Abstract
The combination of early trans-mitral inflow and mitral annular tissue Doppler velocities (E/e′ ratio) is widely applied to noninvasively estimate left ventricular (LV) filling pressures. However, when E/e′ is between 8 and 14 its accuracy decreases substantially. Left atrial (LA) deformation analysis by speckle tracking echocardiography was recently proposed as an alternative approach to estimate LV filling pressures, but its role when E/e′ is between 8 and 14 has been under-investigated. We aimed to assess whether LA strain could help to identify elevated filling pressures in patients with E/e′ between 8 and 14. Among consecutive non-selected patients who underwent a comprehensive echocardiographic evaluation, we enrolled those with E/e′ ratio > 8 and ≤ 14. Exclusion criteria were: organic mitral valve disease or mitral surgery; presence of mitral regurgitation greater than moderate in severity; diseases associated with pre-capillary pulmonary hypertension; and undetectable systolic pulmonary artery pressure (PAP-S). Peak LA longitudinal (PALS) and contraction strain (PACS) values was obtained by averaging all segments, and by separately averaging segments measured in the 4-chamber and 2-chamber views. Seventy-six patients had E/e′ > 8 and ≤ 14 and formed the study cohort. Mean age 69 ± 12 years, LV ejection fraction (LVEF) 54.5 ± 11.2%, mean E/e′ 11.2 ± 1.9, PAP-S 33 ± 7 mmHg, PALS 31.6 ± 11.7%. PALS was significantly associated to PAP-S after adjustment for LVEF, E/e′, septal LV longitudinal shortening velocity (s′), LA volume indexed (p = 0.002) and also for ASE/EACVI diastolic dysfunction classification (p = 0.0002). Furthermore, PALS but not ASE/EACVI diastolic dysfunction grading, resulted independently associated to New York Heart Association (NYHA) class (p = 0.0004). PALS is able to predict increased intra-cardiac pressure and NYHA class in patients characterized by E/e′ between 8 and 14. Therefore, we propose that PALS might be incorporated in a simplified diagnostic algorithm based on E/e′ classes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Careful estimation of left ventricular (LV) filling pressures is a clinical key tool to classify the severity of different heart diseases and to define therapeutic strategies, particularly in patients with heart failure [1, 2]. Although invasive cardiac catheterization is the gold standard to measure LV filling pressure, a good correlation with noninvasive echocardiography markers has been demonstrated [2, 3]. In particular, early trans-mitral flow velocity (E) combined with mitral annular early diastolic velocity (e′) derived from tissue Doppler imaging (E/e′ ratio) has been shown to correlate with invasive capillary wedge pressure, a surrogate for LV filling pressure, in a wide range of cardiac patients [4,5,6,7]. The accuracy of E/e′ is considerable when it is lower than 8 and higher than 14 to identify patients with low and high filling pressures, but when E/e′ ratio value is between 8 and 14, the diagnostic accuracy decreases substantially [6, 8]. Mainly for this reason the American Society of Echocardiography and European Association of Cardiovascular Imaging (ASE/EACVI) suggested a multiparametric approach to the diagnosis and grading of LV diastolic dysfunction [9]. Nevertheless, individual patients may still demonstrate a spectrum of diastolic indices that do not clearly meet the strict definition of a particular diastolic dysfunction type, thereby making interpretation challenging at times [10, 11].
Speckle tracking echocardiography (STE) is a non-Doppler-based method for quantification of myocardial deformation [12] and in contrast to Doppler-derived indexes, STE has the advantage of being angle-independent and to be less affected by artifacts. Recently, some studies have found that a new left atrial (LA) STE-obtained functional parameter, LA strain, has a strong correlation to LV filling pressures and invasive diastolic measurements [13, 14]. Moreover, LA strain has recently shown additional value over LA indexed volume (LAVI) for identifying patients with diastolic dysfunction, particularly in early stages when LA size may not reflect the chronic effect of increased LV filling pressures [15].
There are few data about the ability of LA strain to estimate intra-cardiac pressures in the specific setting of patients in the gray zone defined by the E/e′ value between 8 and 14. The aim of this study was analyze the association between peak LA longitudinal (PALS) and contraction (PACS) functions evaluated by STE, with intracardiac pressure (as defined by systolic pulmonary artery pressure (PAP-S) measured by Doppler) and clinical functional classification in the specific subgroup of patients characterized by E/e′ between 8 and 14.
Methods
Patient population
Consecutive patients aged > 18 years, with some grade of tricuspid regurgitation allowing the estimation of PAP-S level and reported E/e′ measurements, who were referred to the outpatient echocardiographic laboratory of University of Verona Cardiology Department from January 2018 and March 2018 have been retrospectively enrolled. Subsequently we selected only patients with E/e′ ratio > 8 and ≤ 14, who formed the study cohort. Exclusion criteria were: congenital cardiac abnormalities; organic mitral valve disease or previous valve surgery; presence of mitral regurgitation or other valvular diseases greater than moderate in severity; known pre-capillary pulmonary hypertension or pulmonary diseases possibly associated with pre-capillary pulmonary hypertension; inadequate acoustic window to estimation of PAP-S; presence of atrial fibrillation at the time of enrollment or history of catheter ablation for atrial fibrillation; heart rate lower than 60 bpm and > 99 bpm; presence of regional asynergy at the level of the anterolateral and septal wall.
Clinical data included age, gender, New York Heart Association functional class (NYHA), coronary artery disease, history of hypertension (previous diagnosis of hypertension or use of anti-hypertensive drugs), hyperlipidemia (use of lipid-lowering drugs), smoking (current smoking or smoking habit in the past 10 years), and diabetes (previous diagnosis of diabetes mellitus or use of insulin or use of oral diabetic medication). Patients were included in the valve registry of our institution, approved by the local review board.
2D standard echocardiography
All patients underwent a complete transthoracic echocardiography using HD15 or ie33 ultrasound system (Philips, The Netherlands) performed by a board certified echo-cardiologist. Conventional echocardiographic measurement were performed; LV ejection fraction was calculated with the Simpson’s biplane method. For the assessment of LA volume, apical four-chamber were obtained. Maximal LA volume was measured manually using Simpson’s methods at the end of ventricular systole and indexed to the body surface area. Pulsed Doppler echocardiography of the trans-mitral flow was performed by standard methods. Tissue Doppler Imaging derived systolic and diastolic velocities (s′, e′, a′) were recorded from the lateral and septal edge of the mitral annulus and mean E/e′ ratio was calculated. The subjects were in the resting state in the left lateral position. The left ventricular end-diastolic internal dimension (LVIDd), end-diastolic septal thickness (IVSd), and posterior wall thickness (PWTd) were measured at end-diastole by two-dimensional guided M-mode echocardiography. The left ventricular mass (LVM) was calculated according to the equation LVM(g) = 0.8 × {1.04 × (IVSd + LVIDd + PWTd)}3 − (LVID)3 + 0.06. The LVM index was calculated by dividing the LVM(g) by the height in meters to correct the LVM for body surface area. The left ventricular hypertrophy was defined as LVM index (LVMi) as > 95 g/m2 for female and as > 115 g/m2 for male [16]. LV diastolic function was graded as recommended in the latest ASE-EACVI guidelines, taking into account mitral inflow Doppler measurements, tricuspid regurgitation velocity, tissue Doppler indexes and LAVI [9]. PAP-S was obtained using the following formula: PAP-S = 4 × (peak tricuspid regurgitation velocity)2 + right atrial pressure. The tricuspid regurgitation velocity was carefully assessed from multiple acoustic windows to identify the velocity peak. Right atrial pressure was estimated by means of the diameter and collapsibility of the inferior vena cava (3 mmHg if the inferior vena cava diameter was ≤ 21 mm, collapsing more than 50% with inspiration; 15 mmHg if it was > 21 mm, collapsing < 50% with inspiration; and 8 mmHg in the intermediate situations). In this study we used a PAP-S cut-off value of 35 mmHg as index of increased intracardiac pressures, since it was acknowledged that many authors use the previously proposed upper limit of normal PAP-S of > 30–35 mmHg [17, 18].
In the presence of mitral regurgitation, we performed a quantitative assessment obtaining the proximal isovelocity surface area, and Effective Regurgitant Orifice Area (EROA) and was measured according to the last Echocardiographic Guidelines of the ASE [19]. Since the severity of mitral regurgitation may be influenced by hemodynamic conditions, we recorded patient’s blood pressure to better estimate the severity. To better evaluated LA function other than STE-parameters, we measured maximum and minimum atrial volume during cardiac cycle from apical 4-chamber view to calculate LA emptying fraction (LAEF), an acknowledged LA functional parameter [20, 21].
2D-speckle tracking echocardiography (2D-STE)
2D-STE analysis of LA function were performed. 2D greyscale images were acquired in the standard apical four- and two-chamber views at a frame rate of at least 40 frames/s. The off-line analysis was performed by an experienced cardiologist, using dedicated software (QLab 9, Philips, The Netherlands). LA strain was semi-automatically traced and determined as the average value from all segments of the LA in the apical 4-chamber and 2-chamber views. The PALS, which corresponds to the end of the reservoir phase, and the PACS, corresponding to the end of conduit phase in late diastole, were obtained. To assess reproducibility of LA strain, 15 randomly selected cases were re-analyzed more than 30 days later by the same cardiologist and by a second physician of our echo lab team. In this study we used a PALS cut-off value of 23% to distinguish patients with a reduction of LA functions, since this value was demonstrated to be clearly pathologic according to large studies and to well characterize LV diastolic dysfunction [15, 22, 23].
Statistical analysis
Analyses were performed using SPSS software release 20.0 (Statistical Package for the Social Sciences, Chicago, Illinois). Differences between groups were analyzed using unpaired t-test, Chi-squared test, or analysis of variance, as appropriate. Correlations between variables were evaluated with Pearson or Spearman’s coefficients as appropriate. Associations between variables were evaluated using linear or logistic regression analysis as appropriate. Multiple regression analysis was performed in the entire population to explore for independent determinants of PAP-S; on the basis of results of univariate correlations, different echocardiographic parameters were considered for multivariable analysis. To further investigate the diagnostic performances of echocardiographic indices to predict PAP-S, we performed receiver operating characteristics (ROC) curve analysis to obtain optimal cut-offs, as defined by the Youden index. Variables are shown as mean ± standard deviation. A p-value < 0.05 was considered statistically significant.
Results
Population characteristics
One-hundred forty-two patients was retrospectively included. Among them, 76 patients (54%) showed a mean E/e′ ratio > 8 and ≤ 14 and formed the study cohort (mean age 69 ± 12 years; 42% female). Mean systolic and diastolic arterial pressure during echocardiographic examination were respectively 129 ± 11 mmHg and 74 ± 9 mmHg. Overall, 27 patients (35%) had no mitral regurgitation, 40 patients (53%) had mild a and 8 patients (11%) had moderate mitral regurgitation. Table 1 presents a summary of the clinical and echocardiographic data of the study cohort divided according LVEF and PAP-S.
When ASE/EACVI 2016 diagnostic algorithm for diagnosis and grading of diastolic dysfunction (DD grade) was applied in the 76 patients forming the study cohort, 22 patients (29%) had an indeterminate diastole; normal-filling/grade I diastolic dysfunction (normal LA pressure) was found in 36 (47%) patients, grade II in 16 (21%) patients, grade III in 2 (3%) patients.
Relation between PAP-S and echocardiographic parameters
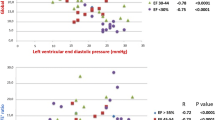
Mean PAP-S was 33 ± 7 mmHg, and 22 patients (29%) had PAP-S > 35 mmHg. In the overall population, patients with higher PAP-S were characterized by higher E/e′ and LAVI and by lower LVEF, septal s′, PALS and PACS (p < 0.05 for all). After adjustment for LVEF, septal s′, E/e′ and LAVI, PALS (p = 0.002) (Fig. 1) and PACS (p = 0.003) remained significantly associated with PAP-S.
As expected, ASE/EACVI DD grade was significantly associated with PAP-S (R2 0.58; p < 0.0001) but in a bivariate model, PALS provided incremental information compared with ASE/EACVI DD increasing R2 up to 0.69 (p = 0.0002).
Using receiver operating characteristic (ROC) curves, PALS demonstrated the highest diagnostic accuracy in predicting PAP-S higher than 35 mmHg (AUC 0.78 (95% CI 66–90), p < 0.0001); the cut-off value of 23% showed an excellent specificity of 90% with a sensibility of 60%. ROC curves for other variables were: PACS (AUC 0.71 (95% CI 0.65–0.85), p < 0.0001), mean E/e′ (AUC 0.70 (95% CI 0.60–0.82%), p = 0.007), EROA (AUC 0.70 (95% CI 0.57–0.82), p = 0.06), LAVI (AUC 0.68 (95% CI 0.56–0.81), p = 0.005), deceleration time of E wave (AUC 0.65 (95% CI 0.55–0.85), p = 0.01).
PALS and NYHA classification
Overall, 41 patients (54%) had heart failure symptoms (NYHA ≥ 2): symptomatic patients were characterized by older age (71 ± 10 vs 66 ± 13 years, p = 0.048), higher PAP-S (35 ± 8 vs 31 ± 5 mmHg, p = 0.018), larger LAVI (45 ± 14 vs 33 ± 9 ml/m2, p < 0.0001) and LV diastolic volume indexed (74 ± 17 vs 65 ± 19 ml/m2, p = 0.028), higher mean E/e′ (12 ± 2 vs 10 ± 2, p = 0.02), lower PALS (27 ± 11 vs 37 ± 10%, p < 0.0001), PACS (12 ± 6 vs 18 ± 6%, p < 0.0001). After adjustment for LVEF, LAVI and E/E′, PALS was the only variable independently associated with NYHA (p = 0.0045). Comparing ASE/EACVI DD with PALS in a bivariate model, only PALS was able to predict NYHA (p = 0.0004). Subdividing symptomatic patients using a cut-off value of LVEF ≥ 50%, 19 patients (46%) had reduced LVEF and 22 patients (54%) had preserved LVEF. In both groups, at univariate analysis, PALS was significantly associated to PAP-S, even if the correlation was slightly stronger in patients with reduced LVEF (r = − 0.66, p = 0.01 vs r = − 0.44, p = 0.02).
Reproducibility of LA strain
Inter-observer agreement was 0.98 and 0.95 for PALS and PACS respectively; the coefficients of variations were 5.1% (3.1–7.2%) for PALS and 15.2% (9.5–22.9%) for PACS. The intra-observer agreement was 0.97 for PALS and 0.96 PACS; the variations were 4.5% (2.5–6.3%) for PALS and 16.7% (10.1–24.6%) for PACS.
Discussion
The main result of the present study is that PALS is highly and independently correlated with intracardiac pressure level, as assessed by PAP-S, in a population of patients characterized by E/e′ ratio between 8 and 14. This result is corroborated by the finding of a strong and independent association between PALS and heart failure symptoms. We believe that these results might support the use of PALS in filling pressure determination and in simplifying the diagnostic algorithm proposed by echocardiographic societies.
Noninvasive estimation of LV filling pressures is fundamental to decide therapeutic strategies and to predict the severity of many heart diseases [24]. Early trans-mitral flow velocity (E) combined with mitral annular early diastolic velocity (e′) derived from tissue Doppler imaging (E/e′ ratio) has been shown to correlate with invasive measurements in different cardiac patients [3]. In patients with normal LVEF, an E/e′ ratio ≥ 14 implies a high probability of elevated LV filling pressures, and a value < 8 is associated to normal LV filling pressures [10]. However, when E/e′ ratio is between 8 and 14, LV filling pressures remain undefined and also there is a lack of data about correlations and meaning of E/e′ ratio in this range of values. Some studies found a good correlation between LA STE-derived functional parameters and LV filling pressure [13, 14, 25]. Moreover, different studies with large populations, provided important insights regarding the usefulness and clinical relevance of adding LA strain to classical algorithms, in the detection of LV diastolic dysfunction [15]. However actually, there are no specific studies about the role of PALS in patients with E/e′ ratio between 8 and 14, which represent a consistent portion of population where a diagnosis of diastolic dysfunction may be missed. Accordingly, in our total population 54% of patients had an E/e′ ration between 8 and 14 and in this specific cohort 29% of patients showed an indeterminate diastolic pattern applying DD grade. According to our data, there is a correlation between intracardiac pressure and LA functional morpho-functional parameters. This is not surprising since the onset of high pulmonary pressure represents the natural history of left-sided heart diseases, preceded by morpho-functional changes of left-sided chambers. Among variables associated to PAP-S, PALS had the highest correlation and degree of accuracy in identifying patients with pulmonary hypertension, even in comparison with DD grading. These data are corroborated by the demonstration of a strong and independent association with heart failure symptoms. Taken together these findings, we might suggest to incorporate PALS in the 2009 ASE/EAE recommendations for filling pressure estimation, which were based on the three E/e′ classes, limiting PALS in the subgroup of patients with E/e′ between 8 and 14 (Fig. 2). This could simplify the application of the diagnostic algorithm and consequently enhance the evaluation of left ventricular diastolic dysfunction.
In conclusion, PALS is a measure of LA function strongly and independently associated with intracardiac pressure in the subgroup of patients characterized by E/e′ between 8 and 14. Our data suggest that PALS could be used together with E/e′ ratio to better characterized diastolic dysfunction categorization. This could simplify the diagnostic algorithm and would help to improve patients care.
Limitations
This was a single-center study with a limited number of patients. The major limitation of the study is that we could not perform invasive measurements of left ventricular filling pressures. However, we have to acknowledge that right and left cardiac catheterization for the purpose of measuring capillary mean pressure or end-diastolic left ventricular pressure are nowadays relatively rare and limited to specific patents populations. The use of PAP-S as a surrogate of intracardiac pressure should be used with cautions. Although patients with previous diagnoses of pre-capillary PH were specifically excluded, a combined pre-capillary component cannot be ruled out in some patients. However, the 2009 and 2016 ASE/EACVI algorithms for classification of diastolic function share the same limitation, given the fact that they used tricuspid regurgitation velocity or PAP-S as one of the cornerstone to diagnose and grade diastolic dysfunction. It is possible that excluding patients with misdiagnosed pre-capillary hypertension, PALS may show even better correlations in this setting.
Data availability
Available.
References
Andersen OS, Smiseth OA, Dokainish H, Abudiab MM, Schutt RC, Kumar A et al (2017) Estimating left ventricular filling pressure by echocardiography. J Am Coll Cardiol 69:1937–1948
Benfari G, Miller WL, Antoine C, Rossi A, Lin G, Oh JK, Roger VL, Thapa P, Enriquez-Sarano M (2019) Diastolic determinants of excess mortality in heart failure with reduced ejection fraction. JACC Heart Fail 7:808–817
Nagueh SF et al (2018) Non-invasive assessment of left ventricular filling pressure. Eur J Heart Fail 20:38–48
Nagueh SF, Lakkis NM, Middleton KJ, Spencer WH, Zoghbi WA, Quiñones MA (1999) Doppler estimation of left ventricular filling pressures in patients with hypertrophic cardiomyopathy. Circulation 99:254–261
Diwan A, McCulloch M, Lawrie GM, Reardon MJ, Nagueh SF (2005) Doppler estimation of left ventricular filling pressures in patients with mitral valve disease. Circulation 111:3281–3289
Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ (2000) Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation 102:1788–1794
Dokainish H, Zoghbi WA, Lakkis NM, Al-Bakshy F, Dhir M, Quinones MA, Nagueh SF (2004) Optimal non-invasive assessment of LV filling pressures: a comparison of tissue Doppler echocardiography and BNP in patients with pulmonary artery catheters. Circulation 109:2432–2439
Park JH, Marwick TH (2011) Use and limitations of e/e′ to assess left ventricular filling pressure by echocardiography. J Cardiovasc Ultrasound 19:169–173
Nagueh SF, Smiseth OA, Appleton CP et al (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 17(1321):1360
Shimron M, Williams L, Hazanov Y, Ghanim D, Kinany W, Amir O, Carasso S (2018) Clinical and echocardiographic characteristics of patients in sinus rhythm, normal left ventricular function, and indeterminate diastolic function. Echocardiography 35:792–797
Kuwaki H, Takeuchi M, Chien-Chia Wu V et al (2014) Redefining diastolic dysfunction grading: combination of E/A ≤ 0.75 and deceleration time >140 ms and E/ε′ ≥10. J Am Coll Cardiol Imaging 7:749–758
Cameli M, Caputo M, Mondillo S, Ballo P, Palmerini E, Lisi M, Marino E, Galderisi M (2009) Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc Ultrasound 7:6
Singh A, Medvedofsky D, Mediratta A et al (2019) Peak left atrial strain as a single measure for the non-invasive assessment of left ventricular filling pressures. Int J Cardiovasc Imaging 35:23–32
Wakami K, Ohte N, Asada K et al (2009) Correlation between left ventricular end-diastolic pressure and peak left atrial wall strain during left ventricular systole. J Am Soc Echocardiogr 22:847–851
Morris DA, Belyavskiy E, Aravind-Kumar R, Kropf M, Frydas A, Braunauer K et al (2017) Potential usefulness and clinical relevance of adding left atrial strain to left atrial volume index in the detection of left ventricular diastolic dysfunction. JACC Cardiovasc Imaging 11:1405–1415
Lang RM, Badano LP, Mor-Avi V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28:1-39 e14
Lam C, Roger V, Rodeheffer R, Borlaug B, Enders F, Redfield M (2009) Pulmonary hypertension in heart failure with preserved ejection fraction: a community based study. J Am Coll Cardiol 53:1119–1126
Vachiéry J-L, Tedford RJ, Rosenkranz S et al (2019) Pulmonary hypertension due to left heart disease. Eur Respir J 53:1801897
Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA et al (2017) Recommendations for non-invasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 30:303–371
Kanagala P, Arnold JR, Cheng ASH, Singh A, Khan JN, Gulsin GS et al (2019) Left atrial ejection fraction and outcomes in heart failure with preserved ejection fraction. Int J Cardiovasc Imaging 36:101–110
Gottdiener JS, Kitzman DW, Aurigemma GP, Arnold AM, Manolio TA (2006) Left atrial volume, geometry, and function in systolic and diastolic heart failure of persons > or = 65 years of age (the cardiovascular health study). Am J Cardiol 97:83–89
Pathan F, D’Elia N, Nolan MT, Marwick TH, Negishi K (2017) Normal ranges of left atrial strain by speckle tracking echocardiography: a systematic review and meta-analysis. J Am Soc Echocardiogr 30:59–70
Morris DA, Takeuchi M, Krisper M et al (2015) Normal values and clinical relevance of left atrial myocardial function analysed by speckle-tracking echocardiography: multicentre study. Eur Heart J Cardiovasc Imaging 16:364–372
Oh JK (2005) Echocardiography as a noninvasive Swan-Ganza catheter. Circulation 111:3192–3194
Cameli M, Mandoli GR, Loiacono F et al (2016) Left atrial strain: a new parameter for assessment of left ventricular filling pressure. Heart Fail Rev 21:65–76
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
Author information
Authors and Affiliations
Contributions
All authors have contributed to the concept of the study, data collection, data analysis and interpretation, drafting, or revision of the letter.
Corresponding author
Ethics declarations
Conflict of interest
The authors declares that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The ethics committee of our institute approved the study.
Consent to participate
All subjects gave their written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cerrito, L.F., Maffeis, C., Inciardi, R.M. et al. How to incorporate left atrial strain in the diagnostic algorithm of left ventricular diastolic dysfunction. Int J Cardiovasc Imaging 37, 945–951 (2021). https://doi.org/10.1007/s10554-020-02070-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-020-02070-6