Abstract
Background
Left ventricular filling pressure (LVFP) is raised by the compromised contraction and impaired ventricular compliance in dilated hearts with systolic dysfunction. Timely recognition and staging of this condition are important for planning of the treatment strategy and making the prognosis. Two-dimensional speckle- tracking echocardiography (2D-STE) has recently enabled the quantification of left atrial (LA) myocardial deformation dynamics. In this study, echocardiographic indicators of increased LVFP and NT-pro-BNP were compared with LA strain measured by 2D-STE.
Methods
A total of 49 nonischemic dilated cardiomyopathy (DCMP) patients were included in the study. All patients underwent standard 2D echocardiography. In the 2D-STE analysis of the LA, global longitudinal LA strain during ventricular systole (GLAs-res) and strain during late diastole (GLAs-pump) were obtained. NT-pro-BNP levels were measured. The patients were divided into two groups—normal (group 1) and increased (group 2) LVFP—according to E/A ratio, E velocity, and E/E’ ratio.
Results
LAVi-max, LAVi-min, and NT-pro-BNP were higher in group 2, whereas LAtotalEF, LAactiveEF, GLAs-res, and GLAs-pump were lower. In univariate analysis, a good negative correlation was seen between GLAs-res vs. NT-pro-BNP, GLAs-res vs. LAVi-max, and GLAs-res vs. E/E’ ratio; a good negative correlation was present between GLAs-pump vs. NT-pro-BNP, GLAs-pump vs. LAVi-max, and GLAs-pump vs. E/E’ ratio. LAVi-max, LAactiveEF, NT-pro-BNP, GLas-res, and GLAs-pump were studied by logistic regression analysis. GLAs-res (p = 0.009, OR = 0.593, 95 % CI 0.4–0.877), NT-pro-BNP (p = 0.028, OR = 1.027, 95 % CI 1.003–1.052), and LAactiveEF (p = 0.022, OR = 0.001, 95 % CI 0.001–0.024) were found to be independent predictors of increased LVFP.
Conclusion
2D-STE-based LA function is impaired in patients with nonischemic DCMP. LA reservoir and pump function parameters together with NT-pro-BNP levels might be useful in estimating LVFP in this patient group.
Zusammenfassung
Hintergrund
Der linksventrikuläre Füllungsdruck (LVFP) ist durch die beeinträchtigte Kontraktion und gestörte ventrikuläre Compliance bei einer Herzdilatation mit systolischer Funktionsstörung erhöht. Zur Planung der Therapiestrategie und Vorhersage der Prognose sind die frühzeitige Erkennung und Klassifizierung dieser Erkrankung von Bedeutung. Durch die 2-D-Speckle-Tracking-Echokardiographie (2D-STE) ist die Quantifizierung der linksatrialen myokardialen Deformationsdynamik seit Kurzem möglich geworden. In der vorliegenden Studie wurden echokardiographische Indikatoren eines erhöhten LVFP und NT-pro-BNP („n-terminal pro brain natriuretic peptide“) mit der per 2D-STE gemessenen linksatrialen Deformierung („strain“) verglichen.
Methoden
Insgesamt wurden 49 Patienten mit nichtischämischer dilatativer Kardiomyopathie (DCMP) in die Studie aufgenommen. Bei sämtlichen Patienten erfolgte eine Standard-2-D-Echokardiographie. Mit der 2D-STE wurden die globale longitudinale linksatriale Deformierung während der Ventrikelsystole (GLAs-res) und die Deformierung während der späten Diastole (GLAs-pump) ermittelt. Auch die NT-pro-BNP-Werte wurden bestimmt. Die Patienten wurden in 2 Gruppen aufgeteilt, je nachdem, ob der LVFP gemäß E/A-Verhältnis, E-Geschwindigkeit und E/E‘-Verhältnis normal (Gruppe 1) oder erhöht (Gruppe 2) war.
Ergebnisse
In Gruppe 2 waren LAVi-max, LAVi-min und NT-pro-BNP höher, LAtotalEF, LAactiveEF, GLAs-res und GLAs-pump dagegen niedriger. In der univariaten Analyse wurde eine gute negative Korrelation zwischen GLAs-res vs. NT-pro-BNP, GLAs-res vs. LAVi-max und GLAs-res vs. E/E’-Verhältnis beobachtet, und eine gute negative Korrelation fand sich zwischen GLAs-pump vs. NT-pro-BNP, GLAs-pump vs. LAVi-max und GLAs-pump vs. E/E’-Verhältnis. LAVi-max, LAactiveEF, NT-pro-BNP, GLas-res und GLAs-pump wurden mittels logistischer Regressionsanalyse untersucht. GLAs-res (p = 0,009; OR = 0,593; 95 %-KI: 0,4–0,877), NT-pro-BNP (p = 0,028; OR = 1,027; 95 %-KI: 1,003–1,052) und LAactiveEF (p = 0,022; OR = 0,001; 95 %-KI: 0,001–0,024) stellten sich als unabhängige Prädiktoren eines erhöhten LVFP heraus.
Schlussfolgerung
Die mit der 2D-STE ermittelte linksatriale Funktion ist bei Patienten mit nichtischämischer DCMP beeinträchtigt. Die Parameter für die Reservoir- und Pumpfunktion des linken Vorhofs könnten zusammen mit den NT-pro-BNP-Werten bei der Abschätzung des LVFP in dieser Patientengruppe von Nutzen sein.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In heart failure patients with low left ventricular ejection fraction (EF), the active myocardial relaxation and elastic recoil are usually affected by the left ventricular dilatation and systolic dysfunction leading to an increase in LVFP. As increased LVFP is associated with a higher NYHA class, worse prognosis, and higher mortality, recognition and staging of this condition are important treatment planning and prediction of the prognosis and mortality [1, 2, 3]. Invasive measurement is the gold standard for evaluation of left ventricular diastolic dysfunction and filling pressure [4, 5, 6]. However, noninvasive echocardiographic measurements could also be used in concordance with invasive parameters, except for some conditions that affect left ventricular filling such as mitral stenosis. Parameters such as E/A ratio and E velocity, increased E/Pv ratio, and E/E’ ratio are some of the echocardiographic indicators of increased LVFP [7, 8, 9, 10, 11]. Brain natriuretic peptide (BNP) that is released in response to myocardial stress caused by pressure and/or volume overload has also been shown to correlate well with diastolic filling pressure in previous studies [12, 13, 14].
The structure and functions of the left atrium (LA) could also be useful in evaluating the filling pressure. LA volumetric parameters, transmitral and pulmonary vein Doppler studies, and myocardial deformation by tissue Doppler examination have been used to depict the effect of increased LVFP on the LA. Recently, strain and strain rate measured by novel two-dimensional speckle-tracking echocardiography (2D-STE) have been used in evaluating various cardiac pathologies. In this study, echocardiographic indicators of increased LVFP and NT-pro-BNP levels were compared with LA strain parameters measured by 2D-STE.
Study population and method
The study included 49 patients with nonischemic dilated cardiomyopathy (DCMP) who presented to the Kosuyolu Heart, Education & Research Hospital between January 2009 and April 2012, and had an EF below 40 % and NYHA class I and II heart disease. Patients with more than 50 % stenosis in the epicardial coronary arteries on coronary angiography performed in the last 6 months, a history of acute coronary syndrome, permanent pacemaker or cardiac resynchronization therapy (CRT), suboptimal medical therapy, chronic hepatic or renal disease, hypo-/hyperthyroidism, brady-/tachyarrhythmias, rheumatic valve diseases, mitral regurgitation of more than moderate degree, chronic alcohol abuse, history of antineoplastic agent usage, radiotherapy and storage diseases were excluded from the study. Written informed consent was obtained from all patients. All work was done in compliance with the Declaration of Helsinki and was performed with the approval of the local ethics committee.
Two-dimensional and Doppler echocardiography
All patients underwent an echocardiographic examination in the left lateral position, using the GE Vivid 7 system (GE Vingmed Ultrasound AS, Horten, Norway) with a 3.5-MHz transducer. Blood pressure and heart rate were monitored during the echocardiographic examination. The data were analyzed offline using EchoPAC (GE Vingmed Ultrasound AS).
Cardiac dimensions and volumes were measured according to the guidelines of the European Society of Cardiology (ESC), and LVEF was calculated using the biplane Simpson method [15]. As defined before in the literature, systolic pulmonary arterial pressure (PAPs) was calculated from the tricuspid regurgitant jet velocity (V) using the Bernoulli equation (4V2) and the estimated right atrial pressure [16]. Transmitral flow velocities (E and A) were obtained by pulsed-wave Doppler in the apical four-chamber view. The ratio of E/A velocity and E-wave deceleration time (DT) were measured. Tissue Doppler imaging was used to measure mitral annular velocities. The early diastolic velocity (E’) was measured at both the mitral septal and lateral annulus, and the mean was taken. The ratio of E/E’ was calculated by using average E’. The patients were divided into two groups—normal and increased LVFP—according to the E/A ratio, E velocity, and E/E’. LVFP was considered to be high in patients with restrictive filling pattern, an E/A ratio of ≥ 1 to < 2 and E/E’ of ≥ 15, or an E/A ratio of < 1 (E > 50 cm/s) and E/E’ of > 15 (group 2). The patients who did not meet these criteria were considered to have normal filling pressures (group 1).
LA volumetric measurements
LA volumes were calculated using the biplane area-length method as described previously {LA volume = 0.85 × [(LA area in 4-chamber view) × (LA area in 2-chamber view)]/LA length} [17]. LA maximum volume (before mitral valve opening), LA pre-A volume (onset of the P-wave on electrocardiography), and LA minimum volume (after atrial contraction) were measured and volumetric parameters were indexed to body surface area (LAVi-max, LAVi-preA, LAVi-mi, respectively). Volumetric parameters of LA systolic function were calculated as follows: LA total EF = 100 × (LAV-max−LAV-min)/LAV-max, LA passive EF = 100 × (LAV-max−LAV-preA)/LAV-max, LA active EF = 100 × (LAV-preA –LAV-min)/LAV-preA.
Speckle-tracking echocardiography
Two-dimensional echocardiographic images for the LA were obtained from the apical four- and two-chamber views. All images were obtained while the patients held their breath and the images were stored in a cineloop format from three consecutive beats. The frame rate was adjusted between 60 and 80 frames/s. The data were analyzed offline using EchoPAC (GE Vingmed Ultrasound AS). The endocardial border was defined manually, and tracing was done by the software automatically for each view. If the obtained tracking segments were adequate for analysis, the software was allowed to read the data, whereas analytically inadequate tracking segments were either corrected manually or excluded from the analysis.
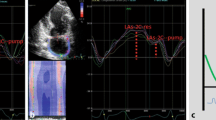
Overall, 552 segments were analyzed (12 segments for each patient). A total of 6 % of segments were excluded from the study because no analysis was done manually and/or automatically. From apical four- and two-chamber views, longitudinal LA strain during ventricular systole (or reservoir phase) (LAs-4C-res and LAs-2C-res) was obtained just before mitral valve opening; strain during late diastole (or pump phase) (LAs-4C-pump and LAs-2C-pump) was obtained at the onset of the P-wave on electrocardiography (see Fig. 1). Global longitudinal LA strain during ventricular systole (GLAs-res) and late diastole (GLAs-pump) were calculated by averaging values observed in all LA segments.
Reproducibility
Intra- and interobserver reproducibility was assessed for both the GLAs-res and the GLAs-pump values. For intraobserver assessment, the measurements were reanalyzed after 4 weeks. The Bland–Altman analysis for interobserver reproducibility [mean difference, 95 % confidence interval (C)] and intraobserver reproducibility (intraclass correlation coefficient, 95%CI) were calculated, and the intraclass correlation coefficient showed good inter- and intraobserver agreement; interobserver and intraobserver agreement was assessed for GLAs-res, − 2.5 [0.7–(− 5.7)] and 0.90 (0.82–0.95), respectively; and for GLAs-pump, 1.2 [4.3–(−1.9)] and 0.89 (0.81–0.94), respectively.
BNP measurement
Blood samples for NT-pro-BNP were obtained from the antecubital vein of all patients before the echocardiographic examination. The samples were sent to the laboratory in citrate tubes without delay. The blood was centrifuged at 3,500 rpm for 5 min. Commercial NT-proBNP assays (Elecsys Roche Diagnostics) were used for plasma NT-pro-BNP level measurement.
Statistical analysis
Continuous variables are expressed as mean (± SD) or median as appropriate. A p value of < 0.05 was taken as significant. The independent Student t test or the Mann–Whitney U test was used to compare parametric continuous variables. For categorical variables, the chi-squared test was used. Correlations between variables were tested by using the Pearson or Spearman correlation tests as appropriate. Stepwise multivariate logistic regression analysis was applied to identify the independent predictors of increased LVFP estimated by echocardiography. Variables with a significant p value on univariate analysis (BNP, LAVimax, LAactiveEF, GLAs-res, and GLAs-pump) were included in the multivariate model. Receiver-operating characteristic (ROC) curves were plotted to determine the optimal cut-off values for individual parameters in order to predict increased LVFP and to establish the optimal cut-off points for use in clinical decision making. Statistical analyses were performed using SPSS, version 15.0 for Windows.
Results
A total of 49 DCMP patients in normal sinus rhythm were included in the study. The mean age was 43.1 ± 12 years and 65.3 % were male. The patients were divided into two groups according to LVFP estimated by echocardiography (group 1: normal LVFP; group 2: increased LVFP). Tab. 1 shows the clinical, echocardiographic, and demographic characteristics of the patients. Baseline demographic and hemodynamic parameters, NYHA class, and drug usage were similar between the two groups, while group 2 patients had higher NT-pro-BNP (229.7 ± 54.8 pg/ml vs. 309.6 ± 56.6 pg/ml, p < 0.001), mitral E velocity (63 ± 15 cm/s vs. 81 ± 19 cm/s p < 0.001), E/A ratio (1.25 ± 0.43 vs. 1.79 ± 0.49 p < 0.001), and E/E’ ratio (10.52 ± 2.82 vs. 17.1 ± 1.96 p < 0.001) but lower E’and A’ by TDI (6.14 ± 1.18 cm/s vs. 4.78 ± 1.21, P < 0.001 and 5.68 ± 2.24 cm/s vs. 4.75 ± 1.27 cm/s, p = 0.088 respectively). LAVi-max (46.7 ± 12 ml/m2 vs. 55.7 ± 14.6 ml/m2, p = 0.023), LAVi-pre (34.4 ± 11 ml/m2 vs. 41 ± 14.3 ml/m2, p = 0.079 and LAVi-min(26.9 ± 8.9 ml/m2 vs. 34.1 ± 12.4 ml/m2, p = 0.022) were higher in group 2, while LA total EF (43.5 ± 6 vs 40.1 ± 7.2, p = 0.075), LA active EF (22 ± 4.7 vs 17 ± 5.2, p = 0.001), GLAs-res (18.2 ± 4.3 vs 12.3 ± 3.6, p < 0.001), and GLAs-pump (8.3 ± 3.5 vs 6.2 ± 1.6, p = 0.014) were lower (see Tab. 2).
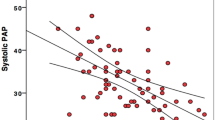
ROC analysis was performed to identify the role of GLAs-res in estimating increased LVFP. The area under the curve (AUC) to predict increased LVFP was 0.844 (95 % GA 0.738–0.951, p < 0.001). GLAs-res < %13.8 predicted increased LVFP with 83 % sensitivity and 61.5 % specificity (see Fig. 2). In univariate analysis, a good negative correlation was seen between GLAs-res vs. NT-pro-BNP (r = − 0.55, p < 0.001), GLAs-res vs. LAVi-max (r = − 0.56, p < 0.001), and GLAs-res vs. E/E’ (r = − 0.68, p < 0.001); a good negative correlation was present between GLAs-pump vs. NT-pro-BNP (r = − 0.57, p < 0.001), GLAs-Pump vs. LAVi-max (r = − 0.68, p < 0.001), and GLAs-pump vs. E/E’ (r = − 0.47, p < 0.001) (see, Fig. 2). A moderate correlation was also seen between LAactiveEF vs. GLAs-res (r = 0.29, p = 0.043), LAactiveEF vs. GLAs-pump (r = 0.3, p = 0.038; see Tab. 3). Parameters found statistically significant in predicting increased LVFP in univariate analysis—namely, LAVi-max, LAactiveEF, NT-pro-BNP, GLas-res, and GLAs-pump—were studied by logistic regression analysis. GLAs-res (p = 0.009, OR = 0.593, 95 % CI 0.4–0.877), NT-pro-BNP (p = 0.028, OR = 1.027, 95 % CI 1.003–1.052), and LAactiveEF (p = 0.022, OR = 0.001, 95 % CI 0.001–0.024) were found to be independent predictors of increased LVFP (see Tab. 4).
Discussion
In the current study, we demonstrated that GLAs-res and GLAs-pump were closely related with LVFP estimated by echocardiography. Moreover, we showed that GLAs-res and GLAs-pump values were negatively correlated with NT-pro-BNP and LA volumetric parameters.
LVFP is raised by the compromised contraction and impaired ventricular compliance in dilated hearts with systolic dysfunction. Timely recognition and staging of this condition are important for planning of the treatment strategy and prediction of the prognosis and mortality of these patients [1, 2, 3]. Invasive measurement is the gold standard for evaluation of left ventricular diastolic dysfunction and filling pressure. However, noninvasive echocardiographic measurements could also be used in concordance with invasive parameters. Parameters such as E/A ratio and E velocity, increased E/Pv ratio, and E/E’ ratio are some of the echocardiographic indicators of elevated LVFP. Another biochemical indicator of elevated LVFP is BNP level. A peptide secreted from both the atrium and the ventricle in response to myocardial stress, BNP has been shown to be associated with LV hypertrophy, systolic and diastolic heart failure, and increased LVFP [12, 13, 14]. In addition, Kurt et al. [18] found an inverse correlation between BNP and LA reservoir and pumping functions. Our study demonstrated significantly higher NT-pro-BNP levels in patients with increased LVFP. A negative correlation was also observed between echocardiographic left atrial parameters and NT-pro-BNP.
Atrial function plays an important role in maintaining optimal cardiac function, and measurement of the LA diameter and volume by 2D echocardiography has been traditionally used for assessment of left atrial function. LA functions are divided into three phases in a cardiac cycle, namely: (1) a reservoir phase, which receives blood from the pulmonary veins during ventricular systole; (2) a passive conduit component during early diastole; and (3) a pumping phase, with active contraction during late diastole. GLAs-res values estimated by 2D-STE serve as a measure of left atrial compliance during the reservoir phase, and GLAs-pump as a measure of active atrial contraction. Increased LVFP occurring as a result of LV systolic or diastolic dysfunction increases LA pressure and causes chamber dilatation and myocardial stretch. Consequently, atrial remodeling occurs, decreasing atrial compliance and contractile functions. LV systolic dysfunction disrupts LA reservoir function not only by elevating LVFP but also by decreasing mitral annular descent from the cardiac base to the apex during LV systole. In addition, the myopathic process in idiopathic dilated cardiomyopathy that affects the ventricle may also affect the atrial myocardium leading to worsening of atrial compliance and contraction [19, 20, 21]. In a study to evaluate LA functions, ischemic and nonischemic cardiomyopathy patients with similar demographic and conventional echocardiographic parameters were included, and the patients with nonischemic cardiomyopathy were found to possess a poorer LA function [22].
In another study, reduced GLAs-res, LVEF, and enlarged LA were found to be independent predictors of LV end diastolic pressure [18]. Our study also demonstrated impaired left atrial function in patients with echocardiographically detected impaired diastolic filling. GLAs-res was found to be an independent predictor of impaired left ventricular diastolic filling. A cut-off value of 13.8% predicted patients with increased LVFP with good sensitivity and specificity. Detection of left atrial function with this novel echocardiographic modality is important in distinguishing patients who are in the gray zone (E/e 8–15) and who might benefit from earlier management strategies [7, 8]. This needs further studies to be clarified.
Limitations
Since there was no specialized software for LA strain analysis, the software for LV analysis was used. This might influence the echocardiographic results. For 2D-STE study of the LA, obtaining optimal images was sometimes challenging especially in obese patients. Although conducted in a very specific patient group, another limitation was the small size of the study population. Moreover, even though echocardiographic estimation of LVFP has been shown to be comparable with invasive methods in evaluating LVFP, invasive evaluation is still the gold standard.
Conclusion
2D-STE-based left atrial function is impaired in patients with nonischemic dilated cardiomyopathy. Left atrial reservoir and pump function parameters together with BNP levels might be useful in estimating left ventricular filling pressure in this patient group.
References
Stevenson WG, Stevenson LW, Middlekauff HR et al (1995) Improving survival for patients with advanced heart failure: a study of 737 consecutive patients. J Am Coll Cardiol 26:1417–1423
Rihal CS, Nishimura RA, Hatle LK et al (1994) Systolic and diastolic dysfunction in patients with clinical diagnosis of dilated cardiomyopathy. Relation to symptoms and prognosis. Circulation 90:2772–2779
Skaluba SJ, Litwin SE (2004) Mechanisms of exercise intolerance: insights from tissue Doppler imaging. Circulation 109:972–977
Haskell RJ, French WI (1988) Accuracy of left atrial and pulmonary artery wedge pressure in pure mitral regurgitation in predicting ventricular end-diastolic pressure. Am J Cardiol 61:136–141
Keogh AM, Baron DW, Hikie JB (1990) Prognostic guides in patients with idiopathic or ischemic dilated cardiomyopathy for cardiac transplantation. Am J Cardiol 65:903–908
Stevenson WG, Stevenson LW, Middlekauff HR et al (1995) Improving survival for patients with advanced heart failure: a study of 737 consecutive patients. J Am Coll Cardiol 26:1417–1423
Nagueh SF, Appleton CP, Gillebert TC et al (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 22(2):107–133
Paulus WJ, Tsche C, Sanderson JE et al (2007) How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 28:2539–2550
Hillis GS, Moller JE, Pellikka PA et al (2004) Noninvasive estimation of left ventricular filling pressure by E/e’ is a powerful predictor of survival after acute myocardial infarction. J Am Coll Cardiol 43:360–367
Mullens W, Borowski AG, Curtin RJ et al (2009) Tissue Doppler imaging in the estimation of intracardiac filling pressure in decompensated patients with advanced systolic heart failure. Circulation 119:62–70
Bruch C, Grude M, Müller J et al (2005) Usefulness of tissue Doppler imaging for estimation of left ventricular filling pressures in patients with systolic and diastolic heart failure. Am J Cardiol 95:892–895
Lee CY, Burnett JC Jr (2007) Natriuretic peptides and therapeutic applications. Heart Fail Rev 12(2):131–142
McCullough PA, Nowak RM, McCord J et al (2002) B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) Multinational Study. Circulation 106(4):416–422
Januzzi JL Jr (2006) Natriuretic peptide testing: a window into the diagnosis and prognosis of heart failure. Cleve Clin J Med 73(2):149–152, 155–157
Lang RM, Bierig M, Devereux RB et al (2006) American Society of Echocardiography’s Nomenclature and Standards Committee; Task Force on Chamber Quantification; American College of Cardiology Echocardiography Committee; American Heart Association; European Association of Echocardiography, European Society of Cardiology. Recommendations for chamber quantification. Eur J Echocardiogr 7(2):79–108
Rudski LG, Lai WW, Afilalo J et al (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23(7):685–713
Kircher B, Abbott JA, Pau S et al (1991) Left atrial volume determination by biplane two-dimensional echocardiography: validation by cine computed tomography. Am Heart J 121(3 Pt 1):864–871
Kurt M, Tanboga IH, Aksakal E et al (2012) Relation of left ventricular end-diastolic pressure and N-terminal pro-brain natriuretic peptide level with left atrial deformation parameters. Eur Heart J Cardiovasc Imaging 13(6):524–530
Ohtani K, Yutani C, Nagata S et al (1995) High prevalence of atrial fibrosis in patients with dilated cardiomyopathy. J Am Coll Cardiol 25:1162–1169
Triposkiadis F, Pitsavos C, Boudoulas H et al (1994) Left atrial myopathy in idiopathic dilated cardiomyopathy. Am Heart J 128(2):308–315
Tigen K, Karaahmet T, Kirma C et al (2010) The association of functional mitral regurgitation and anemia in patients with non-ischemic dilated cardiomyopathy. Cardiol J 17(3):274–280
D’Andrea A, Caso P, Romano S et al (2009) Association between left atrial myocardial function and exercise capacity in patients with either idiopathic or ischemic dilated cardiomyopathy: a two-dimensional speckle strain study. Int J Cardiol 132(3):354–363
Conflict of interest
On behalf of all authors, the corresponding author states that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guler, A., Tigen, K., Dundar, C. et al. Left atrial deformation and nonischemic dilated cardiomyopathy. Herz 39, 251–257 (2014). https://doi.org/10.1007/s00059-013-3817-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00059-013-3817-z