity detected during some surveys.
In this research, conductivity positively correlated with species richness and abundance. Several authors explain the importance of ion concentration in maintaining the osmotic balance between the eggs and the surrounding water (Duellman and Trueb 1994). Water flows through the inner egg membrane into the vitelline chamber providing oxygen to the embryos and flushing away contaminants affecting cell development and functioning (McKibbin et al. 2008). It has been documented that exposure to increased conductivity can affect amphibian behavior, growth and development, increase malformation and decrease larvae survival (Karraker 2007; Karraker et al. 2008; Chambers 2011; Jones et al. 2015). However, most of the previous cited investigations have detected negative effects on amphibians at high ion concentration (mostly around 3000 μS cm−1). The response of amphibians to conductivity varies among species (Viertel 1999; Turtle 2000; Karraker 2007; Klaver et al. 2013) with some of them showing a low tolerance to high conductivity levels whereas others being more tolerant. Yet, when the effect of conductivity on amphibians is apparently species specific, in our area of study water conductivity varied between 150 and 800 μS cm−1 and the sites with higher conductivity level (695–781 μS cm−1) showed higher richness and abundance. This could suggest that the species of the area are tolerant to relatively high ion concentration.
A negative association was found between amphibian metrics and turbidity. Turbidity constitutes a valid and useful water quality measurement that can be used to protect aquatic habitats from sediment pollution (Lloyd 1987). Therefore, the authors used turbidity as an indicator of habitat degradation. Increased sedimentation and siltation often occurs as a consequence of harvesting, road building, mining, urban activity, agriculture, and grazing; resulting in harm to natural habitats, fish, and other aquatic life, and could also impact the hatching success of amphibian embryos. The negative impacts of sediments on aquatic organisms, including amphibians, are well documented (Henley et al. 2000; Rowe et al. 2003; Canals et al. 2011). Suh (2016) reported that the survivorship and the ability for the pacific tree frog Pseudacris regilla tadpoles to find cover in the presence of an invasive predator were diminished in more turbid waters. Thus, a higher predation on tadpoles by native or introduced predators could be expected in areas with lack of a land management plan intended to decrease the effect of erosion and sedimentation in urban rivers.
A weak but significant negative correlation was found between amphibian metrics and TC. It has been widely documented the susceptibility of amphibians to pathogens, including water molds, viruses and mainly Chytridiomycosis disease, caused by the fungus Batrachochytrium dendrobatidis (Blaustein et al. 2003; Skerratt et al. 2007; Gray et al. 2009; Rollins-Smith 2009; Martel et al. 2013; Olson et al. 2013; Berger et al. 2016). However, the literature pertaining to the relationship between amphibians and Total Coliforms (TC) is insufficient. Canals et al. (2011) studied the implementation of livestock ponds as artificial wetlands that could be used by amphibian for reproduction. They found that there was no effect of bacterial load on amphibians since, in their specific case, the period of reproduction was before the peak of contamination. Further research is needed to determine if there is an effect of bacterial load on amphibian species.
A positive correlation was found between DO and amphibian metrics. PFR has the lower values of DO compared with TR and VR, and was also the river with the lower values of richness and abundance of amphibians. However, the values of DO detected in PFR sites and TR4 were above the ones reported as critical by other authors (Wassersug and Seibert 1975; Marian et al. 1980; Sparling 2010). According to literature, the optimal range of DO for amphibians varies among species and life history traits. Low levels of DO concentration can be detrimental to amphibian embryonic development (Wassersug and Seibert 1975; Noland and Ultsch 1981; Schmutzer et al. 2008; Bernal et al. 2011) also affecting egg masses production (Karraker et al. 2008), hatching success, development rate, variations in swimming patterns, and behavior changes that could lead to a higher predation risk (Seymour et al. 2000; Warkentin 2002).
Most of the assessed sites showed some degree of impairment habitat wise, with some of them being severely degraded. On average, VR showed more conserved habitats with higher scores for vegetative cover, riparian width and bank stability categories. Amphibian species show specific microhabitat requirements (Stebbins and Cohen 1997). Amphibians are highly specialized in their uses of lotic microhabitats and terrestrial surroundings for foraging, cover, shelter from predators, overwintering, reproduction, egg laying and development of larvae (Welsh and Ollivier 1998; Semlitsch 2000). HMA takes into account aspects considered important for amphibians, such as water velocity regime, available cover, sediment deposition, vegetative protection of the banks and riparian vegetation width (Porej et al. 2004; Baldwin et al. 2006; Barrett et al. 2010; Kupferberg et al. 2011). Thereby, HMA could be used as a suitable indicator to evaluate the quality of habitat used by amphibians.
The three species detected in VR, Rhinella arenarum, Odontphrynus occidentalis and Boana pulchellus, are species frequently found in highly modified and degraded environments (Agüero et al. 2010; Jofré et al. 2010; Calderon et al. 2014, 2017). The species recorded in PFR and TR included Rhinella arenarum, Odontphrynus occidentalis, Leptodactylus mystacinus, Boana pulchellus and Boana cordobae (endemic species of the Sierras Pampeanas Centrales System (Lescano et al. 2015)). Even when the total richness of TR and PFR rivers were higher than in VR, the activity of the amphibians of VR was more constant throughout the reproduction season. Water characteristics and habitat features could provide amphibians in VR with a habitat stable enough to allow reproduction and development of tadpoles (Villegas Ojeda et al. 2016). Further research is needed, however our data suggests that Odontphrynus occidentalis and Boana cordobae could be less tolerant to the impacts of urbanization as it impacts habitat and water quality.
Several factors have been identified to affect the reproduction behavior of amphibians, apart from the water quality assessed here: temperature and rainfall regime, the nutritional state of females (Duellman and Trueb 1994), stress (Moore and Jessop 2003; Carr 2011), non-native predators of larvae, pesticides input (Davidson et al. 2002; De Solla et al. 2002b), heavy metals (Carr and Patiño 2011), environmental toxicants and their byproducts (Carey and Bryant 1995; Boyer and Grue 1995), pharmaceuticals and, organic pollutants such as Polychlorinated Biphenyls (PCBs), dioxins, and furans, among others (De Solla et al. 2002b; Sparling 2010). However, none of the aforementioned water quality parameters are relevant for the studied area since none have been detected during previous campaigns, which is why they were not included in this study.
Conclusion
Amphibian abundance and richness were negatively affected by the concentration of nitrate, phosphate, turbidity, bacterial load, and the level of habitat degradation. The conservation of amphibians is essential for the conservation of biodiversity since they are major contributors to biomass and the regulation of the trophic structure within ecosystems. Natural lands are increasingly transformed into urban areas, so the determination of the water quality and habitat requirements for amphibians is necessary for the protection of the species persisting in urban settings. This study is intended to provide a first approach to identify the most important water quality aspects affecting amphibians in expanding urban areas; and provides useful information for the design of strategies to minimize the impacts of urbanization on aquatic biodiversity.
References
Agüero NS, Moglia MM, Jofré MB (2010) ¿Se relaciona el patrón de abundancia y distribución de anuros con la estructura de las comunidades de plantas en hábitats acuáticos de la ciudad de San Luis (Argentina)? Neotrop Biol Conserv 5(2)
Almeida CA, Quintar S, González P, Mallea MA (2007) Influence of urbanization and tourist activities on the water quality of the Potrero de los Funes River (San Luis–Argentina). Environ Monit Assess 133:459–465
Almeida C, González SO, Mallea M, González P (2012) A recreational water quality index using chemical, physical and microbiological parameters. Environ Sci Pollut Res 19:3400–3411
American Public Health Association - APHA (2005) Standard methods for the examination of water and wastewater, 21st edn. American Public Health Association, Washington DC, pp 1220
Babini MS, de Lourdes BC, Salinas ZA et al (2018) Reproductive endpoints of Rhinella arenarum (Anura, Bufonidae): populations that persist in agroecosystems and their use for the environmental health assessment. Ecotoxicol Environ Saf 154:294–301
Bagur M, Morales S, López-Chicano M (2009) Evaluation of the environmental contamination at an abandoned mining site using multivariate statistical techniques—the Rodalquilar (Southern Spain) mining district. Talanta 80:377–384
Baldwin RF, Calhoun AJ, deMaynadier PG (2006) Conservation planning for amphibian species with complex habitat requirements: a case study using movements and habitat selection of the wood frog Rana sylvatica. J Herpetol 40:442–453
Barbour MT, Gerritsen J, Snyder BD et al (1999) Rapid bioassessment protocols for use in streams and wadeable rivers: periphyton, benthic macroinvertebrates and fish. US Environmental Protection Agency, Office of Water Washington, DC
Barrett K, Helms BS, Guyer C, Schoonover JE (2010) Linking process to pattern: causes of stream-breeding amphibian decline in urbanized watersheds. Biol Conserv 143:1998–2005
Berger L, Roberts AA, Voyles J, Longcore JE, Murray KA, Skerratt LF (2016) History and recent progress on chytridiomycosis in amphibians. Fungal Ecol 19:89–99
Bernal MH, Alton LA, Cramp RL, Franklin CE (2011) Does simultaneous UV-B exposure enhance the lethal and sub-lethal effects of aquatic hypoxia on developing anuran embryos and larvae? J Comp Physiol B 181:973–980
Blaustein AR, Romansic JM, Kiesecker JM (2003) Ultraviolet radiation, toxic chemicals and amphibian population declines. Divers Distrib 9:123–140
Boyer R, Grue CE (1995) The need for water quality criteria for frogs. Environ Health Perspect 103:352–357
Broggi Colman G, Bellagamba J (2006) Calidad de agua de cursos en el Uruguay y análisis de normativa vigente. Congreso Interamericano de Ingeniería Sanitaria y Ambiental, 30. AIDIS, pp. 1-11
Calderon MR, González P, Moglia M, Oliva Gonzáles S, Jofré M (2014) Use of multiple indicators to assess the environmental quality of urbanized aquatic surroundings in San Luis, Argentina. Environ Monit Assess 186:4411–4422
Calderon M, Moglia M, Nievas R et al (2017) Assessment of the environmental quality of two urbanized lotic systems using multiple indicators. River Res Appl 33:1119–1129
Camargo JA, Alonso A, De La Puente M (2004) Multimetric assessment of nutrient enrichment in impounded rivers based on benthic macroinvertebrates. Environ Monit Assess 96:233–249
Camargo JA, Alonso A, Salamanca A (2005) Nitrate toxicity to aquatic animals: a review with new data for freshwater invertebrates. Chemosphere 58:1255–1267
Canals RM, Ferrer V, Iriarte A, Cárcamo S, Emeterio LS, Villanueva E (2011) Emerging conflicts for the environmental use of water in high-valuable rangelands. Can livestock water ponds be managed as artificial wetlands for amphibians? Ecol Eng 37:1443–1452
Carey C, Bryant CJ (1995) Possible interrelations among environmental toxicants, amphibian development, and decline of amphibian populations. Environ Health Perspect 103:13
Carr JA (2011) Stress and reproduction in amphibians. Amphibians. Elsevier, Hormones and Reproduction of Vertebrates, pp 99–116
Carr JA, Patiño R (2011) The hypothalamus–pituitary–thyroid axis in teleosts and amphibians: endocrine disruption and its consequences to natural populations. Gen Comp Endocrinol 170:299–312
Castaneda AJ (2014) The effects of water and habitat quality on amphibian assemblages in agricultural ditches. Doctoral dissertation, Purdue University. Accessed in: https://pdfs.semanticscholar.org/ee88/e5372e1c48bce4f544b1b8161c2925b294a0.pdf. Accessed April 2019
Chambers DL (2011) Increased conductivity affects corticosterone levels and prey consumption in larval amphibians. J Herpetol 45:219–223
Chen J, Theller L, Gitau MW, Engel BA, Harbor JM (2017) Urbanization impacts on surface runoff of the contiguous United States. J Environ Manag 187:470–481
Chica-Olmo M, Carpintero-Salvo I, García-Soldado M et al (2005) Una aproximación geoestadística al análisis espacial de la calidad del agua subterránea. GeoFocus Revista Internacional de Ciencia y Tecnología de la Información Geográfica:79–93
Cude CG (2001) Oregon water quality index a tool for evaluating water quality management effectiveness. J Am Water Res Assoc 37:125–137
Cushman SA (2006) Effects of habitat loss and fragmentation on amphibians: a review and prospectus. Biol Conserv 128:231–240
Davidson C, Shaffer HB, Jennings MR (2002) Spatial tests of the pesticide drift, habitat destruction, UV-B, and climate-change hypotheses for California amphibian declines. Conserv Biol 16:1588–1601
de Solla SR, Bishop CA, Pettit KE, Elliott JE (2002a) Organochlorine pesticides and polychlorinated biphenyls (PCBs) in eggs of red-legged frogs (Rana aurora) and northwestern salamanders (Ambystoma gracile) in an agricultural landscape. Chemosphere 46:1027–1032
de Solla SR, Pettit KE, Bishop CA, Cheng KM, Elliott JE (2002b) Effects of agricultural runoff on native amphibians in the lower Fraser River Valley, British Columbia, Canada. Environ Toxicol Chem 21:353–360
Dodd CK (2010) Amphibian ecology and conservation: a handbook of techniques. Oxford University Press
Duellman W, Trueb L (1994) Biology of amphibians –John Hopkins University press. Baltimore, London
Earl JE, Whiteman HH (2009) Effects of pulsed nitrate exposure on amphibian development. Environ Toxicol Chem 28:1331–1337
Fabricius KE, Cooper TF, Humphrey C, Uthicke S, de’ath G, Davidson J, LeGrand H, Thompson A, Schaffelke B (2012) A bioindicator system for water quality on inshore coral reefs of the great barrier reef. Mar Pollut Bull 65:320–332
Garcia-Gonzalez C, Garcia-Vazquez E (2012) Urban ponds, neglected Noah's ark for amphibians. J Herpetol 46:507–514
González SO, Almeida C, Calderón M et al (2014) Assessment of the water self-purification capacity on a river affected by organic pollution: application of chemometrics in spatial and temporal variations. Environ Sci Pollut Res 21:10583–10593
Gray MJ, Miller DL, Hoverman JT (2009) Ecology and pathology of amphibian ranaviruses. Dis Aquat Org 87:243–266
Hamer AJ, McDonnell MJ (2008) Amphibian ecology and conservation in the urbanising world: a review. Biol Conserv 141:2432–2449
Hamer AJ, Makings JA, Lane SJ, Mahony MJ (2004) Amphibian decline and fertilizers used on agricultural land in South-Eastern Australia. Agric Ecosyst Environ 102:299–305
Hatch AC, Blaustein AR (2000) Combined effects of UV-B, nitrate, and low pH reduce the survival and activity level of larval cascades frogs (Rana cascadae). Arch Environ Contam Toxicol 39:494–499
Helena B, Pardo R, Vega M, Barrado E, Fernandez JM, Fernandez L (2000) Temporal evolution of groundwater composition in an alluvial aquifer (Pisuerga River, Spain) by principal component analysis. Water Res 34(3):807–816
Helms BS, Feminella JW, Pan S (2005) Detection of biotic responses to urbanization using fish assemblages from small streams of western Georgia, USA. Urban Ecosystems 8:39–57
Henley W, Patterson M, Neves R et al (2000) Effects of sedimentation and turbidity on lotic food webs: a concise review for natural resource managers. Rev Fish Sci 8:125–139
Jofré MB, Cid FD, Caviedes-Vidal E (2010) Spatial and temporal patterns of richness and abundance in the anuran assemblage of an artificial water reservoir from the semiarid central region of Argentina. Amphibia-Reptilia 31:533–540
Jones B, Snodgrass JW, Ownby DR (2015) Relative toxicity of NaCl and road deicing salt to developing amphibians. Copeia 2015:72–77
Karr JR (1991) Biological integrity: a long-neglected aspect of water resource management. Ecol Appl 1:66–84
Karraker NE (2007) Are embryonic and larval green frogs (Rana clamitans) insensitive to road deicing salt? Biology, Herpetological Conservation and
Karraker NE, Gibbs JP, Vonesh JR (2008) Impacts of road deicing salt on the demography of vernal pool-breeding amphibians. Ecol Appl 18:724–734
Klaver RW, Peterson CR, Patla DA (2013) Influence of water conductivity on amphibian occupancy in the greater Yellowstone ecosystem. Western North American Naturalist 73:184–197
Krishnamurthy S, Meenakumari D, Gurushankara H et al (2006) Effects of nitrate on feeding and resting of tadpoles of Nyctibatrachus major (Anura: Ranidae). Aust J Ecotoxicol 12:123–127
Kupferberg SJ, Lind AJ, Thill V, Yarnell SM (2011) Water velocity tolerance in tadpoles of the foothill yellow-legged frog (Rana boylii): swimming performance, growth, and survival. Copeia 2011:141–152
Laposata MM, Dunson WA (2000) Effects of spray-irrigated wastewater effluent on temporary pond-breeding amphibians. Ecotoxicol Environ Saf 46:192–201
Lescano JN, Nori J, Verga E et al (2015) Anfibios de las Sierras Pampeanas Centrales de Argentina: diversidad y distribución altitudinal. Cuadernos de herpetología 29:103–115
Lloyd DS (1987) Turbidity as a water quality standard for salmonid habitats in Alaska. N Am J Fish Manag 7:34–45
Marco A, Quilchano C, Blaustein AR (1999) Sensitivity to nitrate and nitrite in pond-breeding amphibians from the Pacific Northwest, USA. Environ Toxicol Chem 18:2836–2839
Marian MP, Sampath K, Nirmala A et al (1980) Behavioural response of Rana cyanophylictis tadpole exposed to changes in dissolved oxygen concentration. Physiol Behav 25:35–38
Martel A, Spitzen-van der Sluijs A, Blooi M, Bert W, Ducatelle R, Fisher MC, Woeltjes A, Bosman W, Chiers K, Bossuyt F, Pasmans F (2013) Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proc Natl Acad Sci 110:15325–15329
McKibbin R, Dushenko WT, Bishop CA (2008) The influence of water quality on the embryonic survivorship of the Oregon spotted frog (Rana pretiosa) in British Columbia, Canada. Sci Total Environ 395:28–40
McKinney ML (2008) Effects of urbanization on species richness: a review of plants and animals. Urban Ecosystems 11:161–176
Miller JD, Kim H, Kjeldsen TR, Packman J, Grebby S, Dearden R (2014) Assessing the impact of urbanization on storm runoff in a peri-urban catchment using historical change in impervious cover. J Hydrol 515:59–70
Mingo Magro J (1981) La vigilancia de la contaminación fluvial. MOPU, Madrid
Moore IT, Jessop TS (2003) Stress, reproduction, and adrenocortical modulation in amphibians and reptiles. Horm Behav 43:39–47
Morin PJ (1981) Predatory salamanders reverse the outcome of competition among three species of anuran tadpoles. Science 212:1284–1286
Morin PJ (1983) Predation, competition, and the composition of larval anuran guilds. Ecol Monogr 53:119–138
Noland R, Ultsch GR (1981) The roles of temperature and dissolved oxygen in microhabitat selection by the tadpoles of a frog (Rana pipiens) and a toad (Bufo terrestris). Copeia, 645–652
Olson DH, Aanensen DM, Ronnenberg KL, Powell CI, Walker SF, Bielby J, Garner TWJ, Weaver G, The Bd Mapping Group, Fisher MC (2013) Mapping the global emergence of Batrachochytrium dendrobatidis, the amphibian chytrid fungus. PLoS One 8:e56802
Oromí N, Sanuy D, Vilches M (2009) Effects of nitrate and ammonium on larvae of Rana temporaria from the Pyrenees. Bull Environ Contam Toxicol 82:534–537
Ortiz ME, Marco A, Saiz N et al (2004) Impact of ammonium nitrate on growth and survival of six European amphibians. Arch Environ Contam Toxicol 47:234–239
Ortiz-Santaliestra ME, Marco A, Fernández MJ, Lizana M (2006) Influence of developmental stage on sensitivity to ammonium nitrate of aquatic stages of amphibians. Environ Toxicol Chem 25:105–111
Pillsbury FC, Miller JR (2008) Habitat and landscape characteristics underlying anuran community structure along an urban–rural gradient. Ecol Appl 18:1107–1118
Porej D, Micacchion M, Hetherington TE (2004) Core terrestrial habitat for conservation of local populations of salamanders and wood frogs in agricultural landscapes. Biol Conserv 120:399–409
Pounds JA, Bustamante MR, Coloma LA et al (2006) Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439:161–167
Queralt R (1982) La calidad de las aguas en los rios. Tecnologia del agua, 4:49–57
Riley SP, Busteed GT, Kats LB et al (2005) Effects of urbanization on the distribution and abundance of amphibians and invasive species in southern California streams. Conserv Biol 19:1894–1907
Rollins-Smith LA (2009) The role of amphibian antimicrobial peptides in protection of amphibians from pathogens linked to global amphibian declines. Biochim Biophys Acta Biomembr 1788:1593–1599
Rouse JD, Bishop CA, Struger J (1999) Nitrogen pollution: an assessment of its threat to amphibian survival. Environ Health Perspect 107:799–803
Rowe DK, Dean TL, Williams E, Smith JP (2003) Effects of turbidity on the ability of juvenile rainbow trout, Oncorhynchus mykiss, to feed on limnetic and benthic prey in laboratory tanks. N Z J Mar Freshw Res 37:45–52
Rubbo MJ, Kiesecker JM (2005) Amphibian breeding distribution in an urbanized landscape. Conserv Biol 19:504–511
Schmutzer AC, Gray MJ, Burton EC et al (2008) Impacts of cattle on amphibian larvae and the aquatic environment. Freshw Biol 53:2613–2625
Semlitsch RD (2000) Principles for management of aquatic-breeding amphibians. J Wildl Manag 64:615–631
Seymour RS, Roberts JD, Mitchell NJ, Blaylock AJ (2000) Influence of environmental oxygen on development and hatching of aquatic eggs of the Australian frog, Crinia georgiana. Physiol Biochem Zool 73:501–507
Skerratt LF, Berger L, Speare R, Cashins S, McDonald KR, Phillott AD, Hines HB, Kenyon N (2007) Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth 4:125–134
Smith G, Temple K, Vaala D et al (2005) Effects of nitrate on the tadpoles of two Ranids (Rana catesbeiana and R. clamitans). Arch Environ Contam Toxicol 49:559–562
Sowers AD, Mills MA, Klaine SJ (2009) The developmental effects of a municipal wastewater effluent on the northern leopard frog, Rana pipiens. Aquat Toxicol 94:145–152
Sparling DW (2010) Water-quality criteria for amphibians. Amphibians Ecology and Conservation (CD Kenneth, ed.). Oxford University Press, USA 105–117
Stebbins RC, Cohen NW (1997) A natural history of amphibians. Princeton University Press, New Jersey, p 316
Suh D (2016) The impact of turbidity on the predator-prey relationship between red swamp crayfish (Procambarus clarkii) and pacific tree frog (Pseudacris regilla) tadpoles
Taebi A, Droste RL (2004) Pollution loads in urban runoff and sanitary wastewater. Sci Total Environ 327:175–184
Turtle SL (2000) Embryonic survivorship of the spotted salamander (Ambystoma maculatum) in roadside and woodland vernal pools in southeastern New Hampshire. J Herpetol, 60–67
Viertel B (1999) Salt tolerance of Rana temporaria: spawning site selection and survival during embryonic development (Amphibia, Anura). Amphibia-Reptilia 20:161–171
Villegas Ojeda MA, Andrea Espeche B, Jofré MB (2016) Desarrollo larval de anfibios anuros en un río impactado por urbanización: efecto de factores ambientales. Neotropical Biology & Conservation 11
Walsh CJ, Roy AH, Feminella JW, Cottingham PD, Groffman PM, Morgan RP II (2005) The urban stream syndrome: current knowledge and the search for a cure. J N Am Benthol Soc 24:706–723
Warkentin KM (2002) Hatching timing, oxygen availability, and external gill regression in the tree frog, Agalychnis callidryas. Physiol Biochem Zool 75:155–164
Wassersug RJ, Seibert EA (1975) Behavioral responses of amphibian larvae to variation in dissolved oxygen. Copeia 86–103
Weir L, Mossman M (2005) North American amphibian monitoring program (NAAMP)
Welsh HH, Ollivier LM (1998) Stream amphibians as indicators of ecosystem stress: a case study from California’s redwoods. Ecol Appl 8:1118–1132
Zhou F, Huang GH, Guo H, Zhang W, Hao Z (2007) Spatio-temporal patterns and source apportionment of coastal water pollution in eastern Hong Kong. Water Res 41:3429–3439
Zhu B, Wang T, Kuang FH, Luo ZX, Tang JL, Xu TP (2009) Measurements of nitrate leaching from a hillslope cropland in the Central Sichuan Basin, China. Soil Sci Soc Am J 73:1419–1426
Acknowledgements
Authors gratefully acknowledge Instituto de Química de San Luis “Dr. Roberto Olsina”- Consejo Nacional de Investigaciones Científicas y Tecnológicas (INQUISAL-CONICET) and Universidad Nacional de San Luis (Project PROICO 2-1914) for financial support. We thank MSc. Angela Stires for the valuable language and grammar revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Calderon, M.R., Almeida, C.A., González, P. et al. Influence of water quality and habitat conditions on amphibian community metrics in rivers affected by urban activity. Urban Ecosyst 22, 743–755 (2019). https://doi.org/10.1007/s11252-019-00862-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-019-00862-w