Abstract
The temporal variation of ambient SO2 and the chemical composition of particulate matters (PM2.5 and PM10) were studied at National Physical Laboratory (NPL), New Delhi (28°38′N, 77°10′E). Spatial variation of SO2 at seven air quality monitoring stations over Delhi was also studied simultaneously. Wide range of ambient SO2 was recorded during winter (2.55 to 17.43 ppb) compare to other seasons. SO2 mixing ratio was recorded significantly high at industrial sites during winter and summer; however, no significant spatial difference in SO2 mixing ratio was recorded during monsoon. SO 2−4 /(SO2+SO 2−4 ) ratio was recorded high (0.74) during winter and low (0.69) during summer. Monthly variation of PSCF was analyzed using HYSPLIT seven days backward trajectories and daily average SO2 data. PSCF analysis suggests that, during winter (December, January, February) ambient SO2 at the study site might have contributed from long distance sources, located towards west and southwest directions; during monsoon (July, August, September) marine contribution was noticed; whereas, during summer (April, May and June) it was from regional sources (located within few 100 km of study site). During winter there was significant contribution from the long distance sources located in western Asia, northwestern Pakistan, Rajasthan and Punjab provinces of India. Coal used in thermal power plants at Panipat (in the northwestern side) and Faridabad (in the southeastern side), local industries, soil erosion and biomass burning may be major contributing factors for SO2 during summer. The study establishes that the transport sector may not be the major source of ambient SO2 in Delhi.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Ambient air pollution level in most of the Indian metropolitan has increased due to upward trends in power consumption, industrialization and vehicle uses (Census 2001). Delhi ranks amongst ten worst polluted megacities of Asia. Increase of population (9.5 million to 13.8 million) (Chelani and Devotta 2007), intense traffic flow and increased industrial activities (Gurjar and Lelieveld 2005) resulting the pollution level in Delhi, three times higher than the standards of World Health Organization (WHO) and National Air Quality (NEERI 2000; Bhanarkar et al. 2002) over last two decades. Sulfur dioxide (SO2) plays an important role in controlling atmospheric pH as well as atmospheric particle formation. Guttikunda et al. (2003) have reported that emission of SO2 has increased several folds from 1975 to 2000; whereas, Gurjar et al. (2004) have shown no substantial changes in the period of 1990 to 2000. However, Gurjar et al. (2004) have reported that power sector is the major source of ambient SO2 in Delhi; whereas, Garg et al. (2001) have reported that the industrial sector and transport sector are major sources of ambient SO2 in India. Chelani and Devotta (2007) have reported that after implementing the CNG engines, the contribution of transport sector towards ambient SO2 has dwindled in Delhi. Industrial activities including steel, cement, fertilizer and oil refinery are also possible sources of ambient SO2. Yang et al. (2008) and Mittal et al. (2009) have reported that burning of crop stubbles may also influence the rising of ambient SO2 mixing ratio.
SO2 belong to the group of air pollutants collectively termed as oxides of sulfur (SOx) which comprises both gaseous and particulate chemical species. There are four gas-phase compounds, namely; sulfur monoxide (SO), SO2, sulfur trioxide (SO3) and higher sulfur oxides (SO3+x). Among them SO2 occurs at sufficient concentrations in ambient atmosphere and considered as a major atmospheric pollutant. SO2 is emitted into the urban atmosphere mainly from anthropogenic sources; such as, the combustion of sulfur-containing fossil fuels (Reddy and Venkataraman 2002a, b). The particulate phase of SOx consists of strongly and weakly acidic sulfates, namely sulfuric acid (H2SO4) and its products of neutralization with ambient NH3: letovicite [(NH4)3H(SO4)2], ammonium bisulfate (NH4HSO4) and ammonium sulfate [(NH4)2SO4]. Although most of the toxicological database for acidic sulfate involves sulfuric acid as it is the most acidic among particulate sulfates. These species rarely occur alone in ambient air, which generally contains some combination of the various sulfates.
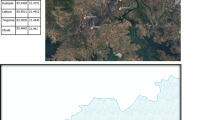
Mixing ratio of ambient SO2, chemical composition of aerosol (PM2.5 and PM10) and meteorological parameters were recorded continuously during the period of January to December in the year 2008 at NPL, New Delhi. Spatiotemporal distribution of ambient SO2 mixing ratio was also recorded at different locations (Fig. 1) in Delhi during the period. PSCF analysis was performed using HYSPLIT seven days back trajectories and daily SO2 mixing ratio to understand sources of SO2 during different months over the study site.
2 Materials and methods
2.1 Site description
The administrative capital of India, New Delhi (28° 39′ N, 77° 13′ E; 218 m MSL) is located in the Municipal Corporation of Delhi (MCD) region in northern India near the Gangetic plain and Thar Desert. The temperature of Delhi varies from 1°C during winter (November to February) to 48°C during summer (March to June). The average rainfall in Delhi is 73 cm, mostly received during monsoon (July to October). There are different small, medium and large scale industries in and around Delhi. There are two thermal power plants: located at Badarpur (1) and Rajghat (2) (southern side) (Fig. 1).
Spatial distribution of ambient SO2 mixing ratio was recorded at eight Air Quality Monitoring (AQM) stations of Central Pollution Control Board (CPCB, India) in Delhi. These locations are: Mayapuri (28°37′N, 77°07′E; site B); Town hall (28°39′N, 77°14′E; site C); Sahadra (28°40′N, 77°16′E; site D); Janakpuri (28°37′N, 77°05′E; site E); Sahajadabagh (28°41′N, 77°10′E; site F); Pritampura (28°42′N, 77°09′E; site G); Nizamuddin (28°35′N, 77°14′E; site H) and Sirifort (28°33′N, 77°13′E; site I) (Fig. 1). Every day, 8 h duration data was recorded at all AQMs. However; ambient SO2 mixing ratio was recorded continuously (24 h) at NPL (28°38′N, 77°10′E; site A) (Fig. 1).
NPL (Site A) is located in the central Delhi. The site has an immense agricultural field in the northwestern and western side; central ridge reserve forest area in the east and south east side. There is no industry in the 5 km radius of this site. The site was chosen to study the atmospheric interactions and diurnal pattern of SO2.
2.2 Sampling
Ambient SO2 mixing ratio was recorded continuously (24 h) at Site A throughout the study period using SO2 analyzer (Model: APSA-360A, Make: HORIBA, Ltd, Japan) at every 5 minutes interval. The instrument follows the principle of ultraviolet fluorescence method. With the irradiation of the sample with UV rays (220 nm), it emits the light of different wavelengths (240 nm to 420 nm with a peak at 320 nm)— these wavelength ranges are referred as fluorescence. To distinguish it from the UV light source, the fluorescence light is detected from the right angle direction as it irradiates in all directions. The accuracy of the instrument was ±1% and minimum detectable limit was 0.5 ppb at a flow rate of 0.8 l min−1.
At other sampling locations (Site B to I) ambient SO2 was collected using high volume air sampler for 8 h period every day during the study period at 0.5 m3 min−1 air flow through glass impinger with 0.04 M potassium tetra-chloromercurte which forms stable di-chlorosulphitomercurate complex with SO2. The complex was stored at 5°C. The sample was analyzed within 30 days by Waste and Gake method (precision: 4.6%; accuracy: 10.4%) in which the complex was allowed to react with pararosaniline and formaldehyde to form intensely colored pararosaniline methylesulphonic acid (Harison et al. 1986). The absorbance of the colored complex was measured in spectrophotometer (Specord 50, Analytic Jena, Germany) at 560 nm.
Ambient particulate matters (PM) were collected at Site A in weekly intervals to study the concentration of different particulate matters in PM2.5 and PM10. PM2.5 (Particulate matter with aerodynamic diameter < 2.5 μm) was collected by using APM550 (PM2.5 APM 550 Make: M/s. Envirotech, India). Ambient air was passed through 47 mm GFF filter paper at a flow rate of 1 m3 h−1 for 8 h during the period of sampling. The instrument was operated based on impactor design standardized by US-EPA. Uncertainties related to flow rate, sampling time and mass depositions on the filter papers was combined to yield a propagated precision of approximately ±13% at 10 μg m−3 and approximately ±5% at 100 μg m−3. The filter deposit mass measurement precision dominates at low concentrations while the flow rate precision dominates at high concentrations. All samples were stored under dry condition at −20°C before further analysis.
PM10 (particulate matter with aerodynamic diameter <10 μm) was collected by using respirable dust sampler (RDS, APM 460NL, Make: M/s. Envirotech, India). Ambient air was passed through a 20.3 cm × 25.4 cm GFF filter paper at a flow rate of 1.12 m3 min−1 (accuracy ±2%) for 8 h during the sampling period. Mass of the filter deposition has typical precision of 2 μg. The difference of weight of the filter paper was calculated to determine the PM10 (μg m−3). After collecting samples, filter papers were stored under dry condition at −20°C. The ion concentration in the PM2.5 and PM10 were analyzed in ion chromatograph (Model: S2000, Dionex, Sunnyvale, CA, USA) with conductivity detector. The lower detection limit of the ion chromatographs for anions were 0.1 ppb and that of Na+, K+ and Mg2+ were 3.0, 0.3 and 0.6 ppb respectively.
Meteorological parameters such as temperature (accuracy: ±1°C), relative humidity (RH) (accuracy: ±2%), wind direction (accuracy: ±3°) and wind speed (accuracy: ±2% of full scale) were recorded at every 5 min interval using calibrated Portable Weather Logger (M/s. RainWise Inc., Bar Harbor, Maine) at site A. All instruments were grounded and operated at 10 m height above the ground.
2.3 Potential source contribution function (PSCF)
PSCF analysis (Malm et al. 1994; Pekney et al. 2006) was used to determine the source region contributing to the SO2 mixing ratio at Site A. The basis of PSCF is that if a receptor site is located at particular lat-long, an air parcel back trajectory passing through that location indicates that the material from other sources can be transported through the trajectory to the receptor site. It follows the following equation,
where, n(ij) is the number of times that the trajectories passed through the cell (i,j) and m(ij) is the number of times that the concentration is high at the receptor site. The criteria for determining m(ij) were either the 75th or 90th percentile highest at the receptor. A cell with PSCF value close to 1 indicates probable source location.
The Hybrid Single-Particle Lagrangian Integrated Trajectory (HYSPLIT) model was used to calculate 7 days air mass back trajectories ending at Site A. HYSPLIT was run every day starting at 06:00 IST (Indian Standard Time), at a starting height of 500 m AGL. The trajectory domain was taken as 0° to 40° N latitude and 40° to 100°E longitude and divided into 0.1° × 0.1° cells, resulting into 40,000 total cells. Monthly PSCF for each cell was calculated using daily data of ambient SO2 mixing ratio at the receptor site (Site A).
3 Results and discussion
3.1 Seasonal variation of SO2 over Delhi
SO2 mixing ratio was recorded significantly low (1.24 ppb to 6.37 ppb) during monsoon and high (2.55 ppb to 17.43 ppb) during winter (Table 1) over Delhi in 2008. Figure 2 shows monthly variation of SO2 mixing ratio at NPL (Site A). Minimum SO2 mixing ratio was recorded during monsoon (1.24 ± 0.21 ppb) and maximum (3.21 ± 0.96 ppb) during winter. Monthly average ambient SO2 mixing ratio shows large variation during winter as compared to monsoon (Fig. 2) at site A. The general meteorology of this region during winter is dominated by high pressure usually centered over Western China causing increased atmospheric stability, which in turn allows for less general circulation and thus more stagnant air masses. Additionally, lack of precipitation during winter reduces the potential for wet deposition and associated cleansing mechanisms.
During winter, average mixing ratio of SO2 (ppb) at different AQM sites has followed the order: B(17.43) > C(15.00) > F(7.43) > D(6.53) > G(4.83) > I(4.75) > H(4.58) > E(3.06) > A(2.55). Industrial activities at site B and site C might have contributed to significantly higher SO2 during the period. During summer, average ambient SO2 mixing ratio was significantly low at almost all AQM stations except site B and C (Table 1). Significantly higher SO2 mixing ratio was recorded at site B (15.55 ppb) during summer. This may attribute to increase in industrial activities during the period. Lioy and Waldman (1989) have reported that the conditions for SO2 oxidation processes are more favorable during the warmer months than during the colder months. During monsoon, the lowest SO2 mixing ratio was recorded at all AQM sites and it may be due to wet deposition of SO2 with rain water.
3.2 Diurnal variation of SO2 mixing ratio and meteorological parameters
Figure 3a shows the monthly variation of diurnal cycle of SO2 mixing ratio at NPL site. Contour plots of ambient temperature (Fig. 3b) and RH (Fig. 3c) were analyzed to correlate variation of SO2 mixing ratio with meteorological parameters. Gamo et al. (1994) have reported significant decrease of atmospheric mixing height (1500 m) over Delhi during winter, which may lead to increase in the mixing ratio of SO2 (Fig. 3a). It was observed that SO2 mixing ratio increases when ambient temperature goes down and humidity goes up and vice versa (Fig. 3b, 3c). The relationship between ambient temperature and SO2 mixing ratio are more prominent in diurnal cycle of SO2 mixing ratio during winter.
During summer, no significant diurnal variation in SO2 mixing ratio was recorded (Fig. 3a). Table 2 establishes a significant negative correlation (r2 =−0.77) between ambient temperature and SO2 mixing ratio throughout the study period. Average ambient temperature (Fig. 3c) during summer was significantly high and there were large differences between day and night temperatures. Increase in oxidation of SO2 during daytime due to higher ambient temperature might have lead to lower SO2 mixing ratio; whereas, higher mixing height (4000 m) (Gamo et al. 1994) during night time allows dispersing of ambient SO2. Higher ambient temperature and higher mixing height together lead to nix of diurnal variation during summer.
During monsoon, no significant diurnal variation in SO2 mixing ratio was recorded (Fig. 3a). Wet deposition during monsoon diminishes the diurnal variation. SO2 mixing ratio shows a significant negative correlation with rainfall (r2 =−0.87) and RH (r2 =−0.59) (Table 2).
3.3 Variation of atmospheric aerosols (PM2.5 and PM10)
Acidic sulfates in the atmosphere consist of strongly acidic H2SO4, (NH4)HSO4 and weakly acidic (NH4)2SO4. When ambient aerosol samples (PM2.5 and PM10) were analyzed, the sulfate ion (SO 2−4 ) measured in the sample is the sum of the above three species. However, alkaline metallic SO 2−4 are also present in the aerosol with much smaller diameter (<2.5 μm) than acidic sulfates. The oxidation of SO2 to sulfuric acid and sulfate salts takes place via two main mechanisms: (i) a slow gas-phase (homogeneous) oxidation and (ii) a gas/particle phase (heterogeneous) oxidation (Hazi et al. 2003). The fine and ultrafine fractions of the ambient PM include the primary and secondary acidic sulfates, which are produced through both homogeneous and heterogeneous processes (Koutrakis and Kelly 1993; Pierson et al. 1989; Winchester and Bi 1984; Tanner et al. 1977). However, secondary sulfates from SO2 are only produced through gas/particle phase oxidation. Since acidic sulfates are mainly secondary pollutants formed through the oxidation processes described above, these pollutants can primarily be found in the fine fraction (PM2.5) of the atmospheric aerosols (Seinfeld and Pandis 1998). Fine acidic sulfates tend to persist in the atmosphere and may be transported long distances from their sources (Rodhe and Grandell 1981, Hazi et al. 2003). Most of the studies have investigated the PM10; however, very few data are available on the amount and the size distribution of sulfate ions within the PM2.5 and PM10 fractions of ambient PM (Hazi et al. 2003).
Chemical composition of aerosol at NPL site (A) during the study period shows higher concentration of SO 2−4 in both PM2.5 (10.54 μg m−3) and PM10 (14.44 μg m−3) mode of particulate matter during winter (Fig. 4). However, remaining ions such as Cl−, Na+, NO −3 , Ca2+ K+, Mg2+ in the PM2.5 fraction were also recorded high during winter (Fig. 4). Particulate Ca2+ concentration was recorded significantly high in the PM10 fraction during the study period (Fig. 4). Higher concentration of Ca2+ in PM10 fraction represents that the Ca2+ may be from the soil erosion from the local agricultural field.
Previous studies have used SO 2−4 /(SO 2−4 +SO2) ratio as a parameter to represent SO 2−4 formation (Kaneyasu et al. 1995). SO 2−4 /(SO 2−4 +SO2) ratio increases with air mass aging because of the chemical conversion of SO2 to SO 2−4 and faster SO2 loss rate than that of SO 2−4 dry deposition (Miyakawa et al. 2007). Significantly high SO 2−4 /(SO 2−4 + SO2) ratio in the PM2.5 (0.69) and PM10 (0.73) was recorded during winter (Fig. 5). Significantly higher mixing ratio of SO2 along with significantly high SO 2−4 /(SO 2−4 +SO2) ratio, suggests that local conversion of SO2 was very slow and particulate SO 2−4 were transported from distant sources during winter. Less sunlight during winter might have restricted the local formation of SO 2−4 from SO2. Significantly higher PM2.5/PM10 ratio (1.27) of Na+ was recorded during winter. It may be possible that during winter most of the SO 2−4 aerosols were present in the form of Na2SO4 which are much stable form of atmospheric sulfates (Hazi et al. 2003).
Significantly low SO 2−4 /(SO 2−4 +SO2) ratio in the PM2.5 (0.47) and PM10 (0.69) during summer (Fig. 5) indicates local conversion of SO2 to particulate SO 2−4 . This is also supported by significantly lower ratio of PM2.5/PM10 of minerals (Ca2+, K+, Mg2+) and SO 2−4 during summer. This is representing significantly higher concentration of mineral (Ca2+, K+, Mg2+) and SO 2−4 in PM10 fraction during summer. Normally bigger size of the particle i.e. PM10, may not be transported from long distance and may be from local soil or dust consisting of CaSO4, MgSO4 and K2SO4. Significant positive correlation of SO 2−4 was also recorded with Ca2+ (r2 = 0.78), Mg+ (r2 = 0.73) and K+ (r2 = 0.81) at PM10 level (Table 2). However, there are also long distance sources of minerals as indicated by significant positive correlation of SO 2−4 with Ca2+ (r2 = 0.50), K+ (r2 = 0.65) and Mg2+ (r2 = 0.49) in PM2.5 fraction (Table 3). Moderate humidity as well as high temperature during summer was also facilitated the local conversion of ambient SO2 to acidic SO 2−4 (Hazi et al. 2003). Significant negative correlation (r2 = 0.59) of RH and ambient SO2 mixing ratio (Table 2) also represents higher conversion of SO2 to acidic SO 2−4 during summer.
All these establish that the source of the SO 2−4 during summer may be from local sources. Energy demand increases during summer in the city which leads to increase use of coal in the power plants around Delhi. Apart from coal, uses of fossil fuel (Kerosene and petrol etc.) in the small scale industries also increases during summer. All these may lead to increase in the ambient SO2 mixing ratio over the area during summer. In addition, positive correlation between K+ and SO 2−4 (Table 2) in the coarse mode (PM10) suggests that SO 2−4 could be from the biomass burning in the nearby agricultural fields as potassium as a tracer of biomass burning. Higher humidity and rainfall during monsoon facilitate the wet deposition of ambient SO2 as acidic SO 2−4 .
3.4 Possible sources of SO2
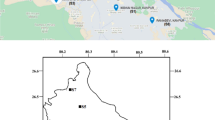
PSCF analysis suggests that during the month of January major contribution of SO2 at Site A was from cells placed between 45 to 65°E longitude and 20 to 25°N latitude (Fig. 6). Similar condition was prevailed upto March. Areas of northwestern Pakistan, eastern Rajasthan, Punjab, Haryana and Uttar Pradesh were probable contributing sources of ambient SO2 at site A (Fig. 7) during winter. Section 3.3 also established long distance contribution to ambient mixing ratio of SO2 during winter at Site A. However, during April and May contribution of SO2 is mostly regional (upto few 100 km of the receptor site) at Site A. With the onset of the southwest monsoon during June, the results of PSCF analysis changes and cells with higher values were reclined towards the south-western part of India. This suggests that part of the peninsular India may contribute to the SO2 mixing ratio at Site A during June. However, there is not much deviation in SO2 mixing ratio during August and September as represented in Fig. 2. During August SO2 mixing ratio over Site A varied between 1.13–1.43 ppb with an average of 1.26 ppb. This leads to higher value for m(ij)/n(ij) for all cells. However, PSCF analysis has recorded that during September three divergent sources exist, (i) source between 5 to 10°N latitude and 45 to 55°E longitude, (ii) source from peninsular and (iii) eastern India (Fig. 6). PSCF analysis also suggests that there may be potential marine contribution from Bay of Bengal and Arabian Sea to the SO2 mixing ratio in the ambient air at site A during monsoon as indicated by Fig. 6. Higher PM2.5/PM10 ratio of Cl− during monsoon (Fig. 4) also indicates that sea may be a major source of Cl− during monsoon.
However, PSCF analysis provides the information about possible sources from different locations. To identify the source at a particular location spatial distribution of SO2 mixing ratio are to be estimated. The methodology provided by Reddy and Venkataraman (2002b) may be useful to determine sources of SO2 at Site A along with this PSCF analysis.
3.5 Ambient SO2 and local wind pattern at site A
During winter, wind flows mainly from the west and northwestern sides at Site A (Fig. 7) and similar pattern was also shown by Goyel and Siddarth (2002). The high pressure zone in the western China impedes the north bound wind to move further. The cold temperature during winter and the high pressure zone together creates low level inversion over the area during winter. Analysis of aerosol chemical compositions and PSCF analysis has established that most of SO2 during winter might have been transported from long distance sources. The power plant in the northwest direction (neighboring states of Haryana, Punjab and Rajasthan) may also contribute to the higher SO2 and SO 2−4 concentration during winter (Kulshrestha et al., 2009). Transportation of SO −24 from long distance sources in western region may also be possible (Fig. 6) during winter.
During monsoon, major wind flow at Site A is from the southeastern direction, although there are occasional wind flow from western and northern directions. As mentioned in section 3.2, wet deposition of SO2 prevails during monsoon. However, PSCF analysis (Fig. 6) also indicates that there were significant contributions from eastern India. Thermal power plants located in Uttar Pradesh and Madhya Pradesh might have also contributed to SO2 over the study region during monsoon.
During summer, the wind speed was comparatively slow and wind flow was from all directions at site A; however, the major wind flow was from the southeast direction. PSCF analysis (Fig. 6) has shown that during summer contribution of SO2 is mostly from regional sources (upto few 100 km) at site A. Towards the southeast side, there are thermal power plants (Fig. 1), which may have contributed to the higher SO2 concentration at site A during summer. Gurjar et al. (2004) have shown that power plants contribute major amount of SO2 in the ambient atmosphere over Delhi. In addition, there may be contribution from Site C, which is an industrial area located towards the southeastern side of Site A. Section 3.3 has also established that the most of the SO 2−4 aerosols during summer are locally originated. The occasional winds from the southwestern direction may carry the pollutant load to the Site A from Site B.
4 Conclusions
The study has demonstrated the monthly variation of the SO2 mixing ratio and particulate SO 2−4 concentration in PM2.5 and PM10 aerosols over Delhi. The mixing ratio of SO2 was significantly high during winter and it may be due to the lower atmospheric mixing height and atmospheric stability due to high pressure zone development over western China. The study has established that the SO2, during winter might have been transported from long distance sources as indicated by combined analysis of SO 2−4 /(SO 2−4 +SO2) ratio and PSCF. The higher mixing ratio of SO2 during winter may also be due to transportation from different sources in the west or northwestern side of Delhi and got impeded in the atmosphere over the study area. Thermal power plants located at Panipat and Faridabad, i.e., in the northwestern side of the study area, might have also contributed to higher SO2 mixing ratio and particulate SO 2−4 concentration. SO 2−4 /(SO 2−4 +SO2) ratio also suggests that the high concentration of SO2 during summer might have been from local sources. Higher emission of SO2 from thermal power plants, industrial area in the southeastern and southern side of the study area might be major contributors to SO2 mixing ratio during summer. The study has also established that the soil erosion and biomass burning in local area may also attribute to SO 2−4 and SO2 during summer. The SO2 mixing ratio was low during the monsoon period and it may be due to wet deposition of SO2.
This study suggests that the transport sector may not be a major source for the ambient SO2 in Delhi. Multi point PSCF analysis along with annual study of SO2 mixing ratio at different locations, particularly at major sources (viz. thermal power plants, industrial area, biomass burning site) are essential to establish sources of ambient SO2 over Delhi.
References
Bhanarkar, A.D., Gajghate, D.G., Hasan, M.Z.: Air pollution concentration in Haryana subregion, India. Bull Environ Cont Toxicol 69(5), 690–695 (2002)
Census: India census: Report of the Registrar General and Commissioner, Govt. of India (2001)
Chelani, A.B., Devotta, S.: Air quality assessment in Delhi: before and after CNG as fuel. Environ Mont Assess 125, 257–263 (2007)
Gamo, M., Goyal, P., Kumari, M., Mohanty, U.C., Singh, M.P.: Mixed-layer characteristics as related to the monsoon climate of New Delhi. Bound-Lay Meteorol 67, 213–227 (1994)
Garg, A., Shukla, P.R., Bhattacharya, S., Dhadhwal, V.K.: Subregion (district) and sectoral level SO2 and NOx emission in India: assessment of inventories and mitigation. Atmos Environ 35, 703–713 (2001)
Goyel, P., Siddarta: Effects of winds on SO2 and SPM concentrations in Delhi. Atmos. Environ. 36, 2925–2930 (2002)
Gurjar, B.R., Lelieveld, J.: New Directions: Megacities and global change. Atmos Environ 39, 391–393 (2005)
Gurjar, B.R., Aardenne, J.A., Lelieveld, J., Mohan, M.: Emission estimate and trend (1990–2000) for megacity Delhi and implication. Atmos Environ 38, 5563–5681 (2004)
Guttikunda, S.K., Charmaichael, G.R., Calori, G., Eck, C., Woo, J.-H.: The contribution of megacities to regional sulphur pollution in Asia. Atmos Environ 37, 11–22 (2003)
Harison, R.M., Perry, R., Young, R.J.: Handbook of air pollution analysis (2nd edition), pp. 241–245. Chapman and Hall, London (1986)
Hazi, Y., Heikkinen, M.S.A., Cohen, B.S.: Size distribution of acidic sulfate ions in fine ambient particulate matter and assessment of source region effect. Atmos Environ 37, 5403–5413 (2003)
NEERI: Ambient Air Quality Status in Ten Cities in India: 1996. National Environmental Engineering Research Institute, Nagpur, India (2000)
Kaneyasu, N., Ohta, S., Murao, N.: Seasonal variation in the chemical composition of atmospheric aerosols and gaseous species in Sapporo, Japan. Atmos Environ 29, 1559–1568 (1995)
Koutrakis, P., Kelly, P.B.: Equilibrium size of atmospheric aerosol sulfates as a function of particle acidity and ambient relative humidity. J Geophys Res 98, 7141–7147 (1993)
Kulshrestha, U.C., Raman, R.S., Kulshrestha, M.J., Rao, T.N., Hazarika, P.J.: Secondary aerosol formation and identification of regional source locations by PSCF analysis in the Indo-gangetic region of India. J Atmos Chem 63, 33–47 (2009)
Lioy, P.J., Waldman, J.M.: Acidic sulfate aerosols: characterization and exposure. Environ Health Perspect 79, 15–34 (1989)
Malm, W.C., Sisler, J.F., Huffamn, D., Eldered, R.A., Cahil, T.A.: Spatial and seasonal treands in particle concentration and optical extraction in the United States. J Geophys Res 99, 1347–1370 (1994)
Mittal, S.K., Singh, N., Agarwal, R., Awasthi, A., Gupta, P.K.: Ambient air quality during wheat and rice straw stubble crop burning episodes in Patiala. Atmos Environ 43, 238–244 (2009)
Miyakawa, T., Takegawa, N., Kondo, Y.: Removal of sulfur dioxide and formation of sulfate aerosol in Tokyo. J Geophys Res 112, 13209–13221 (2007)
Pekney, N.J., Davidson, C.I., Zhou, L., Hopke, P.K.: Application of PSCF and CPF to PMF-Modeled Sources of PM2.5 in Pittsburgh. Aerosol Sci Technol 40, 952–961 (2006)
Pierson, W.R., Brachazec, W.W., Gorse Jr., R.A., Japar, S.M., Norbeck, J.M., Keeler, G.J.: Atmospheric acidity measurements on Allegheny Mountain and the origins of ambient acidity in the northeastern United States. Atmos Environ 23, 431–459 (1989)
Reddy, S.M., Venkataraman, C.: Inventory of aerosol and sulphur dioxide emissions from India: I— fossil fuel combustion. Atmos Environ 36, 677–697 (2002a)
Reddy, S.M., Venkataraman, C.: Inventory of aerosol and sulphur dioxide emissions from India. Part II— biomass combustion. Atmos Environ 36, 699–712 (2002b)
Rodhe, H., Grandell, J.: Estimates of characteristic times for precipitation scavenging. Atmos Sci J 38, 370–386 (1981)
Seinfeld, H.J., Pandis, N.S.: Atmospheric Chemistry and Physics. Wiley, New York (1998)
Tanner, R.L., Cederwall, R., Garber, R., Leah, D., Marlow, W., Meyers, R., Philip, M., Newman, L.: Separation and analysis of aerosol sulfate species at ambient concentrations. Atmos Environ 11, 955–966 (1977)
Winchester, J.W., Bi, M.T.: Fine and coarse aerosol composition in an urban setting: a case study in Beijing, China. Atmos Environ 18, 1399–1409 (1984)
Yang, S., He, H., Lu, S., Chen, D., Zhu, J.: Quantification of crop residue burning in the field and its influence on ambient air quality in Suqian, China. Atmos Environ 42(9), 1961–1969 (2008)
Acknowledgement
We are thankful to Head, Radio & Atmospheric Sciences Division, NPL; Directors, NPL, New Delhi and Indian Institute of Tropical Meteorology, Pune for providing facilities to conduct the study. CSIR (India) is greatly appreciated for providing research fellowship to A. Datta and T. Saud during the study. CPCB, New Delhi is appreciated for extending their support for the development of the ambient SO2 dataset at different locations in Delhi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Datta, A., Saud, T., Goel, A. et al. Variation of ambient SO2 over Delhi. J Atmos Chem 65, 127–143 (2010). https://doi.org/10.1007/s10874-011-9185-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10874-011-9185-2