Abstract
Chemical characterization of PM2.5 [organic carbon, elemental carbon, water soluble inorganic ionic components, and major and trace elements] was carried out for a source apportionment study of PM2.5 at an urban site of Delhi, India from January, 2013, to December, 2014. The annual average mass concentration of PM2.5 was 122 ± 94.1 µg m−3. Strong seasonal variation was observed in PM2.5 mass concentration and its chemical composition with maxima during winter and minima during monsoon. A receptor model, positive matrix factorization (PMF) was applied for source apportionment of PM2.5 mass concentration. The PMF model resolved the major sources of PM2.5 as secondary aerosols (21.3 %), followed by soil dust (20.5 %), vehicle emissions (19.7 %), biomass burning (14.3 %), fossil fuel combustion (13.7 %), industrial emissions (6.2 %) and sea salt (4.3 %).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
It is well documented that atmospheric particulate matter (PM) significantly affects atmospheric chemistry, ambient air quality, visibility and the earth’s radiation budget (Ramgolam et al. 2009; Pope et al. 2009). Several studies have revealed that aerosols, especially fine mode particles (<2.5 µm) can lead to serious human health effects like cardiovascular and respiratory disorders (Pope and Dockery 2006). Therefore, identification of PM sources is necessary to develop air quality improvement strategies in order to be able to control and reduce ambient PM concentrations through targeted action (Waked et al. 2014). To address this issue, many tools or receptor models have been used for identification and quantification of PM sources (Paatero 1997; Ulbrich et al. 2009).
In the recent past, receptor models have been applied in many studies and have shown the ability to accurately identify the potential emission sources of ambient PM at a receptor site (Waked et al. 2014). The PMF model is highly recommended when sources are not formally known, but it requires post-treatment source identification. Recently, the PMF model has been improved significantly and a new approach was developed by Paatero and Tapper (1994), using a least squares approach. PMF solves the problem arising in factor analysis by integrating non-negativity constraints in the optimization process and utilizing the error estimates for each data value as a point-by-point weight (Begum et al. 2004). The PMF model has been applied successfully worldwide (Kim and Hopke 2004; Karanasiou et al. 2009; Sharma et al. 2015). In this study, we report the chemical characteristics and source apportionment of PM2.5 at an urban site in Delhi, India. The PMF model was applied for the source apportionment using chemical composition (organic carbon, elemental carbon, major ions, metals and trace elements) of PM2.5 mass.
Materials and Methods
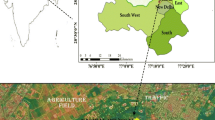
PM2.5 samples were collected periodically at CSIR-National Physical Laboratory, New Delhi (28°38′N, 77°10′E; 218 m amsl) from January, 2013, to December, 2014. The sampling site is amenable to free wind flow from all the directions and represents a typical urban atmosphere, surrounded by huge roadside traffic (~100 m) and agricultural fields in the southwest direction (~500 m). Roadside vehicles, industrial emission and biomass burning could be major sources of carbonaceous aerosols and several other pollutants. The occasional occurrence of dust storms may contribute the presence of mineral dust significantly to the aerosol loading in summertime (Ram et al. 2010). The sampling area is under the influence of air mass flow from north-east to north-west in winter and from south-east to south-west in the summer. The temperature of Delhi varied from maximum in summer (March to June) to minimum in winter (November to February). The average rainfall in Delhi during monsoon (July to October) was of the order of ~900 mm. A detailed description of sampling site is available in Sharma et al. (2015).
PM2.5 samples (n = 140) were collected on pre-combusted (at 550°C) and dessicator-stored quartz fibre filters (QM-A, Whatman, GE Healthcare UK Limited, Buckinghamshire, UK) by using a fine particle sampler (APM 550, Envirotech, Delhi, IN) at 10 m height above ground level. Ambient air was passed through the quartz filter (47 mm) at a flow rate of 1 m3 h−1 (accuracy ±2 %) for 24 h. The filters were weighed before and after the sampling during the experiment in order to determine the mass of PM2.5 collected. The quantitative elemental analysis (Mg, Al, S, Si, Cl, K, Ca, Ti, Cr, Mn, Fe, Zn, Cr, Br, As and Pb) of PM2.5 samples was carried out first using non-destructive X-ray fluorescence spectroscopy with a Rigaku ZSX Primus wavelength dispersive X-ray fluorescence spectrometer (ZSX Primus WD-XRF, The Woodland, TX, USA). Then 6.25 cm2 (2.5 × 2.5 cm2) of each filter was used for analysis of water soluble inorganic ions (WSIC) by ion chromatograph (Dionex ICS-3000, Sunnyvale, CA, USA). The remainder of each filter was used for organic carbon (OC)/elemental carbon (EC) analysis with a carbon analyzer (DRI 2001A, Atmoslytic Inc., Calabasas, CA, USA). A more detailed description of the analytical methods, calibration procedures, etc. are available in our earlier paper (Sharma et al. 2015). Statistical analysis of PM2.5 and its chemical species data was done using standard recommended methods and seasonal significance difference of chemical species of PM2.5 was analyzed by one-way ANOVA test (Datta et al. 2010).
In the present study, PMF (v3.0) was used to quantify the contribution of various emission sources to PM2.5 mass concentration (USEPA 2008). The model requires two input files: one of the measured concentrations of the species and another for the estimated uncertainty of the concentration. A detailed description of the PMF model has been presented in Paatero and Tapper (1994); Paatero (1997). A speciated data set can be viewed as a data matrix X of i by j dimensions, in which i number of samples and j chemical species are measured. The aim of multivariate receptor modeling (e.g., PMF) is to identify a number of factors p, the species profile f of each source, and the amount of mass g contributed by each factor to each individual sample which is given as:
where e ij is the residual for each sample/species.
Results are constrained so that no sample can have a negative source contribution. PMF allows each data point to be individually weighed. This feature allows the analyst to adjust the influence of each data point, depending on the confidence in the measurement. For example, data below the detection limit can be retained for use in the model, with the associated uncertainty adjusted so these data points have less influence on the solution than measurements above the detection limit. The PMF solution minimizes the object function Q, based upon these uncertainties (u) as follows.
where Xij are the measured concentration (in µg m−3), uij are the estimated uncertainty (in µg m−3), n is the number of samples, m is the number of species and p is the number of sources including in the analysis. In this study, information on chemical properties of 140 PM2.5 samples has been used as input to the PMF model for a total of 23 parameters. Categorization of quality of data was based on the signal to noise ratio (S/N) and the percentage of sample method detection limit (MDL). Those species which have S/N ≥ 2 were categorized as strong in data quality. Those with S/N between 0.2 and 2 were categorized as weak in quality. These species are not likely to provide enough variation in concentration and therefore contribute to the noise in the results. Those species with an S/N ratio below 0.2 are classified as bad values and were thus excluded from further analysis. In the present case, signal to noise ratio (S/N) estimated as >0.6 and the model performance in a base run showed determination coefficient (R 2) between the modeled and experimental concentration of PM2.5, OC, and EC of 0.97, 0.94 and 0.96, respectively and most of the other chemical species are also well reconstructed. These results are within the range of those presented in many PMF studies. For example, R 2 values of 0.71 were reported for a study in Spain (Cusack et al. 2013) and of 0.96 for a study in Germany (Beuck et al. 2011) for PM2.5 mass reconstruction. Scaled residuals between −3 and +3 were obtained for all of the major components, and the value of Q robust is strictly identical to the value of Q true, all of these showing that no specific event was affecting the results and that the base run could be regarded as stable.
Results and Discussion
The temporal variation in mass concentration of PM2.5, OC, EC and inorganic ions during the study are depicted in Fig. 1. The average concentration of PM2.5 was 122 ± 94.1 µg m−3 (range 25.1–430 µg m−3) from January, 2013, to December, 2014. The mass concentrations of almost all the chemical constituents were highest during the months of January, 2013, and January, 2014. The seasonal variation and average mass concentrations of OC, EC, WSIC and major and trace elements (Na, Mg, Al, S, Cl, K, Si, Ca, Cr, Ti, Fe, Zn, Mn, Br and Pb) of PM2.5 with maxima and minima are summarized in Table 1. The average concentration of OC and EC of PM2.5 was recorded as 19.9 ± 14.3 µg m−3 (~15 % of PM2.5) and 10.4 ± 8.0 µg m−3 (~8 % of PM2.5), respectively. The annual average of total carbon (TC = OC + EC) concentration contributed ~23 % of PM2.5. Perrino et al. (2011) reported similar percentage contributions of OC (~12 % of PM10) and EC (~3 % of PM10) of PM10 at Delhi, whereas Mandal et al. (2014) reported higher percentage contributions of OC (28 % of PM2.5) and EC (9 % of PM2.5) of PM2.5 in an industrial area of Delhi. In the present study, the average concentration of major and trace elements in PM2.5 was recorded as 29.0 ± 2.3 µg m−3 (22.3 % of PM2.5).
The mass concentrations of PM2.5, OC and EC varied significantly during winter, summer and monsoon seasons at Delhi (Table 1). During winter, the concentrations of PM2.5, OC and EC were recorded as being more than twice the concentrations during the summer and monsoon seasons. This may be due to the source strength and prevailing meteorological conditions at the sampling site. Significant lowering of mixing height of the boundary layer during winter season may also contribute to the higher concentration of PM2.5 during winter (Datta et al. 2010). Strong positive linear relationships between OC and EC (R 2 = 0.91; at p < 0.05), OC versus PM2.5 (R 2 = 0.87) and EC versus PM2.5 (R2 = 0.82) were recorded during the study. A significant linear correlation between OC and EC of PM2.5 is usually indicative of their common sources like vehicular traffic and biomass burning (Salma et al. 2004; Sharma et al. 2014).
The average concentration of WSIC and its seasonal variability are summarized in Table 1. During the study, the concentrations of NH4 +, SO4 2− and NO3 − of PM2.5 was recorded as 9.4 ± 8.6, 12.9 ± 8.1 and 10.0 ± 9.8 µg m−3, respectively. The water soluble inorganic ionic species (WSIC) accounted for ~40 % of PM2.5 concentration during the study with seasonal variability (Table 1). The study revealed positive linear correlations of molar mass between NH4 + and SO4 2− (R 2 = 0.65), NH4 + and NO3 − (R 2 = 0.69) of PM2.5, as well as for charge balance between SO4 2− and NH4 +; NO3 − and NH4 +; SO4 2−, NO3 − and NH4 +; and SO4 2−, NO3 −, Cl− and NH4 +. The above correlations indicate the possible formation of secondary aerosols [(NH4)2SO4, NH4NO3 and NH4Cl] at the sampling site. NH4 + generally combines with NO3 − and SO4 2− in the atmosphere to form NH4NO3 and (NH4)2SO4, respectively. Figure 2 shows the charge balance between SO4 2− and NH4 + (R 2 = 0.58); NO3 − and NH4 + (R 2 = 0.67); SO4 2−, NO3 − and NH4 + (R 2 = 0.67); SO4 2−, NO3 −, Cl− and NH4 + (R 2 = 0.73) in Delhi during the study. The charge balance (in Fig. 3 a-c) is well below a 1:1 relationship, indicating an excess of NH4 + compared to anions (Behra and Sharma 2010). The charge balance between SO4 2−, NO3 −, Cl− and NH4 + (R 2 = 0.73) in Fig. 3d was closer to the 1:1 line confirming that most of the time sufficient NH4 + was present to neutralize the acidic components (H2SO4, HNO3 and HCl) to form (NH4)2SO4, NH4NO3 and NH4Cl (Sharma et al. 2015).
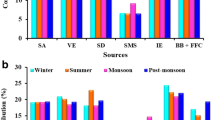
The PMF was applied to the analyzed data set consisting of 23 species and 140 PM2.5 samples collected at sampling site. For the final analysis, PMF was applied to the data sets using factors and the resultant change in the Q values was examined. In this study, the theoretical Q value was to be approximately 3220 (i.e., 140 × 23). In seven factor solutions, more than 95 % of Q values were quite close to 3220. Based on an evaluation of the model results for the Q value variations, a seven-factor solution provided the most feasible results. The descriptions of the model and source apportionment of PM have been discussed in detail in our previous paper (Sharma et al. 2015). The mass fraction distribution of species was used to identify the sources, which were soil dust, vehicular emission, sea salt, industrial emission, secondary aerosol, biomass burning and fossil fuel combustion for PM2.5 mass (Fig. 3).
A discussion follows of the seven major sources of PM2.5 air pollution at the sampling site.
Source 1 Present PMF analysis shows that secondary aerosols have contributed to about 21.3 % for PM2.5 mass concentrations, respectively. Secondary aerosols are mainly composed of ammonium sulphate and nitrate deriving primarily from the gaseous precursors NH3, SO2 and NOx. The abundance of gaseous NH3, SO2 and NOx at Delhi (Sharma et al. 2012) supports the presence of secondary aerosols over the region. The key markers of secondary aerosols are NO3 −, SO4 2− and NH4 +, as shown in Fig. 3.
Source 2 PMF analysis showed that soil dust contributed 20.5 % of aerosol mass in PM2.5 at sampling site. Soil dust includes most of the crustal elements and has high concentrations of Fe, Ca, Na, Mg, Al and K (Lough et al. 2005). The concentration of Ca in PM2.5 is associated with its resuspension from agricultural fields or bare soils by local winds. Crustal elements typically used as tracers for soil and/or crustal resuspension include Al, Si, Ca, Mg, Fe and Na (Begum et al. 2006). A whole array of element tracers has been used in India for identification of this source type, including Al, Si, Ca, Ti, Fe, Pb, Cu Cr, Ni, Co and Mg (Sharma et al. 2014).
Source 3 Vehicle exhaust is generally dominated by elemental carbon, Cu, Zn, Ba, Sb, Pb, Mn, Mo and Ni which are widely used as markers of vehicular sources. In the present study, Cu, Zn, Mn, Pb and EC contributed 32.7 %, 42.6 %, 26.9 %, 43.5 % and 51.3 %, respectively, indicating vehicle emissions as the source. PMF analysis indicated that vehicle emissions contributed 19.7 % in PM2.5 at Delhi. Internationally, EC (Lee and Hopke 2006) is used extensively as a marker for diesel exhaust. In India, Cu, V, Mn, Co, Pb and Zn have been used as tracer elements for identification of vehicular emission. Vehicular emissions are a major source of the PM and research indicates that they contribute 10 %–80 % to PM in cities across India.
Source 4 Biomass burning, wood burning and vegetative burning have been characterized as having high concentrations of K+ and SO4 2− by various source studies (Wu et al. 2007). The potassium ion has been used in many source apportionment studies conducted in Europe and Asia as an indicator of biomass burning (Pant and Harriso 2012). The PMF analysis showed that biomass burning contributed 14.3 % for PM2.5 mass in the present study. In India, K+ has been used as a key marker for biomass/wood combustion for TSP, PM10 and PM2.5 (Shridhar et al. 2010), whereas levoglucosan is the key organic marker (Chowdhury et al. 2007).
Source 5 The higher concentrations of Al, Cl, Fe, Zn, Cr and SO4 2− at the sampling site clearly indicate the source of fossil fuel combustion of PM2.5. The PMF analysis showed that fossil fuel burning contributed 13.7 % for PM2.5 in the present study. In international studies, a key marker for coal combustion includes As, Se, Te and SO4 2− and it has contributed 6 %–30 % to PM in different studies (Gupta et al. 2007; Sharma et al. 2007).
Source 6 The results of the PMF analysis show that industrial emissions accounted for about 6.2 % for PM2.5 mass concentration. Begum et al. (2006) used Ni, Pb and S as markers for industrial emission, whereas Song et al. (2006) used Ni, Cr, Fe and Mn, and Tauler et al. (2009) used Zn, Fe, Mn and Cd. Generally Zn, Cu, Mn, S, Ni, Cd, Fe, Mo and Cr are used as tracers for industrial emission IE in India (Sharma et al. 2014).
Source 7 Higher concentrations of Na, K and Cl (52.2 %, 27.2 % and 47.6 %, respectively) in PM2.5 indicate the possible contribution of sea salt, which is supported by PMF analysis. The use of K offers possible confusion with wood/biomass burning combustion and Cl with coal burning, but a combination of the four elements (Na, K, Cl− and Mg) should provide a reliable signature. In the present study, PMF analysis shows that sea salt contributed to about 4.3 % for PM2.5 mass concentration.
References
Begum BA, Kim E, Biswas SK, Hopke PK (2004) Investigation of sources of atmospheric aerosol at urban and semi-urban areas in Bangladesh. Atmos Environ 38:3025–3038
Begum BA, Akhter S, Sarker L, Biswas SK (2006) Gravimetric analysis of air filters and quality assurance in weighing. Nucl Sci Appl 15:36–41
Behra SN, Sharma M (2010) Investigating the potential role of ammonia in ion chemistry of fine particulate matter formation for an urban environment. Sci Total Environ 408:3569–3575
Beuck H, Quass U, Klemm O, Kuhlbusch TA (2011) Assessment of sea salt and mineral dust contributions to PM10 in NW Germany using tracer models and positive matrix factorization. Atmos Environ 45:5813–5821
Chowdhury Z, Zheng M, Schauer JJ, Sheesley RJ, Salmon LG, Cass GR, Russell AG (2007) Speciation of ambient fine organic carbon particles and source apportionment of PM2.5 in Indian cities. J Geophys Res 112:D15303
Cusack M, Perez N, Pey J, Alastuey A, Querol X (2013) Source apportionment of fine PM and sub-micron particle number concentrations at a reginal background site in the western Mediterranean: a 2.5 year study. Atmos Chem Phys 13:5173–5187
Datta A, Saud T, Goel A, Tiwari S, Sharma SK, Saxena M, Mandal TK (2010) Variation of ambient SO2 over Delhi. J Atmos Chem 65:127–143
Gupta AK, Karar K, Srivastava A (2007) Chemical mass balance source apportionment of PM10 and TSP in residential and industrial sites of an urban region of Kolkata, India. J Hazard Mater 142:279–287
Karanasiou AA, Siskos PA, Eleftheriadis K (2009) Assessment of source apportionment by positive matrix factorization analysis on fine and coarse urban aerosol size fractions. Atmos Environ 43:3385–3395
Kim E, Hopke PK (2004) Source apportionment of fine particles in Washington, DC, utilizing temperature-resolved carbon fractions. J Air Waste Manag Assoc 53:773–785
Lee JH, Hopke PK (2006) Apportioning sources of PM2.5 in St. Louis, MO using speciation trends network data. Atmos Environ 40:360–377
Lough GC, Schauer JJ, Park JS, Shafer MM, De Minter JT, Weinstein JP (2005) Emissions of metals associated with motor vehicle roadways. Environ Sci Technol 39:826–836
Mandal P, Saud T, Sarkar R, Mandal A, Sharma SK, Mandal TK, Bassin JK (2014) High seasonal variation of atmospheric C and particulate concentrations in Delhi, India. Environ Chem Lett. doi:10.1007/s10311-013-0438-y
Paatero P (1997) Least squares formulation of robust nonnegative factor analysis. Atmos Environ 37:23–35
Paatero P, Tapper U (1994) Positive matrix factorization: a non-negative factor model with optimal utilization of error estimates of data values. Environmetrics 5:111–126
Pant P, Harriso RM (2012) Critical review of receptor modelling of particulate matter: a case study of India. Atmos Environ 49:1–12
Perrino C, Tiwari S, Catrambone M, Torre SD, Rantica E, Canepari S (2011) Chemical characterization of atmospheric PM in Delhi, India during different periods of the year including Diwali festival. Atmos Pollut Res 2:418–427
Pope CA, Dockery DW (2006) Health effects of fine particulate air pollution: lines that connect. JAPCA 56:709–742
Pope CA, Ezzati M, Dockery DW (2009) Fine-particulate air pollution and life expectancy in the United States. N Engl J Med 360:376–386
Ram K, Sarin MM, Tripathi SN (2010) One-year record of carbonaceous aerosols from an urban location (Kanpur) in the Indo-Gangetic Plain: characterization, sources and temporal variability. J Geophys Res. doi:10.1029/2010JD014188
Ramgolam K, Favez O, Cachier H, Gaudichet A, Marano F et al (2009) Size-partitioning of an urban aerosol to identify particle determinants involved in the proinflammatory response induced in airway epithelial cells. Part Fibre Toxicol 6:1–12
Salma I, Chi XG, Maenhaut W (2004) Elemental and organic carbon in urban canyon and background environments in Budapest, Hungary. Atmos Environ 38:2517–2528
Sharma M, Kishore S, Tripathi SN, Behra SN (2007) Role of atmospheric ammonia in the formation of inorganic secondary particulate matter: a study at Kanpur, India. J Atmos Chem 58:1–17
Sharma SK, Singh AK, Saud T, Mandal TK, Saxena M, Singh S, Ghosh S, Raha S (2012) Study on water soluble ionic composition of PM10 and trace gases over Bay of Bengal during W_ICARB campaign. Meteorol Atmos Phys 118:37–51
Sharma SK, Mandal TK, Saxena M, Rashmi Rohtash, Sharma A, Gautam R (2014) Variation of OC, EC, WSIC and trace metals of PM10 in Delhi. J Atmos Sol Terr Phys 113:10–22
Sharma SK, Sharma A, Saxena M, Choudhary N, Masiwal R, Mandal TK, Sharma C (2015) Chemical characterization and source apportionment of aerosol at an urban area of Central Delhi, India. Atmos Pollut Res. doi:10.1016/j.apr.2015.08.002
Shridhar V, Khillare PS, Agarwal T, Ray S (2010) Metallic species in ambient particulate matter at rural and urban location of Delhi. J Hazard Mater 175:600–607
Song Y, Zhang Y, Xie S, Zeng L, Zheng M, Salmon LG, Shao M, Slanina S (2006) Source apportionment of PM2.5 in Beijing by positive matrix factorization. Atmos Environ 40(1):1526–1537
Tauler R, Viana M, Querol X, Alastuey A, Flight RM, Wentzell PD, Hopke PK (2009) Comparison of the results obtained by four receptor modelling methods in aerosol source apportionment studies. Atmos Environ 43:3989–3997
Ulbrich IM, Canagaratna MR, Zhang Q, Worsnop DR, Jimenez JL (2009) Interpretation of organic components from positive matrix factorization of aerosol mass spectrometric data. Atmos Chem Phy 9:2891–2918
USEPA (2008) EPA positive matrix factorization (PMF) 3.0 fundamentals and user guide. USEPA Office of Research and Development, Washington, DC
Waked A, Favez O, Alleman LY, Piot C, Petit JE, Delaunay T et al (2014) Source apportionment of PM10 in a north-western Europe regional urban backgroung site (Lens, France) using positive matrix factorization and including primary biogenic emission. Atmos Chem Phy 14:3325–3346
Wu CF, Larson TV, Wu SY, Williamson J, Westberg HH, Liu LJS (2007) Source apportionment of PM2.5 and selected hazardous air pollutants in Seattle. Sci Total Environ 386:42–52
Acknowledgments
The authors are thankful to the Director, CSIR-NPL, New Delhi and Head, Radio and Atmospheric Sciences Division (RASD), CSIR-NPL, New Delhi for their encouragement. The authors also acknowledge the Council of Scientific and Industrial Research, New Delhi for providing partial financial support for this study (PSC-0112 Project). Authors thankfully acknowledge to Ms. Nikki Choudhary, Ms. Renu Masiwal, Dr. Anshu Gupta and Dr. N.C. Gupta, University School of Environment Management, GGS IP University, Delhi, India for partial sample collection and discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, S.K., Mandal, T.K., Jain, S. et al. Source Apportionment of PM2.5 in Delhi, India Using PMF Model. Bull Environ Contam Toxicol 97, 286–293 (2016). https://doi.org/10.1007/s00128-016-1836-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-016-1836-1