Abstract
The present study investigated the comprehensive chemical composition [organic carbon (OC), elemental carbon (EC), water-soluble inorganic ionic components (WSICs), and major & trace elements] of particulate matter (PM2.5) and scrutinized their emission sources for urban region of Delhi. The 135 PM2.5 samples were collected from January 2013 to December 2014 and analyzed for chemical constituents for source apportionment study. The average concentration of PM2.5 was recorded as 121.9 ± 93.2 μg m−3 (range 25.1–429.8 μg m−3), whereas the total concentration of trace elements (Na, Ca, Mg, Al, S, Cl, K, Cr, Si, Ti, As, Br, Pb, Fe, Zn, and Mn) was accounted for ∼17% of PM2.5. Strong seasonal variation was observed in PM2.5 mass concentration and its chemical composition with maxima during winter and minima during monsoon seasons. The chemical composition of the PM2.5 was reconstructed using IMPROVE equation, which was observed to be in good agreement with the gravimetric mass. Source apportionment of PM2.5 was carried out using the following three different receptor models: principal component analysis with absolute principal component scores (PCA/APCS), which identified five major sources; UNMIX which identified four major sources; and positive matrix factorization (PMF), which explored seven major sources. The applied models were able to identify the major sources contributing to the PM2.5 and re-confirmed that secondary aerosols (SAs), soil/road dust (SD), vehicular emissions (VEs), biomass burning (BB), fossil fuel combustion (FFC), and industrial emission (IE) were dominant contributors to PM2.5 in Delhi. The influences of local and regional sources were also explored using 5-day backward air mass trajectory analysis, cluster analysis, and potential source contribution function (PSCF). Cluster and PSCF results indicated that local as well as long-transported PM2.5 from the north-west India and Pakistan were mostly pertinent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Delhi, the capital of India, has ranked as one of the megacities with the worst air quality in the world (WHO 2016). Grave air pollution and its allied health impacts have grown to be one of the major and foremost perturb in India. According to World Health Organization report (2015), India’s air quality is among the worst in the world, almost equivalent to China in respect of the population’s share susceptible to average air pollution levels exceeding World Health Organization thresholds. Particulate matter (PM) is crucial urban ambient air pollutant, especially fine mode particles (PM2.5), which are mainly produced by combustion processes, transformation of gaseous species, and forest fires (Sharma et al. 2007; Kong et al. 2011; Zheng et al. 2013; Sharma et al. 2016a, b). Fine mode particles have been reported to cause extensive detrimental effects on human health, environment, and climate (Schwartz and Dockery 1992; Buseck and Posfai 1999; Davidson et al. 2005; Ito et al. 2006; WHO 2009; Seinfeld and Pandis 2016). Several epidemiological studies conducted in recent years have revealed fine particulates to be causative of broad range of health effects, ranging from allergies, respiratory, cardiopulmonary, and cardiovascular diseases to premature mortality in severe cases (Ozkaynak and Thurston 1987; Pope and Dockery 2006; Mauderly and Chow 2008; Russell and Brunekreef 2009; Tie et al. 2009; Yan et al. 2009; Habre et al. 2011; Vernile et al. 2013), since fine particles have the ability to penetrate much deeper into the lungs and enter into bloodstream through human respiratory tract (Pope et al. 2002; Pope and Dockery 2006; Brauer et al. 2015; Van Donkelaar et al. 2015).
Particulate matter also plays an important role in climate change (IPCC 2013), and it tends to influence global climate change by absorption and scattering of solar radiation (Buseck and Posfai 1999; Seinfled and Pandis 2016). Furthermore, PM has other several pernicious influences on our environment like formation of smog/haze, material corrosion and damage, and ecosystem damage and visibility impairment (Reddy and Venkatraman 2000; Zhang et al. 2002). The small particle size of PM2.5 leads to a greater atmospheric residence time and a propensity to spread over a much larger geographic region (Wolff et al. 1985; Eldred and Cahill 1994; Wilson and Suh 1995). Since, the physical and chemical properties of these fine particles vary significantly with time, region, meteorology, and source category (Chow et al. 1994; Malm et al. 1994; Chow et al. 1996; Watson and Chow 2002; Chu 2004). Thus, the exigency to comprehend the potential source categories and their contributions (source apportionment) has become imperative to reduce the PM pollution (Zheng et al. 2005; Sharma et al. 2014b, c; Sharma et al. 2016a; Panda et al. 2016). Source apportionment results can furnish the scientific auxiliary for air quality management resolutions.
To deal with this matter, many tools have been employed for identification and quantification of PM sources (Paatero and Tapper 1994; Paatero 1997; Ulbrich et al. 2009) including receptor modeling that proffers a manner to complete the process by evaluating the pollutant concentrations at a sampling site. Receptor models have been globally accepted to quantitatively apportion and identify the sources of PM in the atmosphere (Watson and Chow 2005; Hopke et al. 2006; Mazzei and Prati 2009; Kong et al. 2010; Pant and Harrison 2012; Belis et al. 2013; Bove et al. 2014; Banerjee et al. 2015). Two major classes of models that have been used worldwide are (i) chemical mass balance (CMB) model and (ii) multivariate factor analysis models (including principal component analysis with absolute principal component scores (PCA/APCS), UNMIX, and positive matrix factorization (PMF)) (Hopke et al. 2003; Pandolfi et al. 2008; Alleman et al. 2010; Amodio et al. 2013). Both receptor and source profile data are required to be put in former class, whereas source profiles and their contributions can be extracted using latter set of models (Hopke 2003). The detailed principles and applications for PCA/APCS, UNMIX, and PMF models have been documented in literature (Thurston and Spengler 1985; Paatero 1997, 1999; Garcia et al. 2006; Song et al. 2006a; Chen et al. 2007; Gildemeister et al. 2007; Zheng et al. 2007; Olson and Norris 2008; Begum et al. 2010; Harrison et al. 2011; Gugamesetty et al. 2012; Wang et al. 2012; Lelpo et al. 2014; Shi et al. 2014) and our previous publications (Sharma et al. 2014b, c; Sharma et al. 2015; Sharma et al. 2016a, b). The detailed analysis of source apportionment of PM2.5 using PMF model is also available in our previous paper and reference therein (Sharma et al. 2016b).
The strengths and weaknesses for these models have been recapitulated in literature (Callen et al. 2009; Pant and Harrison 2012; Banerjee et al. 2015). Briefly, PCA/APCS is an exploratory tool for investigating structure in multivariate data sets by combining factor analysis with multilinear regression which aids in quantifying particulate source contribution (Viana et al. 2008). It identifies individual components’ group (PCs) using orthogonal decomposition, and loading factors connect these PCs with variables. PCs further connect with Varimax rotation to connect individual variables to different components (Chanand Mozurkewich 2007; Belis et al. 2013), whereas the UNMIX estimates the source number by minimizing the dimensionality of data by using singular value decomposition (SVD) method. High correlation exists within each component, while least or no correlation exists between individual components. PCA/APCS is considerably the most common model for source apportionment studies possibly because of its simplistic analytic procedure and have been frequently employed in the past for various source apportioning studies, but recently, a shift from PCA and classic factor analysis to PMF was observed mainly due to the ability of the model to provide better and more accurate results than PCA as PMF takes into account the uncertainty in the experimental data. PMF uses least squares approach and integrate non-negativity constraints in the optimization process in attempt to unravel the problem arising in factor analysis and utilize estimates of error for each data value as a point-by-point weight (Begum et al. 2004; Belis et al. 2013).
The present work aims to perform the comprehensive characterization of PM2.5 for an urban site of Delhi, India, during the period of January 2013 to December 2014. The inter-comparison of PM2.5 source apportionment results achieved using three receptor models (PCA/APCS, UNMIX, and PMF) to study their performances in source identification and in the quantification of source contributions are performed. Furthermore, the trajectory analysis and potential source contribution function (PSCF) are incorporated with secondary aerosol (SA) results to qualify the contribution of each identified local and long-range transport sources in a more accurate way (Kim et al. 2003; Kang et al. 2006; Lee and Hopke 2006; Heo et al. 2009).
Material and methods
Site description
Delhi, the capital of India, is surrounded by four different climatic zones (Himalayas in the north, central hot plains in the south, the Thar desert in the west, and the Indo-Gangetic Plain in the east), which influence its semi-arid climate and considered as one of the most polluted megacities in the world. PM2.5 samples were collected at CSIR-National Physical Laboratory, New Delhi (28° 38′ N, 77° 10′ E; 216 m amsl) (Fig. 1), India. The sampling site represents a typical urban environment surrounded by a heavy roadside traffic and agricultural fields in the southwest direction. The area is under the influence of air mass flow from north-east to north-west in winter, south-east to south-west in summer, and south-east to south-west in monsoon (Goyal and Sidhartha 2002). Although the traffic could be one of the major sources of PM in Delhi, significant contributions from other sources, i.e., roadside dust, secondary aerosol, biomass burning, and industrial emissions, have also been observed (Sharma et al. 2014b, c). It also observes the alarming vehicular growth rate with around 8.29 million of registered vehicles in 2013–2014 (Statistical Abstract of Delhi 2014). The ambient temperature of sampling site varies from minimum (∼3 °C) in winter (November to February) to maximum (∼47 °C) in summer (March to June). The average rainfall in Delhi during monsoon (July to September) was of the order of ∼900 mm. The detailed descriptions of sampling site including meteorology are available in Sharma et al. (2016a).
Sampling method
PM2.5 samples were collected (n = 135) periodically on pre-combusted (∼550 °C for at least 5 h to eradicate organic impurities) quartz micro-fiber filters (QM-A) during January 2013 to December 2014, using fine particle sampler (model APM 550, M/s. Envirotech, India), which was operated at an average flow rate of 1 m3 h−1 (accuracy ±2%) at a height of 10 m (above ground level) for 24 h. The flowmeter of the sampler was calibrated (with the accuracy of ±2% of full scale) with Air Flow Calibrator traceable to National Standard. The pre-combusted filters were desiccated for 24 h before measuring the initial and final weight by a micro-balance (M/s. Sartorius, resolution ±10 μg). After collecting samples, filters were stored under dry conditions in the deep freezer at −20 °C prior to analysis.
Chemical analysis
The quantitative elemental analyses of PM2.5 filters were carried out first by a non-destructive method using Rigaku ZSX Primus wavelength dispersive X-ray fluorescence spectrometer (WD-XRF). Then, ∼6.92 cm2 (size ½ of the filter) of QM-A filters was used for analysis of water-soluble inorganic ionic component (WSIC) using ion chromatograph, and rest of the filter was used for organic carbon (OC)/elemental carbon (EC) analysis using OC/EC carbon analyzer. Details of the PM2.5 filters analysis are given below.
WD-XRF (Rigaku ZSX Primus) was used for quantitative analysis of major and trace elements in PM2.5 (Al, Mg, S, Si, Cl, K, Ca, Ti, Cu, Mn, Fe, Zn, Br, Cr, As, and Pb). The spectrometer consists of scintillation counter (SC) and flow proportional counter (F-PC), detectors for heavy and light elements, respectively, sealed X-ray tube for excitation, end window, and an Rh target. The readings were taken in vacuum conditions at a temperature of 36 °C and a tube rating of 2.4 kW. The scan was made to identify all elements in the loaded filter except Si. The k–α X-spectral lines identify elements (Al, Mg, S, K, Cl, Ca, Ti, Si, Cr, Zn, Fe, and Mn) under particular conditions, i.e., F-PC detector for Mg, Al, P, S, Cl, K, and Ca; RX25 analyzer crystal for Mg; PET analyzer crystal for Al; Ge analyzer crystal for P, Cl, and S; LiF (200) analyzer crystal for Ca and K; and SC detector and LiF(200) analyzer crystal for Ti, Cr, Mn, Fe, and Zn. Measurements on blank filter were also taken, and correction in the intensities was done for loaded filters. Quantitative analysis was carried out using parameter method through ZSX software (Rigaku Corporation, Japan).
The EC and OC of PM2.5 were analyzed using OC/EC carbon analyzer (model DRI 2001A; Atmoslytic Inc., Calabasas, CA, USA) following the USEPA “IMPROVE protocol” with negative pyrolysis areas zeroed (Chow et al. 2004). The OC/EC carbon analyzer (DRI 2001A) works on the principle of the preferential oxidation of OC and EC, in which the sample heated at four different temperatures (140, 280, 480, and 580 °C) in pure helium and at three different temperatures (580, 740, and 840 °C) in 98% helium and 2% oxygen, since OC can be volatilized from the sample in a non-oxidizing helium atmosphere and EC is volatilized through combustion by an oxidizer. Correction for pyrolysis and charring of OC compounds into EC are predominantly carried by the optical component (laser reflectance and transmittance) of the analyzer (Chow et al. 2004). Approximately 0.536-cm2 area of QM-A filter was taken, cutting though the proper punch, and the values are given in μg cm−2 by the analysis software (CarbonNet). Each filter was analyzed triplicate with blank filters to get the concentrations of OC and EC in PM2.5 mass. The OC/EC analyzer was calibrated every day before analysis of the samples using mixture of 5% CH4 + balance helium standard gases (for OC/EC peak verification). The process was repeated after every five-sample analysis. A mixture of 4.8% CO2 + balance helium standard gases were used periodically for calibration of OC/EC analyzer in addition to calibration with KHP and sucrose. Details of OC and EC analysis of PM have been given in Sharma et al. (2014b).
For estimation of WSIC (Li+, Na+, NH4 +, K+, Ca2+, Mg2+, F−, Cl−, NO3−, and SO4 2−) of PM2.5, filters (∼6.92 cm2) were extracted in de-ionized water (resistivity 18.2 MΩ cm) for 90 min in ultrasonic extractor. The extract was filtered through 0.22-μm nylon membrane filters and transferred to polypropylene sample bottles and analyzed by ion chromatograph (model DIONEX-ICS-3000, Sunnyvale, CA, USA). The concentrations of F−, Cl−, NO3 −, and SO4 2− were determined by using an Ion Pac-AS11-HC analytical column with a guard column, ASRS-300 4-mm anion micro-membrane suppressor, 20 mM sodium hydroxide (NaOH; 50% w/w) as eluent, and de-ionized water as regenerator. Li+, Na+, NH4 +, K+, Ca2+, and Mg2+ were evaluated by using a guard column with a separation column, suppressor CSRS-300, and 5 mM methane sulfonic acid (MSA) as eluent. Chromeleon® software was used to process the chromatograms, and data of chromatography was collected at 5 Hz. Calibration standards have been prepared by National Institute of Standards and Technology (NIST, USA), traceable certified standards for calibration of ion chromatograph (Sharma et al. 2014a). The blank filters were also analyzed for cations (Li+, Na+, NH4 +, K+, Ca2+, and Mg2+) and anions (F−, Cl−, NO3−, and SO4 2−). The analytical error (repeatability) was approximated to be 3–5% based on triplicate analysis of each filter. Detailed principle and analytical procedure of WSIC of PM are described in Sharma et al. (2012a, b).
Descriptions of PCA/APCS, UNMIX, and PMF
Principal component analysis (PCA) is a statistical tool that identifies patterns in data, revealing their differences and similarities. It was performed to identify the possible sources of PM2.5 mass over the selected site. Orthogonal transformation method with Varimax rotation in PCA was employed in present study. The lowest eigenvalue for extracted factors was restricted to more than 1. Total 22 constituents/species of PM2.5 were used as variable in the data set. In PCA, dimensionless standardized form has been transformed from the chemical data:
where i = 1, …, n samples; j = 1, …m elements; C ij is the concentration of element j in sample i; and C j and σ j are the arithmetic mean concentration and the standard deviation for element j, respectively. The PCA model is expressed as
where k = 1, …, p sources and g ik and h kj are the factor loadings and the factor scores, respectively. Eigenvector decomposition helps in solving this equation (Song et al. 2006).
Source profiles and source contributions are then estimated quantitatively based on factor loading scores of PCA by using APCS method (Thurston and Spengler 1985; Henry and Hidy 1979). Since the data for PCA results are normalized, thus for each factor score, the true zero is derived as
The re-scale scores are known as APCS and further source contribution is obtained by linear regression using the following equation:
where M i is the measured mass concentrations in sample i and ζ 0 is the mass contribution made by sources unaccounted for in the PCA. APCS ki is the rotated absolute component score for source k in sample i, and ζ k APCS ki is the mass contribution in the sample i made by the source k. It follows the regression of the sample concentrations on these APCS to get each identified source’s estimated mass contribution, i.e., regression between C ij and APCS ki (Song et al. 2006).
UNMIX is a multivariate model with non-negativity constraints. It evaluates the source number by means of reducing data space dimensionality m to p through the SVD method (Henry 2003). The UNMIX model can be represented as
where V, U, and D are the p × m matrices and n × p and p × p diagonal, respectively. Ε ij is the error term comprising C ij variability, which is not accounted for by the first principal component (p). UNMIX uses self-modeling curve resolution to make sure that the results follow (within error) the non-negative constraints on source compositions and contributions. It ensures that all the species are on the same scale with a mean of 1 by normalizing the data set and then projected it perpendicular to the first axis of p-dimensional space. UNMIX finds edges in m-dimensional space using principal component analysis which are then used to compute vertices, where m is the number of ambient species. These vertices with the SVD decomposed matrices are used to find source profiles. The UNMIX version used for this study was the stand-alone EPA UNMIX 6.0.
PMF is a multivariate factor analysis receptor model that distributed the speciated sample data matrix into two matrices, which are factor contributions and factor profiles. The PMF in detail has been described in Paatero and Tapper (1994) and Paatero (1997). A speciated data set can be considered as a data matrix X of i by j dimensions, in which the number of samples (i) and chemical species (j) are measured. The purpose of PMF is to recognize the species profile (f) of each source, factors (p), and the amount of mass (g) contributed to each individual sample by each factor which is given as
where e ij is the residual for each sample/species.
For samples to have no negative contribution, the results are constrained accordingly. PMF weighs each data point individually, which permits the adjustment of the influence of each data point. Perhaps, below detection limit data can be taken in the model for use, with the associated uncertainty adjusted so these data points have less influence on the solution than the above detection limit measurements. The object function (Q) gets minimized by the PMF solution, based upon these uncertainties (u) as follows:
where X ij are the measured concentration (in μg m−3), u ij are the estimated uncertainty (in μg m−3), n is the number of samples, m is the number of species, and p is the number of sources included in the analysis. The detail descriptions of EPA PMF v3.0 are described in Gugamsetty et al. (2012) and EPA PMF User Guide (2008).
In this study, information on chemical properties of 135 PM2.5 samples has been used as input to the PMF model for the total of 22 parameters. The method detection limit (MDL) of each chemical species (OC, EC, WSIC, and major and trace elements) is calculated as 3 times of the average standard deviation of 10 replicates of filter blanks analysis. Overall, MDL were reported in Table 1. The signal-to-noise ratio (S/N ratio) is calculated by PMF model as per EPA PMF User Guide (2008), wherein species which have S/N ≥ 2 were categorized as strong in data quality. Species with S/N between 0.2 and 2 indicate weak data quality and are improbable to provide enough variation in concentration and consequently contribute to the noise in the results (if the S/N ratio of species are below 0.2, then they are classified as bad values and are thus excluded from further analysis). In the present case, S/N ratios of the species are estimated as >0.6 (Pb and Cu were estimated as 0.62 and 0.78, respectively).
In the present study, the model performance in a base run showed determination coefficient (R 2) between the modeled and experimental concentrations of PM2.5, OC, EC, NH4 +, SO4 2−, and NO3 − of 0.87, 0.86, 0.87, 0.97, 0.76, and 0.83, respectively (Table 1). Most of the other chemical species are also well reconstructed, except for some trace elements like Cr (R 2 = 0.55), Cu (R 2 = 0.57), and Al (R 2 = 0.54) (Table 1). These results are within the range of those presented in many PMF studies, with for example, values 0.71 reported for a study in Spain (Cusack et al. 2013) and of 0.96 for a study in Germany (Beuck et al. 2011) for PM2.5 mass reconstruction. Scaled residuals between −3 and +3 are obtained for all of the major components, and the value of Q robust is strictly identical to the value of Q true, all of these showing that no specific event is affecting the results and that the base run can be regarded as stable.
Air mass back trajectory and cluster analysis
In order to identify and trace the trans-boundary movement of PM2.5 from their potential source of origin to the receptor site, 24-h backward air mass trajectories for each experimental day (sampling was performed at 1030 h IST), starting at 0500 h Universal Coordinated Time (UTC) at height of 500 m above ground level (agl) at the sampling site, were plotted employing the National Oceanic and Atmospheric Administration (NOAA) Air Resource Laboratory’s (ARL) Hybrid Single-Particle Lagrangian Integrated Trajectory (HYSPLIT) model (http://ready.arl.noaa.gov/HYSPLIT.php) with the Global Data Assimilation System (GDAS) data as input (Draxler and Rolph 2003; Wang et al. 2015).
Cluster analysis aids in classifying trajectories with similar pathways, differentiating the mean transport pathway from the numerous existent trajectories to the receptor site, thereby demarcating trajectories into the clusters carrying maximum homogeneity within and maximum heterogeneity between themselves (Brankov et al. 1998; Wang et al. 2009). The groups of trajectories have been clustered together to represent the four major directions of trans-boundary migration of polluted air mass and to present an enhanced visualization for recognition of source regions. It must be taken into consideration that cluster analysis can only depict the general directions in which the potential sources may lie, but fall short in indicating the precise locations of the sources.
PSCF analysis
To identify the potential source regions contributing to PM2.5 mass over the observational site, the PSCF was employed for the study period, using air mass trajectory statistics software, TrajStat (version 1.2.2.6). PSCF is a hybrid receptor model applied to estimate the air parcel carrying the certain level of pollutant concentration advancing through explicit upwind source area (Ashbaugh et al. 1985; Hwang and Hopke 2007). The probable source area is divided into a gridded i by j array. PSCF follows the following equation:
where PSCF ij is the probability that air mass originates in the ijth cell on days with high species concentrations, m(ij) is the number of times that the concentration is higher than the pre-determined criterion value (75th percentile of PM2.5 is taken in the present study) at the receptor site, and n(ij) is the number of times that the trajectories passed through the cell (i, j). A cell with PSCF value close to 1 indicates probable source location.
Results and discussions
Elemental concentration and reconstructed PM2.5
The mass concentration of PM2.5 has ranged from 25.1 to 429.8 μg m−3 with an average value of 121.9 ± 93.3 μg m−3 during January 2013 to December 2014. The seasonal variation and average concentrations of OC, EC, WSIC, and major and trace elements of PM2.5 with maxima and minima are listed in Table 2. Mandal et al. (2014) also reported the similar average concentration of PM2.5 (142.50 ± 23.57 μg m−3) for an industrial area of Delhi, whereas Panda et al. () reported higher concentration of PM2.5 (186.25 ± 47.46 μg m−3) at Delhi. Other studies have also reported higher ambient PM2.5 concentrations in New Delhi (Singh et al. 2011; Tiwari et al. 2014; Trivedi et al. 2014), and most of them exceed the National Ambient Air Quality Standards (60 μg m−3 for 24-h average and 40 μg m−3 for annual average).
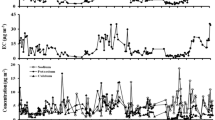
The average concentrations of OC and EC of PM2.5 were 17.6 ± 14.1 μg m−3 (∼14.4% of PM2.5 mass) and 10.2 ± 7.5 μg m−3 (∼8.4% of PM2.5 mass), respectively. Whereas the contribution of total carbon (TC = OC + EC) was ∼22.8% of PM2.5 mass during the study. Panda et al. (2016) have reported the lower values of percentage contribution of TC in PM2.5 (∼15.0% of PM2.5 mass) at Delhi while higher values were obtained at Bhubaneswar (∼30.4% of PM2.5 mass), whereas Mandal et al. (2014) reported the higher percentage of TC in PM2.5 (37.3% of PM2.5 mass) in an industrial area of Delhi. Ram and Sarin (2011) observed higher percent contribution of TC in PM2.5 at Kanpur (∼50% of PM2.5 mass). The emissions from vehicles and biomass burning (agricultural waste burning and wood burning) significantly contribute to the atmospheric concentrations of OC and EC (Ram and Sarin 2011; Sharma et al. 2016a). The concentrations of OC and EC of PM2.5 varied significantly during winter, summer, and monsoon seasons (Table 2). During winter, the concentrations of OC and EC of PM2.5 were recorded more than twice of the concentrations of OC and EC during summer and monsoon seasons. The scatter plots between OC vs. EC, OC vs. PM2.5, and EC vs. PM2.5 for the winter, summer, and monsoon seasons are depicted in Fig. 2. A significant positive linear trend between OC and EC have been observed during winter (R 2 = 0.81; p < 0.05), summer (R 2 = 0.75; p < 0.05), and monsoon (R 2 = 0.96; p < 0.05) seasons, which is indicative of their common sources, i.e., vehicular emissions and biomass burning (Salma et al. 2004; Sharma et al. 2014c). While weakly correlated values of OC and EC signify the formation of secondary aerosols in the atmosphere through a photochemical reaction under conditions favorable for gas to particle conversion of VOCs (Begum et al. 2010).
The average concentration of major and trace elements in PM2.5 was recorded as 20.2 ± 2.4 μg m−3 which accounts for ∼17% of PM2.5, whereas the WSIC accounts for ∼38% of PM2.5 mass concentration. The average concentrations of NH4 +, SO4 2−, and NO3 − of PM2.5 were recorded as 9.4 ± 8.2, 12.8 ± 8.0, and 9.7 ± 7.8 μg m−3, respectively (Table 2). Figure 3 shows the charge balance between NH4 + and SO4 2−, NO3 −, and Cl− of PM2.5 during winter (R 2 = 0.68), summer (R 2 = 0.12), and monsoon (R 2 = 0.07) seasons. During winter, the molar mass of SO4 2−, NO3 −, and Cl− are significantly correlated with molar mass of NH4 +, which indicates the possible formation of secondary aerosols [(NH4)2SO4, NH4NO3, and NH4Cl] (Sharma et al. 2012b; Sharma et al. 2015), whereas during summer and monsoon seasons, molar mass of SO4 2−, NO3 −, and Cl− are non-significantly correlated with molar mass of NH4 + ions. In (NH4)2SO4, the molar ratio of NH4 + to SO4 2− is 2:1. The molar ratio of greater than 2 signifies the abundance of NH4 +, which has potential to combine with NO3 − or other ions (Sharma et al. 2012b; Sharma et al. 2014a, c). In the present study, the equivalent molar ratio of NH4 +/SO4 2− is >2, which shows the complete neutralization of atmospheric acid gas H2SO4 and predominant to aerosol formation during winter season. Meteorological conditions (temperature and RH) play an important role in the formation of secondary aerosols (NH4NO3, NH4HSO4, and (NH4)2SO4). The reaction of H2SO4 with NH3 is favored leading to the formation of (NH4)2SO4 and NH4HSO4, left over NH3, then react with HNO3 to form NH4NO3 (Meng et al. 2011; Li et al. 2013; Sharma et al. 2014b). In summer and monsoon, NH4 + was not significantly correlated with anions; this particular observation alludes towards the active role of NH4 + in particulate formation during winter seasons (Saxena et al. 2017).
Reconstructed mass of PM2.5
Chemical constituents/species of PM2.5 mass were re-constructed (RCPM2.5) to determine the relative contribution of measured chemical species to the measured PM2.5 mass and their respective relationships using IMPROVE equation (Chan et al. 1997; Malm et al. 2007). In the present study, RCPM2.5 was calculated using Eq. 9.
where AS is the ammonium sulfate, AN is the ammonium nitrate, POM is the particulate organic matter, LAC is the light-absorbing carbon, and SS is the sea salt. The concentrations of the respective factors in Eq. 9 are calculated by multiplying their individual concentration with their conversion factors as described in Table S-1 (in supplementary information). The mass difference (dM = PM2.5 − RCPM2.5) of PM2.5 was calculated by subtracting RCPM2.5 from measured PM2.5 (Chan et al. 1997; Malm et al. 2007; Sharma et al. 2014c). The average mass difference between PM2.5 and RCPM2.5 was recorded as 18.0 μg m−3 (∼15% of PM2.5 mass) during the study period which may be attributable to unidentified mass (UM) of PM2.5. The mass difference (UM) could be due to carbonate-rich minerals, calcium sulfate, and alumino-silicates (Zhang et al. 2010; Ram et al. 2012). The observed average re-constructed concentration of all factors and their percent contributions to PM2.5 are given in Table S-1 and Fig. 4, respectively. The result showed that the contributions of AN and AS to PM2.5 were ∼10 and ∼15%, respectively. Majorly, AS is produced in the atmosphere through the chemical reactions of SO2, which emits from combustion of fossil fuels (coal and diesel) and AN is produced through reversible reactions of gas-phase NH3 and HNO3, aided by the formation of oxidized nitrogen through combustion of fossil fuels and vehicular emissions (Ram et al. 2012; Sharma et al. 2015, 2016a). The POM (1.6 [OC]) contributed 23% of RCPM2.5. The sources of POM in the atmosphere are both primary (combustion of fossil fuel or biomass) and secondary (secondary organic aerosols formed through oxidation of gas phase precursors) formation. POM was found to be the most abundant species in PM2.5, which is similar with other urban or roadside measurements (Phuleria et al. 2007; Chen et al. 2010; Gugamsetty et al. 2012; Sharma et al. 2014c). The annual average LAC was estimated as 10.2 μg m−3 (∼8% of RCPM2.5). LAC (black carbon, elemental carbon, or graphite carbon) is largely emitted through incomplete combustion of fossil fuels and biomass burning. The observed contribution of soil dust was 13%, which is expected to be higher at sampling location due to impact of local as well as trans-boundary transport from the Thar desert and other Asian regions. The sequence of percentage contribution of these components to PM2.5 mass is POM > SS > AS > soil > AN > LAC (Fig. 3).
Source apportionment
In the present study, PCA, UNMIX, and PMF analyses have been done to estimate source profiles and contributions to PM2.5 and results have been inter-compared. PCA/APCS, UNMIX, and PMF extracted sources and their source contributions are summarized in Tables 3 and 4, respectively. Summary of the average percent contribution of major source types of PM2.5 mass at Delhi, India, and other countries are summarized in Table 5.
PCA/APCS
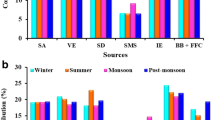
In total, 22 variables (constituents of PM2.5) with 135 PM2.5 samples have been analyzed through PCA coupled with APCS using software package SPSS version 11.5 and Varimax rotation has been employed. All variables were typified and transformed into a dimensionless standardized form prior to statistical analysis, and the lowest eigenvalue for extracted factors was restricted to more than 1 that explained the 72.4% of the variance of the data. PCA/APCS analysis identified five different sources of PM2.5 based on the loading of the variables in the factor (Table 3).
The first factor was dominated by high nitrate, sulfate, and ammonium, which marks the presence of secondary sulfate and secondary nitrate mixture. These secondary products are formed in the atmosphere, being emitted either by natural or anthropogenic sources. This source has explained about 23.4% variance and contributed to about 27.2% for PM2.5 mass concentration. Also, the abundances of elements like As, Zn, Fe, Cu, Cr, Pb, and S indicate the emissions arriving from the industrial source, thus making this factor to be a mixed type source (SA + industrial emission (IE)). The secondary nitrate is formed by the oxidation of NOx and is favored by low temperature (Li et al. 2004), while high temperature and strong solar radiations favor the formation of secondary sulfates through photochemical reactions (Seinfield and Pandis 2016). The presence of secondary aerosols over Delhi is in agreement with the studies done on trace gases, i.e., NH3, SO2, and NOx by Sharma et al. (2012b) and Sharma et al. (2014b). NH4 + and SO4 2− have also been used as a marker for biomass burning and coal combustion, respectively (Pant and Harrison 2012). Kumar et al. (2001) used Cu, Mn, and Ni as tracers for industrial emissions in Mumbai; Sharma et al. (2014b) used Cu, Cr, Mn, Ni, Co, and Zn as industrial emission tracers for metal manufacturing plants in Delhi; Kulshrestha et al. (2009) used a combination of Ni, Cu, Fe, and Cr as a marker for construction activities in Agra; and Kar et al. (2010) used Zn, Cu, and Ni as tracers of galvanizing, metallurgy, and electroplating industries while Cr from tannery industry in Kolkata.
The second factor was associated to vehicular emission and it explained 22.4% of the variance. The high loading of OC, EC, Zn, Fe, Mn, and Pb attributed to vehicular emissions contributes ∼23.5% to PM2.5 mass. EC is used extensively as marker for diesel exhaust (Song et al. 2006; Yin et al. 2010) and it is predominantly emitted from combustion sources and shows limited chemical transformations, while OC can be contributed directly by primary emission sources (vaporization and combustion of solvents and fuel) and through chemical reactions among primary gaseous OC in the atmosphere (Turpin et al. 1991; Ho et al. 2003; Behera and Sharma 2010). The source represents the abundances of Pb, Zn, Fe, Mn, Cu, and Al, which are also related to the traffic source profiles. Zn comes from two stroke engines as it is used as a fuel additive and tire wear (Kothai et al. 2008), Fe from wear and tear of break and wear of metal in the exhaust system (Gupta et al. 2007; Karar and Gupta 2007), and Pb from gasoline additives, brake pads, and road dust re-suspensions (Pant and Harrison 2012). Mn is used as an additive in unleaded gasoline (Kulshrestha et al. 2009), and combinations of Cu and Al signify emissions from brake lining wearing (Srimuruganandam and Nagendra 2012a, 2012b).
Third factor is rich in Na+, Cl−, Mg2+, and K+, representing the sea salt source, and it explained 12.2% of variance. None of the less, the use of some markers is rather perplexing and may be influenced by different sources like K+ from biomass and wood burning, Cl− from coal burning, and Mg from crustal emissions. The presence of NO3 − in this source concurs with the possibility of its marine origin through condensation of HNO3 (Srimuruganandam and Nagendra 2012a, 2012b). PCA analysis reveals that sea salt has contributed 15.4% to PM2.5 concentration.
Fourth factor represents the soil-related sources with crustal elements, i.e., Al, Si, Ca, Mn, Mg, and Fe. It explained 8.4% variance and 21.5% contribution to PM2.5 concentration. Wide range of elements (Al, Ca, Si, Ti, Mg, Pb, Cu, Cr, Ni, Co, Na, K, and Zn) have been used as tracer species for crustal elements in India (Balachandran et al. 2000; Khillare et al. 2004; Chelani et al. 2008; Chakrobarty and Gupta 2010; Shridhar et al. 2010). The presence of Cl− and SO4 2− with OC and TC signifies the soil dust emissions, while occurrence of Fe with OC and TC marked for road dust (Gupta et al. 2007; Banerjee et al. 2015). Hence, the presence of both combinations in the present study manifests the mixed emissions from road and soil dust.
Fifth factor is dominated by K+ and NH4 +, signifying the source of biomass burning emissions, and it explained 7.4% variance. In India, biomass burning commonly alludes to combination of cow dung and fuel wood burning, agricultural burning, and wildfire. K+ is used in many source apportionment studies conducted in Asia and Europe as a marker of biomass burning (Rodriguez et al. 2004; Andersen et al. 2007; Pant and Harrison 2012). Also, NH4 + with K+ has been used as a marker combination to identify the burning emissions (Khare and Baruah 2010; Sharma et al. 2014c), which justifies the presence of NH4 + with K+ in the present study and thus representing this source to be of biomass burning emission. Biomass burning contributes 12.3% to PM2.5 mass concentration in the present study.
UNMIX
The 22 species were taken into account and PM2.5 was considered as the total mass in UNMIX model, which identified four sources. Silicon (Si) was discarded by the model according to suggested exclusion. Numerical values for the solution’s diagnostic indicators (R 2 = 0.76 and S/N ratio = 2.07) were consistent with recommendations. UNMIX re-samples the data 100 times and uses the bootstrap method to calculate uncertainties.
Source 1: This source was inferred as soil/road dust (SD) due to high composition of crustal elements (Al, Ti, Fe, Ca, and Mg) in the UNMIX results, and the results are comparable with PCA and PMF output. UNMIX analysis showed that soil dust has contributed 24.3% to PM2.5 mass. Crustal elements are major constituents of airborne soil and road dust and generally contribute to coarse aerosols, including Al, Si, Ca, Ti, Mg, Fe, and Na that are used as tracers for soil dust and/or crustal re-suspension (Lough et al. 2005; Begum et al. 2011; Yin et al. 2010; Pant et al. 2012).
Source 2: Higher concentrations of OC, EC, Zn, Fe, K+, Na+, Cl−, Ca, Mg, and SO4 2− indicate that this source may have been a mixture of vehicular emissions, biomass burning, coal combustion, and aged sea salt and contributed to about 32.2% for PM2.5 mass concentration. Higher concentrations of OC, EC, Zn, and Fe indicate the possible contribution of vehicular emission (Yin et al. 2010; Sharma et al. 2016b). Biomass burning and coal combustion are characterized by the high concentrations of K+, Cl−, and SO4 2− (Pant and Harrison 2012; Banerjee et al. 2015), since the results of UNMIX showed the high concentrations of Na+ and Mg2+ along with K+ and Cl−. Hence, it is considered as a mixed source, whereas PCA and PMF results well differentiated the two sources (biomass burning and sea salt).
Source 3: This source corresponds to secondary aerosols due to the presence of high concentrations of nitrate, sulfate, and ammonium. Thus, this source was identified as a mixture of secondary nitrate and secondary sulfate in UNMIX analysis. UNMIX analysis shows 30% contribution of secondary aerosols to PM2.5 in the present study.
Source 4: It is observed as IE source as UNMIX analysis results show high-factor loading by the heavy metal elements like Cr, Zn, As, Fe, and Cu, which were also dominant in the PMF industry source. Some industrial sources such as metal manufacturing plants and storage are located near the sampling site, and these could probably affect it. Largely, Zn, Cu, As, Fe, Cr, Cd, Mo, Ni, and S have been used as marker species for industrial emanations (Sharma et al., 2016a, b). Present UNMIX analysis recognizes that IE have contributed to about 13.4% for PM2.5 mass concentration.
PMF
The PMF model identified 7 sources (secondary aerosol, soil dust, vehicular emission, biomass burning, fossil fuel combustion, industrial emissions, and sea salt) using 22 chemical species of 135 PM2.5 samples collected during the study period (Fig. 5 and Table 4). In order to diminish the influence of extreme values in the PMF solution, model was made to run in the default robust mode and different numbers of sources were identified using a trial-and-error method to find the optimal number of sources. Further, the data sets using factors were entered into the PMF model for final analysis and the resultant change in the Q values was examined. In this study, the theoretical Q value was estimated to be approximately 2970 (i.e., 135 × 22). Robust Q value was the value for which the impact of outlier was minimized, while true Q value was the value for which the influence of extreme values was not controlled. The robust Q values were close to the true Q values in this study, which entails the reasonable fitness of the model with the outlier. Also, it is imperative that the range of Q values should be adequately small from the random runs (100 runs in the present study) to corroborate the attainment of a similar global minimum and thus confirms the fitness of outliers equally well for each random run. At the 7% of the error constant, over 95% of Q values were nearly to 2970 for seven-factor solution, indicating the global minimum of the Q value. Based on the estimation of the model results, the Q value variations in the model, the seven-factor solution delivered the most viable results. The descriptions of the PMF model and source apportionment of PM2.5 have been discussed in detail in our previous paper Sharma et al. (2016b) and reference therein.
Source 1: PMF analysis shows that secondary aerosols have contributed to about 21.3% for PM2.5 mass concentrations with the dominated key markers of NO3 −, SO4 2−, and NH4 + (Fig. 5). The secondary aerosols are mainly composed of (NH4)2SO4 and NH4NO3 deriving from the gaseous precursors NH3, SO2, and NOx. The study evidenced the abundances of ambient NH3, NOx, and SO2 over Delhi (Sharma et al. 2014a).
Source 2: Soil dust includes most of the crustal elements and has high concentrations of Fe, Si, Ca, Na, Cu, Mg, and Al. PMF analysis showed that soil dust has contributed 20.5% to PM2.5 mass. Many other researchers cite these elements (Fe, Si, Ca, Na, Mg, Cu, Ni, and Al) as a soil dust source (Moreno et al. 2013; Mustaffa et al. 2014; Shen et al. 2010; Waked et al. 2014). A whole array of marker elements that have been used in India for identification of soil dust include Al, Si, Ca, Ti, Fe, Pb, Cu, Cr, Ni, Co, and Mn (Killare et al. 2004; Chelani et al. 2008; Gupta et al. 2012; Sharma et al. 2016a). Cu, Zn, and Ba are associated with road dust due to the release of these marker elements from cars and from non-exhaust sources.
Source 3: In this source, Cu, Zn, Mn, Pb, and EC contributed significantly, which have been considered as an indicator of vehicle emission. PMF analysis indicates that vehicle emissions have contributed 19.7% in PM2.5 at sampling site of Delhi. Vehicular emissions are a major source of the PM, and research indicates that they contribute 10 to 80% to PM in cities across India (Sharma et al. 2014c; Banerjee et al. 2015; Sharma et al. 2016b). But various studies have computed different vehicular sources (exhausts, re-suspension, abrasion, etc.) which make their comparison more complicated. A study by Begum et al. (2010) conducted in Dhaka and by Santoso et al. (2013) at roadside in Jakarta defined Pb in PM2.5 releasing from the pre-existing road dust by PMF. Choi et al. (2013) also introduced Pb in PM2.5 as a tracer for motor vehicle source. Further, Zn in PM2.5 appeared to have a motor vehicle as resolved by PMF (Brown et al. 2007).
Source 4: Emission from biomass burning, wood burning, and vegetative burning have been characterized as presence of high concentration of K+ (Ram et al. 2010; Pant and Harison. 2012). The K+ ion has been widely cited in the literature as an excellent tracer representing a wood or biomass burning source (Mustaffa et al. 2014). In India, K+ has also been used as a key marker of biomass burning for PM (Ram et al. 2010), whereas levoglucosan is the key organic marker (Chowdhury et al. 2007). PMF analysis reveals that biomass burning has contributed 14.3% for PM2.5 mass in the present study. Depending on the season and location, biomass burning has been assessed to contribute in the range of 7–20% (Chowdhury et al. 2007), which has been reported to be one of the major sources in Delhi, predominantly in winter due to combustion of wood (Sharma et al. 2003; Srivastava and Jain 2007).
Source 5: Abundance of marker elements Al, Cl, Fe, Zn, Cr, and SO4 2− at the sampling site indicate the source of fossil fuel combustion to PM2.5 mass. Zn and Se are marker species for coal combustion and oil-fired power plants, respectively (Lee et al. 2002), whereas Ni and V are tracer species for the combustion of heating oils (Vallius et al. 2005). PMF analysis shows that fossil fuel combustion has contributed 13.7% for PM2.5 mass in the present study. Gupta et al. (2007) used As, Se, Te, and SO4 2− as markers for coal combustion and reported the contribution of 6–30% to PM mass.
Source 6: The industrial emissions are characterized by high concentrations of Cr, Mn, Zn, and S, possibly emanates from metal manufacturing plants and storage which are located near the sampling location. An array of tracer species (Ni, Cr, Co, Cd, Zn, As, Fe, Cu, Mn, S, and Mo) have been used in India to identify specific industrial emissions (Banerjee et al. 2015; Sharma et al. 2014c). In this study, PMF resolved 6.2% contribution to industrial emissions for PM2.5 mass.
Source 7: The higher concentrations of Na+, K+, and Cl− in PM2.5 mass indicate the contribution of sea salt. The present study shows that sea salt contributed to about 4.3% for PM2.5. The presence of K+ along with Na+ and Cl− in PM2.5 offers possible confusion with wood/biomass/coal burning, but a combination of four elements (Na, K, Cl, and Mg) should provide a reliable signature (Sharma et al. 2016a). Begum et al. (2010) identified sea salt in PM2.5 by PMF in Dhaka based on the presence of Na+ and Cl−. Choi et al. (2013) defined sea salt source in Seoul, Korea, due to the high contribution of Na+ and Cl− in PM2.5 concentration. Several other studies in East, Southeast, and South Asia assigned a sea salt source in PM2.5 considering Na+ and Cl− from the model output of PMF (Lee et al., 1999; Santoso et al. 2013; Senevirante et al. 2011).
Model comparison
The present study depicts the analysis by three different receptor models of same PM2.5 data set which provides an opportunity to shape the decision about evaluation of the degree to which these models concur, differ, or complement one another. The PMF analysis identified seven sources (SD, vehicular emission (VE), SA, biomass burning (BB), SS, IE, fossil fuel combustion (FFC)), whereas UNMIX and PCA showed four sources [SD, mixed type (VE + BB + SS), SA, and IE] and five sources (SD, mixed type (SA + IE), VE, SS, BB), respectively. The UNMIX analysis resulted in one mixed type source, combining traffic, biomass burning, and sea salt precursors together and PCA resulted in mixed secondary aerosol and industrial emission markers, whereas in PMF results, these sources were well differentiated by their corresponding tracers. Each receptor model identifies sources differently, majorly based on the considerations of the models to choose the species selected as variables. PCA and PMF extracted similar sources; however, the former was unable to differentiate the fossil fuel factor from the vehicular factor. Comparable study was also done by Callen et al. (2009) and similar results were observed (Cesari et al. 2016). It is noteworthy that the primary disparity between PCA and PMF lies in the non-negativity of loadings and score factors (built in PMF) and inclusion of individual data uncertainties. In many ways, PMF resembles PCA except in exclusion of all the negative entries (Paatero and Tappert 1994). Additionally, it has an edge over PCA in handling missing or below detection level data. UNMIX can be easily used in data sets which do not have comprehensive source profile information. It is helpful in distinguishing the most influential sources, whereas it underperforms to make agreement between expected and estimated contributions for weaker sources (Henry 2003). UNMIX differs from PCA; in that, it uses a new conversion method to derive significant factors based on the self-modeling curve resolution technique. The UNMIX solution is greatly reliant on the selected species, and on the other hand, this model showed problem in identifying the sources with low percentage to the total mass corroborating the weakness of UNMIX related to identify infrequent and relatively small sources. Moreover, it does not account uncertainties in ambient measurements and is unable to process samples with missing data (Banerjee et al. 2015), whereas PMF analyzes individual data points separately, normalizing the influences of each data points on the basis of measurement confidence (USEPA 2008).
Since, PMF employs a point-by-point least squares minimization scheme and not the correlation matrix information. Thus, the resulting profiles can be compared directly to the input matrix without transformation. However, PMF requires a large data set, preferably much more than the numbers of concerned factors, and allocation of weighing factor associated with each measurement is to be done. Contrastingly in PCA, the load matrix is non-dimensional and has to be combined with multilinear regression analysis to procure the source profiles. PCA tends to generalize the information that the data set originally have, as it depends on statistical association of data and not on chemical nature of particulates. PCA does not account a non-negativity constraint which makes it different from UNMIX and PMF. Another drawback of PCA is the use of single marker for multiple sources or availability of specific tracers (Belis et al. 2013). Furthermore, the model assumes the normal distribution of data set which may not be effective for all the cases, but error on the reconstructed concentration matrix is less when the APCS is used (Cesari et al. 2016). UNMIX provides a relatively coarse means of down-weighting outliers and PMF employs point-by-point error estimation in the data, allowing down-weighting of missing observations and outliers. This limitation is related to the models (PCA and UNMIX) because they do not take into account the uncertainty in the experimental data. That could be one of the reasons why PMF model provided better results than PCA and UNMIX model (Banerjee et al. 2015; Sharma et al. 2015).
Backward air mass trajectory and cluster analysis
It has become evident from Fig. 6 that majority of the air mass parcel during the study period is approaching to receptor site from arid landscapes of Rajasthan (Thar desert), Pakistan, Afghanistan, Indo-Gangetic Plain (IGP) region, and its surrounding areas during winter and summer seasons, whereas during monsoon season, the approaching air mass is transported from IGP region, Bay of Bengal, and Arabian Sea through Thar desert. The trajectories have been plotted to trace the origin and transport pathways of air masses as they are ascribed to letting influx of pollutants and their precursors in the city. These groups of trajectories have been clustered together (Fig. 6) to evince the major transport pathways of the polluted air mass flow, so as to provide an envisage of dominant source regions precisely. It can be discerned that the source regions are both trans-boundary and locally originated from continental landmass, which is also supported by the chemical composition of the pollutants found at the observational site. Sharma et al. (2014a) had also observed the similar trajectories at Delhi.
PSCF
To delineate the probable source regions which could be causative of augmenting PM2.5 concentration at the receptor site, a PSCF analysis was done (Fig. 6) for the study period. In the present study, the pollution criterion value was considered to be 75th percentile of PM2.5 concentration. The grids with a probability of <0.1 are transparent, and different colors (as shown in the PSCF map) denote the lowest to highest probability grids. Delhi was observed to have a significant amount of potential source areas within highly polluted and populated regions of north-western India such as Punjab, Haryana, Rajasthan, Uttar Pradesh, and parts of Pakistan and Afghanistan which contain key oil fields, refineries, and major thermal power plants (Naja et al. 2014) during winter and summer seasons and IGP region including coastal areas of Bay of Bengal and Arabian Sea during monsoon season. Therefore, the air mass parcels traveling from these regions are inevitably carry pollutant-laden air masses to the receptor site.
Conclusion
This study attempts to investigate the comprehensive characterization, and source apportionment of PM2.5 at an urban site of Delhi, India, from January 2013 to December 2014 provides the following points:
-
During the study, the average concentration of PM2.5 was 121.9 ± 93.2 μg m−3 with a range of 25.1–429.8 μg m−3. The average concentrations of OC and EC of PM2.5 were recorded as 17.6 ± 14.1 and 10.2 ± 7.5 μg m−3, respectively. Strong seasonal variation was recorded in PM2.5 concentration and its chemical composition with maxima during winter and minima during monsoon season.
-
The chemical composition of the PM2.5 was reconstructed using IMPROVE equation from the analyzed chemical composition. The highest contribution comes from particulate organic matter (23%), followed by sea salt (16%), ammonium sulfate (15%), soil/crustal matter (13%), ammonium nitrate (10%), and light-absorbing carbon (8%).
-
All the three models (PCA/APCS, UNMIX, and PMF) agreed on the sources of PM2.5 and majority to be soil dust, secondary aerosols, vehicle emissions, industrial emissions, and biomass burning. PCA/APCS extracted five sources of PM2.5 (soil dust, vehicle exhaust, secondary aerosols + industrial emissions, biomass burning, and sea salt), whereas PMF model identified seven sources of PM2.5 (secondary aerosol, soil dust, vehicular emission, fossil fuel combustion, biomass burning, industrial emission, and sea salt). UNMIX model revealed four sources of PM2.5 (soil dust, secondary aerosols, vehicle exhaust + biomass burning + sea salt, and industrial emission).
-
The 24-h backward trajectories were traced and cluster analysis was performed on a seasonal basis to trace the air mass flow pathways, and PSCF was also done to identify the potential source regions. North-west India, regions of Pakistan, and Afghanistan were found to be more dominant during summer and winter seasons, and IGP region, coastal areas of Bay of Bengal, and Arabian Sea were more prevailing during monsoon season.
-
This study can help the stakeholders and policymakers to know more about the attributes of PM2.5, the influence of regional and local sources, and their effect on air quality of the region. A definitive objective is to upgrade emanation control strategies, enhance general well-being, and to improve the overall quality of ambient air.
References
Alleman LY, Lamaison L, Perdrix E, Robache A, Galloo JC (2010) PM10 metal concentrations and source identification using positive matrix factorization and wind sectoring in a French industrial zone. Atmos Res 96(4):612–625
Amodio M, Andriani E, De Gennaro G, Di Gilio A, Ielpo P, Placentino CM, Tutino M (2013) How a steel plant affects air quality of a nearby urban area: a study on metals and PAH concentrations. Aerol Air Qual Res 13(2):497–508
Andersen ZJ, Wahlin P, Raaschou-Nielsen O, Scheike T, Loft S (2007) Ambient particle source apportionment and daily hospital admissions among children and elderly in Copenhagen. J Expo Sci Environ Epidemiol 17(7):625–636
Ashbaugh LL, Malm WC, Sadeh WZ (1985) A residence time probability analysis of sulfur concentrations at Grand Canyon National Park. Atmos Environ 19(8):1263–1270
Balachandran S, Meena BR, Khillare PS (2000) Particle size distribution and its elemental composition in the ambient air of Delhi. Environ Internat 26(1):49–54
Banerjee T, Murari V, Kumar M, Raju MP (2015) Source apportionment of airborne particulates through receptor modeling: Indian scenario. Atmos Res 164:167–187
Begum BA, Kim E, Biswas SK, Hopke PK (2004) Investigation of sources of atmospheric aerosol at urban and semi-urban areas in Bangladesh. Atmos Environ 38(19):3025–3038
Begum BA, Biswas SK, Markwitz A, Hopke PK (2010) Identification of sources of fine and coarse particulate matter in Dhaka, Bangladesh. Aerosol Air Qual Res 10(4):345–353
Begum BA, Biswas SK, Hopke PK (2011) Key issues in controlling air pollutants in Dhaka, Bangladesh. Atmos Environ 45(40):7705–7713
Behera SN, Sharma M (2010) Investigating the potential role of ammonia in ion chemistry of fine particulate matter formation for an urban environment. Sci Total Environ 408(17):3569–3575
Belis CA, Karagulian F, Larsen BR, Hopke PK (2013) Critical review and meta-analysis of ambient particulate matter source apportionment using receptor models in Europe. Atmos Environ 69:94–108
Beuck H, Quass U, Klemm O, Kuhlbusch TA (2011) Assessment of sea salt and mineral dust contributions to PM10 in NW Germany using tracer models and positive matrix factorization. Atmos Environ 45:5813–5821
Bove MC, Brotto P, Cassola F, Cuccia E, Massabò D, Mazzino A, Prati P (2014) An integrated PM2.5 source apportionment study: positive matrix factorisation vs. the chemical transport model CAMx. Atmos Environ 94:274–286
Brankov E, Rao ST, Porter PS (1998) A trajectory-clustering-correlation methodology for examining the long-range transport of air pollutants. Atmos Environ 32(9):1525–1534
Brauer M, Freedman G, Frostad J, Van Donkelaar A, Martin RV, Dentener F, Balakrishnan K (2015) Ambient air pollution exposure estimation for the global burden of disease 2013. Environ Sci Technol 50(1):79–88
Brown SG, Frankel A, Raffuse SM, Roberts PT, Hafner HR, Anderson DJ (2007) Source apportionment of fine particulate matter in Phoenix, AZ, using positive matrix factorization. J Air Waste Manage Assoc 57:741–752
Buseck PR, POsfai M (1999) Air borne minerals and related aerosol particles: effects on climate and the environment. Proc Natl Acad Sci 96(7):3372–3379
Callén MS, De La Cruz MT, López JM, Navarro MV, Mastral AM (2009) Comparison of receptor models for source apportionment of the PM10 in Zaragoza (Spain). Chemosphere 76(8):1120–1129
Cesari D, Amato F, Pandolfi M, Alastuey A, Querol X, Contini D (2016) An inter-comparison of PM10 source apportionment using PCA and PMF receptor models in three European sites. Environ Sci Poll Res:1–16
Chakraborty A, Gupta T (2010) Chemical characterization and source apportionment of submicron (PM1) aerosol in Kanpur region, India. Aero Air Qual Res 10(5):433–445
Chan YC, Simpson RW, McTainsh GH, Vowles PD, Cohen DD, Bailey GM (1997) Characterisation of chemical species in PM2.5 and PM10 aerosols in Brisbane, Australia. Atmos Environ 31(22):3773–3785
ChanT W, Mozurkewich M (2007) Application of absolute principal component analysis to size distribution data: identification of particle origins. Atmos Chem Phy 7(3):887–897
Chelani AB, Gajghate DG, Devotta S (2008) Source apportionment of PM10 in Mumbai, India using CMB model. Bull Environ Contami Toxicol 81(2):190–195
Chen LWA, Watson JG, Chow JC, Magliano KL (2007) Quantifying PM2.5 source contributions for the San Joaquin Valley with multivariate receptor models. Environ Sci Technol 41(8):2818–2826
Chen LWA, Watson JG, Chow JC, DuBois DW, Herschberger L (2010) Chemical mass balance source apportionment for combined PM2.5 measurements from US non-urban and urban long-term networks. Atmos Environ 44(38):4908–4918
Choi JK, Heo JB, Ban SJ, Yi SM, Zoh KD (2013) Source apportionment of PM2.5 at the coastal area in Korea. Sci Total Environ 447:370–380
Chow JC, Watson JG, Fujita EM, Lu Z, Lawson DR, Ashbaugh LL (1994) Temporal and spatial variations of PM2.5 and PM10 aerosol in the Southern California air quality study. Atmos Environ 28(12):2061–2080
Chow JC, Watson JG, Lu Z, Lowenthal DH, Frazier CA, Solomon PA, Magliano K (1996) Descriptive analysis of PM2.5 and PM10 at regionally representative locations during SJVAQS/AUSPEX. Atmos Environ 30(12):2079–2112
Chow JC, Watson JG, Chen LWA, Arnott WP, Moosmüller H, Fung K (2004) Equivalence of elemental carbon by thermal/optical reflectance and transmittance with different temperature protocols. Environ Science Technol 38(16):4414–4422
Chowdhury Z, Zheng M, Schauer J J, Sheesley R J, Salmon L G, Cass G R, Russell A G (2007) Speciation of ambient fine organic carbon particles and source apportionment of PM2.5 in Indian cities. J Geophy Res: Atmos 112(D15)
Chu SH (2004) PM2.5 episodes as observed in the speciation trends network. Atmos Environt 38(31):5237–5246
CPCB February (2010) Air quality monitoring, emission inventory and source apportionment study for Indian cities. Central Pollution Control Board. India
Cusack M, Perez N, Pey J, Alastuey A, Querol X (2013) Source apportionment of fine PM and sub-micron particle number concentrations at a reginal background site in the western Mediterranean: A 2.5 year study. Atmos Chem Phy 13:5173–5187
Davidson CI, Phalen RF, Solomon PA (2005) Air borne particulate matter and human health: a review. Aero Sci Techn 39(8):737–749
Draxler R R, Rolph G D (2003) HYSPLIT (HYbrid Single-Particle Lagrangian Integrated Trajectory) model access via NOAA ARL READY website (http://www.arl.noaa.gov/ready/hysplit4.html). NOAA Air Resources Laboratory, Silver Spring
Eldred RA, Cahill TA (1994) Trends in elemental concentrations of fine particles at remote sites in the United States of America. Atmos Environ 28(5):1009–1019
EPA PMF User Guide (2008) EPA Positive matrix Factorization (PMF) 3.0 Fundamentals and User Guide, US-EP Office of Research and Development
García JH, Li WW, Cárdenas N, Arimoto R, Walton J, Trujillo D (2006) Determination of PM2.5 sources using time-resolved integrated source and receptor models. Chemosphere 65(11):2018–2027
Gildemeister AE, Hopke PK, Kim E (2007) Sources of fine urban particulate matter in Detroit, MI. Chemosphere 69(7):1064–1074
Goyal P, Sidhartha (2002) Effect of winds on SO2 and SPM concentarion in Delhi. Atmos Environ 36:2925–2930
Gugamsetty B, Wei H, Liu CN, Awasthi A, Hsu SC, Tsai CJ et al (2012) Source characterization and apportionment of PM10, PM2.5 and PM0.1 by using positive matrix factorization. Aero Air Qual Res 12:476–491
Gupta AK, Karar K, Srivastava A (2007) Chemical mass balance source apportionment of PM10 and TSP in residential and industrial sites of an urban region of Kolkata, India. J Hazardous Materials 142(1):279–287
Gupta I, Salunkhe A, Kumar R (2012) Source apportionment of PM10 by positive matrix factorization in urban area of Mumbai, India. The Science of World Journal doi:10.1100/2012/585791
Habre R, Coull B, Koutrakis P (2011) Impact of source collinearity in simulated PM2.5 data on the PMF receptor model solution. Atmos Environ 45(38):6938–6946
Harrison RM, Beddows DC, Dall’Osto M (2011) PMF analysis of wide-range particle size spectra collected on a major highway. Environ Science Tech 45(13):5522–5528
Henry RC (2003) Multivariate receptor modeling by N-dimensional edge detection. Chemom Intell Lab Syst 65(2):179–189
Henry RC, Hidy GM (1979) Multivariate analysis of particulate sulfate and other air quality variables by principal components—I. Annual data from Los Angeles and New York. Atmos Environ 13:1581–1596
Heo JB, Hopke PK, Yi SM (2009) Source apportionment of PM2.5 in Seoul, Korea. Atmos Chem Phy 9(14):4957–4971
Ho KF, Lee SC, Chow JC, Watson JG (2003) Characterization of PM10 and PM2.5 source profiles for fugitive dust in Hong Kong. Atmos Environ 37(8):1023–1032
Hopke PK (2003) Recent developments in receptor modeling. J Chemometrics 17(5):255–265
Hopke PK, Ito K, Mar T, Christensen WF, Eatough DJ, Henry RC, Liu H (2006) PM source apportionment and health effects: intercomparison of source apportionment results. J Exposure Sci Environ Epidemiol 16(3):275–286
Hwang I, Hopke PK (2007) Estimation of source apportionment and potential source locations of PM2.5 at a west coastal IMPROVE site. Atmos Environ 41(3):506–518
Ielpo P, Paolillo V, de Gennaro G, Dambruoso PR (2014) PM10 and gaseous pollutants trends from air quality monitoring networks in Bari province: principal component analysis and absolute principal component scores on a two years and half data set. Chemistry Central Journal 8(1):1
IPCC (2013) Climate Change 2013: The Physical Science Basis, contribution of Working Group I to the Fifth Assessment Report of the IPCC
Ito K, Christensen WF, Eatough DJ, Henry RC, Kim E, Laden F, Thurston GD (2006) PM source apportionment and health effects: 2. An investigation of inter-method variability in associations between source-apportioned fine particle mass and daily mortality in Washington, DC. J Exposure Sci Environ Epidemiol 16(4):300–310
Jin X, Xiao C, Li J, Huang D, Yuan G, Yao Y, Wang P (2016) Source apportionment of PM2.5 in Beijing using positive matrix factorization. J Radioanalytical and Nuclear Chemistry 307(3):2147–2154
Kang CM, Kang BW, Lee HS (2006) Source identification and trends in concentrations of gaseous and fine particulate principal species in Seoul, South Korea. J the Air & Waste Management Association 56(7):911–921
Kar S, Maity JP, Samal AC, Santra SC (2010) Metallic components of traffic-induced urban aerosol, their spatial variation, and source apportionment. Environ Monit Asses 168(1–4):561–574
Karanasiou AA, Siskos PA, Eleftheriadis K (2009) Assessment of source apportionment by positive matrix factorization analysis on fine and coarse urban aerosol size fractions. Atmos Environ 43:3385–3395
Karar K, Gupta AK (2007) Source apportionment of PM10 at residential and industrial sites of an urban region of Kolkata, India. Atmos Res 84(1):30–41
Khare P, Baruah BP (2010) Elemental characterization and source identification of PM2.5 using multivariate analysis at the suburban site of north-east India. Atmos Res 98(1):148–162
Khillare PS, Balachandran S, Meena BR (2004) Spatial and temporal variation of heavy metals in atmospheric aerosol of Delhi. Environ Monit Asses 90(1–3):1–21
Kim E, Hopke PK, Edgerton ES (2003) Source identification of Atlanta aerosol by positive matrix factorization. J the Air & Waste Management Association 53(6):731–739
Kong S, Ding X, Bai Z, Han B, Chen L, Shi J, Li Z (2010) A seasonal study of polycyclic aromatic hydrocarbons in PM2.5 and PM2.5–10 in five typical cities of Liaoning Province, China. J Hazardous Materials 183(1):70–80
Kong S, Ji Y, Lu B, Chen L, Han B, Li Z, Bai Z (2011) Characterization of PM10 source profiles for fugitive dust in Fushun—a city famous for coal. Atmos Environ 45(30):5351–5365
Kothai P, Saradhi IV, Prathibha P, HopkeP K, Pandit GG, Puranik VD (2008) Source apportionment of coarse and fine particulate matter at Navi Mumbai, India. Aerosol Air Qual Res 8(4):423–436
Kulshrestha A, Satsangi PG, Masih J, Taneja A (2009) Metal concentration of PM2.5 and PM10 particles and seasonal variations in urban and rural environment of Agra, India. Sci Total Environ 407(24):6196–6204
Kumar AV, Patil RS, Nambi KSV (2001) Source apportionment of suspended particulate matter at two traffic junctions in Mumbai, India. Atmos Environ 35(25):4245–4251
Lee JH, Hopke PK (2006) Apportioning sources of PM2.5 in St. Louis, MO using speciation trends network data. Atmos Environ 40:360–377
Lee E, Chan CK, Paatero P (1999) Application of positive matrix factorization in source apportionment of particulate pollutants in Hong Kong. Atmos Environ 33:3201–3212
Lee JH, Yoshida Y, Turpin BJ, Hopke PK, Poirot RL, Lioy PJ, Oxley JC (2002) Identification of sources contributing to mid-Atlantic regional aerosol. J Air & Waste Management Association 52(10):1186–1205
Li Z, Hopke PK, Husain L, Qureshi S, Dutkiewicz VA, Schwab JJ, Demerjian KL (2004) Sources of fine particle composition in New York city. Atmos Environ 38(38):6521–6529
Li X, Wang Y, Guo X, Wang Y (2013) Seasonal variation and source apportionment of organic and inorganic compounds in PM2.5 and PM10 particulates in Beijing, China. J Environ Sci 25:741–750
Lough G C, Schauer JJ, Park JS, Shafer MM, DeMinter JT, Weinstein JP (2005) Emissions of metals associated with motor vehicle roadways. Environ Sci Technol 39:826–836
Malm WC, Sisler JF, Huffman D, Eldred RA, Cahill TA (1994) Spatial and seasonal trends in particle concentration and optical extinction in the United States. J Geophyl Res:Atmos 99(D1):1347–1370
Malm WC, Pitchford ML, McDade C, Ashbaugh LL (2007) Coarse particle speciation at selected locations in the rural continental United States. Atmos Environ 41(10):2225–2239
Mandal P, Sarkar R, Mandal A, Saud T (2014) Seasonal variation and sources of aerosol pollution in Delhi, India. Environ Chem Letts 12(4):529–534
Mauderly JL, Chow JC (2008) Health affects of organic aerosols. Inhal Toxicol 20(3):257–288
Maykut NN, Lewtas J, Kim E, Larson TV (2003) Source apportionment of PM2.5 at an urban IMPROVE site in Seattle, Washington. Environ Sci Technol 37(22):5135–5142
Mazzei F, Prati P (2009) Coarse particulate matter apportionment around a steel smelter plant. J Air & Waste Management Association 59(5):514–519
Meng ZY, Lin WL, Jiang XM, Yan P, Wang Y, Zhang YM, Jia XF, Yu XL (2011) Characteristics of atmospheric ammonia over Beijing, China. Atmos Chem Phys 11:6139–6151
Moreno T, Karanasiou A, Amato F, Lucarelli F, Nava S, Calzolai G, Chiari M, Coz E, Artíñano B, Lumbreras J, Borge R, Boldo E, Linares C, Alastuey A, Querol X, Gibbons W (2013) Daily and hourly sourcing of metallic and mineral dust in urban air contaminated by traffic and coal-burning emissions. Atmos Environ 68:33–44
Murillo JH, Ramos AC, Carcia FA, Jimenez SB, Cardenas B, Mizohata A (2012) Chemical composition of PM2.5 particles in Salamanca, Guanajuato Mexico: source apportionment with receptor models. Atmos Res 107:31–41
Mustaffa N, Latif M, Ali M, Khan M (2014) Source apportionment of surfactants in marine aerosols at different locations along the Malacca Straits. Environ Sci Pollut Res 21:6590–6602
Naja M, Mallik C, Sarangi T, Sheel V, Lal S (2014) SO2 measurements at a high altitude site in the central Himalayas: role of regional transport. Atmos Environ 99:392–402
Ogundele LT, Owoade OK, Olise FS, Hopke PK (2016) Source identification and apportionment of PM2.5 and PM2.5–10 in iron and steel scrap smelting factory environment using PMF, PCFA and UNMIX receptor models. Environ Monit Asses 188(10):574
Olson DA, Norris GA (2008) Chemical characterization of ambient particulate matter near the World Trade Center: source apportionment using organic and inorganic source markers. Atmos Environ 42(31):7310–7315
Özkaynak H, Thurston GD (1987) Associations between 1980 US mortality rates and alternative measures of airborne particle concentration. Risk Anal 7(4):449–461
Paatero P (1997) Least squares formulation of robust non-negative factor analysis. Chemom Intell Lab Syst 37(1):23–35
Paatero P (1999) The multilinear engine—a table-driven, least squares program for solving multilinear problems, including the n-way parallel factor analysis model. J Comput Graph Stat 8(4):854–888
Paatero P, Tapper U (1994) Positive matrix factorization: a non-negative factor model with optimal utilization of error estimates of data values. Environmetrics 5(2):111–126
Panda S, Sharma SK, Mahapatra PS, Panda U, Rath S, Mahapatra M, Das T (2016) Organic and elemental carbon variation in PM2.5 over megacity Delhi and Bhubaneswar, a semi-urban coastal site in India. Nat Hazards 80(3):1709–1728
Pandolfi M, Viana M, Minguillón MC, Querol X, Alastuey A, AmatoF ME (2008) Receptor models application to multi-year ambient PM10 measurements in an industrialized ceramic area: comparison of source apportionment results. Atmos Environ 42(40):9007–9017
Pant P, Harrison RM (2012) Critical review of receptor modelling for particulate matter: a case study of India. Atmos Environ 49:1–12
Phuleria HC, Sheesley RJ, Schauer JJ, Fine PM, Sioutas C (2007) Roadside measurements of size-segregated particulate organic compounds near gasoline and diesel-dominated freeways in Los Angeles, CA. Atmos Environ 41(22):4653–4671
Pope CA III, Dockery DW (2006) Health effects of fine particulate air pollution: lines that connect. J Air & Waste Management Association 56(6):709–742
Pope CA III, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD (2002) Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 287(9):1132–1141
Ram K, Sarin MM (2011) Day–night variability of EC, OC, WSOC and inorganic ions in urban environment of Indo-Gangetic Plain: implications to secondary aerosol formation. Atmos Environ 45(2):460–468
Ram K, Sarin MM, Tripathi SN (2010) One-year record of carbonaceous aerosols from an urban location (Kanpur) in the Indo-Gangetic Plain: characterization, sources and temporal variability. J Geophys Res doi:10.1029/2010JD014188
Ram K, Sarin MM, Sudheer AK, Rengarajan R (2012) Carbonaceous and secondary inorganic aerosols during wintertime fog and haze over urban sites in the Indo-Gangetic Plain. Aerosol Air Qual Res 12:359–370
Reddy MS, Venkataraman C (2000) Atmospheric optical and radiative effects of anthropogenic aerosol constituents from India. Atmos Environ 34(26):4511–4523
Rodrıguez S, Querol X, Alastuey A, Viana MM, Alarcon M, Mantilla E, Ruiz CR (2004) Comparative PM10–PM2.5 source contribution study at rural, urban and industrial sites during PM episodes in eastern Spain. Sci Total Environ 328(1):95–113
Russell AG, Brunekreef B (2009) A focus on particulate matter and health. Environ Sci Technol 43(13):4620–4625
Salma I, Chi X, Maenhaut W (2004) Elemental and organic carbon in urban canyon and background environments in Budapest, Hungary. Atmos Environ 38(1):27–36
Santoso M, Lestiani DD, Markwitz A (2013) Characterization of airborne particulate matter collected at Jakarta roadside of an arterial road. J Radioanal Nucl Chem 297:165–169
Saxena M, Sharma A, Sen A, Saxena P, Saraswati, Mandal TK, Sharma SK (2017) Water soluble inorganic species of PM10 and PM2.5 at an urban site of Delhi, India: seasonal variability and sources. Atmos Res 184:112–125
Schwartz J, Dockery DW (1992) Increased mortality in Philadelphia associated with daily air pollution concentrations. Am Rev Respir Dis 145(3):600–604
Seinfeld J H, Pandis S N (2016) Atmospheric chemistry and physics: from air pollution to climate change. John Wiley & Sons
Seneviratne M, Waduge VA, Hadagiripathira L, Sanjeewani S, Attanayake T, Jayaratne N, Hopke PK (2011) Characterization and source apportionment of particulate pollution in Colombo, Sri Lanka. Atmos Pollut Res 2:207–212
Sharma DN, Sawant AA, Uma R, Cocker DR (2003) Preliminary chemical characterization of particle-phase organic compounds in New Delhi, India. Atmos Environ 37(30):4317–4323
Sharma M, Kishore S, Tripathi SN, Behera SN (2007) Role of atmospheric ammonia in the formation of inorganic secondary particulate matter: a study at Kanpur, India. J Atmos Chem 58(1):1–17
Sharma SK, Singh AK, Saud T, Mandal TK, Saxena M, Singh S, Raha S (2012a) Study on water-soluble ionic composition of PM10 and related trace gases over Bay of Bengal during W_ICARB campaign. Meteorol Atmos Phy 118(1–2):37–51
Sharma SK, Saxena M, Saud T, Korpole S, Mandal TK (2012b) Measurement of NH3, NO, NO2 and related particulates at urban sites of Indo-Gangetic Plain (IGP) of India. J Sci Indust Res 71(5):360–362
Sharma SK, Kumar M, Gupta NC, Saxena M, Mandal TK (2014a) Characteristics of ambient ammonia over Delhi, India. Meteorol Atmos Phy 124(1–2):67–82
Sharma SK, Mandal TK, Saxena M, Sharma A, Datta A, Saud T (2014b) Variation of OC, EC, WSIC and trace metals of PM10 in Delhi, India. J Atmos Solar-Terres Phy 113:10–22
Sharma SK, Mandal TK, Saxena M, Sharma A, Gautam R (2014c) Source apportionment of PM10 by using positive matrix factorization at an urban site of Delhi, India. Urban Climate 10:656–670
Sharma SK, Sharma A, Saxena M, Choudhary N, Masiwal R, Mandal TK, Sharma C (2015) Chemical characterization and source apportionment of aerosol at an urban area of central Delhi, India. Atmos Poll Res 7(1):110–121
Sharma SK, Mandal TK, Srivastava MK, Chatterjee A, Jain S, Saxena M, Ghosh SK (2016a) Spatio-temporal variation in chemical characteristics of PM10 over Indo-Gangetic Plain of India. Environ Sci Poll Res 23(18):18809–18822
Sharma SK, Mandal TK, Jain S, Saraswati SA, Saxena M (2016b) Source apportionment of PM2.5 in Delhi, India using PMF model. Bull Environ Contamin Toxicol 97(2):286–293
Shen G, Wang W, Yang Y, Zhu C, Min Y, Xue M, Wang R (2010) Emission factors and particulate matter size distribution of polycyclic aromatic hydrocarbons from residential coal combustions in rural northern China. Atmos Environ 44(39):5237–5243
Shi GL, Liu GR, Peng X, Wang YN, Tian YZ, Wang W, Feng YC (2014) A comparison of multiple combined models for source apportionment, including the PCA/MLR-CMB, UNMIX-CMB and PMF-CMB models. Aero Air Qual Res 14(7):2040–2050
Shridhar V, Khillare PS, Agarwal T, Ray S (2010) Metallic species in ambient particulate matter at rural and urban location of Delhi. J Hazardous Materials 175(1):600–607
Singh DP, Gadi R, Mandal TK (2011) Characterization of particulate-bound polycyclic aromatic hydrocarbons and trace metals composition of urban air in Delhi, India. Atmos Environ 45(40):7653–7663
Song Y, Xie S, Zhang Y, Zeng L, Salmon LG, Zheng M (2006) Source apportionment of PM2.5 in Beijing using principal component analysis/absolute principal component scores and UNMIX. Sci Total Environ 372(1):278–286
Srimuruganandam B, Nagendra SS (2012a) Source characterization of PM10 and PM2.5 mass using a chemical mass balance model at urban roadside. Sci Total Environ 433:8–19
Srimuruganandam B, Nagendra SS (2012b) Application of positive matrix factorization in characterization of PM10 and PM2.5 emission sources at urban roadside. Chemosphere 88(1):120–130
Srivastava A, Jain VK (2007) Seasonal trends in coarse and fine particle sources in Delhi by the chemical mass balance receptor model. J Hazard Mater 144(1):283–291
Statistical Abstract of Delhi 2014 Directorate of Economics and Statistics. Govt. of National Capital Delhi. www.delhi.gov.in
Thurston GD, Spengler JD (1985) A quantitative assessment of source contributions to inhalable particulate matter pollution in metropolitan Boston. Atmos Environ (1967) 19(1):9–25
Tie X, Wu D, Brasseur G (2009) Lung cancer mortality and exposure to atmospheric aerosol particles in Guangzhou, China. Atmos Environ 43(14):2375–2377
Tiwari S, Bisht DS, Srivastava AK, Pipal AS, Taneja A, Srivastava MK, Attri SD (2014) Variability in atmospheric particulates and meteorological effects on their mass concentrations over Delhi, India. Atmos Res 145:45–56
Trivedi DK, Ali K, Beig G (2014) Impact of meteorological parameters on the development of fine and coarse particles over Delhi. Sci Total Environ 478:175–183
Turpin BJ, Huntzicker JJ (1991) Secondary formation of organic aerosol in the Los Angeles Basin: a descriptive analysis of organic and elemental carbon concentrations. Atmos Environ Part A General Topics 25(2):207–215
Ulbrich IM, Canagaratna MR, Zhang Q, Worsnop DR, Jimenez JL (2009) Interpretation of organic components from positive matrix factorization of aerosol mass spectrometric data. Atmos Chem Phys 9(9):2891–2918
USEPA (2008) EPA positive matrix factorization (PMF) 3.0 fundamentals & user guide. United States Environmental Protection Agency, Washington, DC
Vallius M, Janssen NAH, Heinrich J, Hoek G, Ruuskanen J, Cyrys J, Pekkanen J (2005) Sources and elemental composition of ambient PM2.5 in three European cities. Sci Total Environ 337(1):147–162
Van Donkelaar A, Martin R V, Brauer M, Boys B L (2015) Use of satellite observations for long-term exposure assessment of global concentrations of fine particulate matter (Doctoral dissertation, University of British Columbia).
Vernile P, Tutino M, Bari G, Amodio M, Spagnuolo M, de Gennaro G, de Lillo E (2013) Particulate matter toxicity evaluation using bioindicators and comet assay. Aerosol Air Qual Res 13(1):172–178
Viana M, Pandolfi M, Minguillón MC, Querol X, Alastuey A, Monfort E, Celades I (2008) Inter-comparison of receptor models for PM source apportionment: case study in an industrial area. Atmos Environ 42(16):3820–3832
Waked A, Favez O, Alleman LY, Piot C, Petit JE, Delaunay T, Verlinden E, Golly B, Besombes JL, Jaffrezo JL, Leoz-Garziandia E (2014) Source apportionment of PM10 in a north-western Europe regional urban background site (Lens, France) using positive matrix factorization and including primary biogenic emissions. Atmos Chem Phys 14:3325–3346
Wang YQ, Zhang XY, Draxler RR (2009) TrajStat: GIS-based software that uses various trajectory statistical analysis methods to identify potential sources from long-term air pollution measurement data. Environ Model Softw 24(8):938–939
Wang ZS, Wu T, Shi GL, Fu X, Tian YZ, FengY C, Zhang WJ (2012) Potential source analysis for PM10 and PM2.5 in autumn in a northern city in China. Atmos Environ 43(14):2375–2377
Wang L, Liu Z, Sun Y, Ji D, Wang Y (2015) Long-range transport and regional sources of PM2.5 in Beijing based on long-term observations from 2005 to 2010. Atmos Res 157:37–48
Watson JG, Chow JC (2002) A wintertime PM2.5 episode at the Fresno, CA, supersite. Atmos Environ 36(3):465–475
Watson J G, Chow J C (2005) Receptor models. Air Quality Modeling–Theories, Methodologies, Computational Techniques, and Available Databases and Software 2: 455–501
Wilson W E, Suh H H (1995) Differentiating fine and coarse particles: definitions and exposure relationships relevant to epidemiological studies. In: Trends in Aerosol Research IV: New Approaches in Aerosol Science and Technology, Proceedings of the Seminar. Gerhard Mercator University, Duisburg, Germany 57–71
Wolff GT, Korsog PE, Stroup DP, Ruthkosky MS, Morrissey ML (1985) The influence of local and regional sources on the concentration of inhalable particulate matter in southeastern Michigan. Atmos Environ 19(2):305–313
World Health Organization (2009) Global health risks: mortality and burden of disease attributable to selected major risks. World Health Organization
World Health Organization (2015) World Health Statistics. World Health Organization
World Health Organization (2016) WHO Global Urban Ambient Air Pollution Database. World Health Organization
Yan B, Zheng M, Hu Y, Ding X, Sullivan AP, Weber RJ, Russell AG (2009) Roadside, urban, and rural comparison of primary and secondary organic molecular markers in ambient PM2.5. Environ Sci Technol 43(12):4287–4293
Yin J, Harrison RM, Chen Q, Rutter A, Schauer JJ (2010) Source apportionment of fine particles at urban background and rural sites in the UK atmosphere. Atmos Environ 44(6):841–851
Zhang XY, Cao JJ, Li LM, Arimoto R, Cheng Y, Huebert B, Wang D (2002) Characterization of atmospheric aerosol over XiAn in the south margin of the Loess Plateau, China. Atmos Environ 36(26):4189–4199
Zhang QH, Zhang JP, Xue HW (2010) The challenge of improving visibility in Beijing. Atmos Chem Phy 10(16):7821–7827
Zhang R, Jing J, Tao J, Hsu SC, Wang G, Cao J et al (2013) Chemical characterization and source apportionment of PM2.5 in Beijing: seasonal perspective. Atmos Chem Phy 13(14):7053–7074
Zheng M, Salmon LG, Schauer JJ, Zeng L, Kiang CS, Zhang Y, Cass GR (2005) Seasonal trends in PM2.5 source contributions in Beijing, China. Atmos Environ 39(22):3967–3976
Zheng M, Cass GR, Ke L, Wang F, Schauer JJ, Edgerton ES, Russell AG (2007) Source apportionment of daily fine particulate matter at Jefferson Street, Atlanta, GA, during summer and winter. J Air & Waste Management Association 57(2):228–242
Zheng J, Che W, Zheng Z, Chen L, Zhong L (2013) Analysis of spatial and temporal variability of PM10 concentrations using MODIS aerosol optical thickness in the Pearl River Delta region, China. Aerosol Air Qual Res 13(3):862–876
Acknowledgements
The authors would like to thank the Director, CSIR-NPL, New Delhi, and Head, Environmental Sciences and Biomedical Metrology Division, for their support and encouragement. Authors are also thankful to the Council of Scientific and Industrial Research, New Delhi, for providing the partial financial support for this study (PSC-0112 Network Project). One of the authors, Srishti Jain, thankfully acknowledge Department of Science and Technology (DST), New Delhi, for awarding the INSPIRE Fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Electronic supplementary material
.
ESM 1
(DOCX 12 kb)
Rights and permissions
About this article
Cite this article
Jain, S., Sharma, S.K., Choudhary, N. et al. Chemical characteristics and source apportionment of PM2.5 using PCA/APCS, UNMIX, and PMF at an urban site of Delhi, India. Environ Sci Pollut Res 24, 14637–14656 (2017). https://doi.org/10.1007/s11356-017-8925-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-8925-5