Abstract
Establishment and maintenance of the reproductive division of labor within social insect colonies relies on clear communication between nestmates. Fertile members convey their status to prevent others from becoming reproductively active. Recent findings in some basal termites indicate that cuticular hydrocarbon profiles may indicate reproductive state, but there is little evidence to show a direct link between reproductive status and hydrocarbon production—a prerequisite for an “honest” fertility signal. Here, we report that the putative signaling mechanism is influenced by juvenile hormone (JH), a primary regulator of gonadal development and activity in insects. Topical application of a JH-analog (pyriproxyfen) to reproductively inactive alates of the basal dampwood termite Zootermopsis nevadensis induced both females and males to express significantly more of a reproductive-specific hydrocarbon (6,9,17-tritriacontatriene). However, the JH-analog did not significantly enhance gonadal development or activity in treated termites beyond what is usually observed in maturing alates released from the inhibitory stimuli of their natal nest. These results suggest that a rise in JH following disinhibition drives the expression of reproductive-specific hydrocarbons, but that an individual’s hydrocarbon profile is not directly linked to its gonadal state. Rather than directly driving the expression of reproductive-specific hydrocarbons, the gonads may act indirectly through their influence on circulating JH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Within the eusocial insects, signals of fertility status are used to maintain a reproductive division of labor, ensuring that only one or a few individuals in a colony reproduce, while the rest serve as a functionally sterile workforce (Wilson 1971; Gadagkar 1994; Sherman et al. 1995). The current model suggests that functional reproductives produce a chemical signature linked with their fertility (Heinze 2004; Monnin 2006; Le Conte and Hefetz 2008; Peeters and Liebig 2009; Liebig 2010; Van Oystaeyen et al. 2014; Bagnères and Hanus 2015), and that this chemical signature prevents other colony members from reproducing via self-regulated inhibition (Seeley 1979, 1985; Keller and Nonacs 1993). The means by which the production of such fertility signals is coordinated with an individual’s reproductive status remains to be fully elucidated, but elucidation of this mechanism is crucial to understanding how a stable reproductive division of labor might have evolved in social species.
For basal termites, cuticular hydrocarbons appear to be a major conduit of information needed for colony organization. At a rudimentary level, hydrocarbon profiles appear to encode species and colony identity (Howard et al. 1982; Haverty et al. 1988; Howard and Blomquist 2005). Within the colony, individual profiles appear to convey caste- (Sevala et al. 2000; Klochkov et al. 2005; Darrouzet et al. 2014) and gender-specific (Bordereau et al., 2010) information. Recently, reproductive-specific hydrocarbons have been identified in Cryptotermes secundus (Weil et al. 2009) and Zootermopsis nevadensis (Liebig et al. 2009, 2012). These compounds may play a role as primer pheromones, ensuring that nestmates do not develop into supernumerary reproductives in the presence of active kings and queens. Although there is not yet clear evidence of their impact on conspecific development in termites, cuticular hydrocarbons are used for reproductive regulation through fertility signaling in a number of ant species (Endler et al. 2004; Smith et al. 2009; Holman et al. 2010; Smith et al. 2013; Van Oystaeyen et al. 2014). Similar patterns in other eusocial Hymenoptera suggest a widespread use of such hydrocarbon-based signals (Monnin 2006; Le Conte and Hefetz 2008; Peeters and Liebig 2009; Liebig 2010; Van Oystaeyen et al. 2014). There is also evidence that removal of individuals bearing reproductive-specific hydrocarbons quickly evokes a pronounced behavioral response in Z. nevadensis larval helpers, suggesting that they are attuned to the chemical signatures of their nestmates (Penick et al. 2012).
To establish that the identified hydrocarbons signal reproductive status, it is necessary to show a mechanistic connection between signal production and reproductive activity. Crucial to both insect reproduction and the expression of cuticular hydrocarbons is the activity of the neuroendocrine system, with juvenile hormone (JH) playing a central role. In termites, JH is involved in determining an individual’s reproductive fate (Lüscher 1972; Wanyonyi 1974; Yin and Gillot 1975; Lenz 1976; Okot-Kotber 1982; Okot-Kotber and Prestwich 1991a, b; Miura et al. 2003; Scharf et al. 2005a; Cornette et al. 2008; Elliott and Stay 2008; Korb et al. 2009a; Leniaud et al. 2011), and this hormone also appears to be associated with gonadal activity (Greenberg and Tobe 1985; Brent et al. 2005, 2006; Scharf et al. 2005a; Elliott and Stay 2007; Saiki et al. 2015). Cockroaches, which belong to the same group as termites (Kambhampati 1995; Thorne 1997; Inward et al. 2007), also use JH to regulate gonadal activity (Engelmann 1959; Stay et al. 1980; Koeppe 1981; Koeppe et al. 1985; Schal et al. 1997), and to stimulate hydrocarbon synthesis, storage and transport to the cuticle (Schal et al. 1994; Fan et al. 2002; reviewed in Tillman et al. 1999; Schal et al. 2003). Although the specific relationship between JH, gonadal activity, and hydrocarbon expression remains to be elucidated in termites, we anticipate that the association follows a pattern similar to that of the cockroaches.
In Zootermopsis alates, the winged imagoes that found new colonies, JH titers remain high so long as alates remain in their natal nest exposed to stimuli inhibiting reproductive maturation. A few days after an alate has been separated from the inhibitory stimuli and paired with a mate, JH production drops sharply and remains low for more than a month (Brent et al. 2005). Subsequently, JH release increases, corresponding with an increase in gonadal activity (Brent and Traniello 2001a, b). During this transition, females and males begin to express reproductive-specific cuticular hydrocarbons, namely alkadienes and alkatrienes (Liebig et al. 2009, 2012). In transcriptomic analyses, mRNA reads of an elongase and a desaturase, both needed for the production of long-chained alkadienes or alkatrienes, were most expressed in reproductive males and females (Terrapon et al. 2014). Of the four such compounds identified, 6,9,17-tritriacontatriene has the most pronounced peak in the cuticular hydrocarbon profile. The expression level of these compounds was found to be positively associated with gonadal development, at least for males (Brent et al. 2005). Collectively, these data suggest that there is at least a correlational relationship between JH titer, gonadal activation and hydrocarbon expression. However, further evidence is needed to establish a clear regulatory link between reproductive state and odorant profile before any conclusions can be drawn about the role of these hydrocarbons as a fertility signaling mechanism. Accordingly, the current study was undertaken to investigate the role that JH may have in linking the expression of the reproductive-specific cuticular hydrocarbon with gonadal development in females and males of Z. nevadensis.
Methods and materials
Animals
Colonies of Zootermopsis nevadensis were collected in Pebble Beach near Monterey, California in 2009. Collection location and the cuticular hydrocarbon profiles of the colonies indicate that they belong to the subspecies nuttingi (Haverty and Thorne 1989). Colonies were extracted from wood logs in the laboratory and transferred into nests consisting of several pre-cavitated 5-mm-thick layers of moistened Spruce Pine (Pinus glabra) sheets that were bolted together. This allowed ready access to the termites by disassembling and subsequently reassembling the wood nest. Nests were periodically sprayed with ddH2O to maintain moisture, and were kept in transparent plastic boxes under a 12L:12D light cycle at 25.2 °C.
Hormone treatment and experimental conditions
Alate females and males were collected from 16 laboratory stock colonies. Because alates turn dark brown as their cuticle becomes sclerotized following their imaginal molt, choosing alates with similarly dark pigmentation minimized pretreatment developmental differences. Zootermopsis alates maintained in their natal nest have inhibited gonadal development and refrain from oogenesis (Castle 1934; Brent and Traniello 2001a), and they do not express reproductive-specific hydrocarbons (Liebig et al. 2009). Only once they are removed from stimuli produced by functional reproductives and other alates will they complete their maturation. Therefore, prior to treatment, alates were separated from the colony, and held in plastic containers with moistened tissue paper for 7–10 days. This period has been found to be sufficiently long to induce development toward a fertile state (Brent et al. 2005). Subsequently, the alates were gently dealated by folding their wings anteriorly over the wing suture. They were then treated once with either 10 µg of the juvenile hormone analog (JHA) pyriproxyfen (technical grade, 98.9 %; Syngenta, Greensboro, NC, USA) dissolved in 1 µl acetone (Sigma-Aldrich, St. Louis, MO), or with 1 µl acetone only (control). Topical applications were made to the dorsal abdomen 3 using 5-µl graduated glass pipettes. Pyriproxyfen was chosen for its demonstrated effects on termite development (Ogino et al. 1993; Miura et al. 2003; Scharf et al. 2003), and its long-term pharmacological activity in vivo. This latter quality permitted use of a single high-dosage application, whereas use of JHIII, the primary homolog in Zootermopsis (Meyer et al. 1976; Greenberg and Tobe 1985; Brent et al. 2005), or a short-lived JH-analog, like methoprene, would have necessitated multiple periodic topical applications. Repeated disturbances can have stress effects on development and hormone levels, with potential to negatively impact fertility and hydrocarbon expression.
After treatment, heterosexually paired individuals that had both received the same treatment were placed in clear circular plastic boxes (diameter 50 mm) containing a pinewood ring (diameter 45 mm, height 6 mm) and a piece of paper towel. The wooden ring had a central hole (diameter 27 mm) to serve as a nesting chamber and had been presoaked in water for a minimum of 24 h. The ring and paper towel were moistened as needed. The rearing chambers were kept under the same ambient conditions as the stock colonies. Different subsets of the treated individuals were collected at 10, 30 and 50 days after the start of the experiment to assess their hydrocarbon profile. To avoid sampling bias, the individuals used for each day and treatment condition were drawn from between 6 and 10 stock colonies. The females and males examined after 50 days were also dissected to assess reproductive development. The experiment was limited to 50 days, because that duration is normally sufficient for Zootermopsis primaries that have been recently released from reproductive inhibition to become mature enough to produce gametes and mate (Heath 1903; Castle 1934; Greenberg and Stuart 1979; Brent and Traniello 2001a, b, c). At the time of sampling, neither eggs nor any larvae were found in the nest boxes or the wood.
Analysis of cuticular hydrocarbon patterns and gonad development
The hydrocarbon profiles of females and males were obtained by hexane extraction. The whole insect was gently shaken in a Teflon-capped 2-ml borosilicate glass vial containing 100 μl hexane (Sigma-Aldrich, St. Louis, MO) for 2 min. One-μl aliquots of the hexane extracts were injected into an Agilent 6890N GC (Agilent, Santa Clara, CA, USA) coupled with an Agilent 5975 Mass Selective Detector, operated in the electron impact ionization mode. The GC was operated in splitless injection mode with helium as carrier gas at 1 ml min−1 flow rate. It was fitted with a 30-m × 0.25-mm (ID) × 0.1-μm DB-1MS non-polar column (Agilent). The oven temperature was programmed to rise from 60 to 200 °C at 40 °C min−1 after an initial delay of 2 min including a splitless time of 0.5 min. Subsequently, the temperature rose from 200 to 320 °C at 5 °C min−1. Injector temperature was 260 °C, MS quad 150 °C, MS source 230 °C, and transfer line 300 °C. For the comparison of peak areas, we standardized to 100 % the sum of the major four peaks of the termite cuticular hydrocarbon profile (C21, C23:2, C23, and C25), and calculated the relative amount of 6,9,17-tritriacontatriene in each sample as a percentage. Tritriacontatriene was previously identified as being the most prevalent of four reproductive-specific hydrocarbons, which makes it the most reliable indicator compound (Liebig et al. 2009, 2012). The remaining three reproductive-specific peaks are generally expressed at much lower levels (Liebig et al. 2009). In this experiment, 6,9-nonacosadiene and 6,9,17-dotriacontatriene were below detection limits and 6,9-hentriacontadiene only rarely rose above detection limits.
After being subjected to hexane washing, test individuals from 50 days post-treatment were stored in 50 % ethanol until they could be dissected under a stereomicroscope to determine gonadal development. The ovaries were assessed by counting the total number of functional ovarioles and the number of vitellogenic terminal oocytes, as described in Brent and Traniello (2001b). Ovarioles were considered functional if they were not filamentous and contained oocytes at some stage of development. Oocytes were considered vitellogenic if yolk protein could be observed and the volume equaled or exceeded 0.01 mm3 (Hewitt et al. 1972; Brent and Traniello 2001b). Male testis diameter, an indicator of sperm production capacity (Brent and Traniello 2001a), was measured using an ocular micrometer. Dissections were conducted blind to the treatment group.
Statistical analyses
The association between JHA treatment and the occurrence of the reproductive-specific hydrocarbon was investigated using a generalized linear mixed model (Baayan 2008) with binomial error structure and logit link function. The model was fitted in R (R Development Core Team 2009) using the function lmer of the R-package lme4 (Bates and Maechler 2010). For the analysis, a trial was treated as a qualitative success if at least one of the members of the pair had a detectable 6,9,17-tritriacontatriene peak; replicates in which only one individual survived were excluded from the analysis. Treatment, collection day, and their interaction were included in the model as fixed factors, while colony was included as a random effect. Collection day was z-transformed before loading the model. We began with a random effects structure that accounted for random intercepts, random slopes, and a correlation between them. Variance inflation factors were derived using the function ‘vif’ of the R-package car (Fox and Wiesberg 2011) applied to the standard linear model excluding the random effect. VIFs were moderately high (mean = 2.56, maximum = 3.37). Model stability was evaluated by excluding cases one by one and comparing the estimates derived from the reduced models with those obtained for the full model. One influential case was found to exist: a day-10 female in the control condition was found to express the 6,9,17-tritriacontatriene peak. Since this case was conservative with regards to our hypothesis, it was not removed from the model. After checking the assumptions, we evaluated the model’s random effects structure by sequentially comparing the full model with a reduced model using likelihood ratio tests; no significant differences were detected between the models, and thus, we used the simplest model, which included only random intercepts. As an overall test of the effect of treatment, we used a likelihood ratio test comparing the full model with a null model comprising only the fixed effect of collection day and the same random effects structure. After assessing the significance of the full model, we assessed the significance of the interaction term. It was found to be non-significant and was, therefore, removed from the model to allow direct interpretation of the main effects. Inter-gender comparisons of peak expression were not performed due to the non-independent nature of the data collected.
Comparisons of gonadal development (numbers of ovarioles and vitellogenic oocytes; testis diameter) between treatments were made by a nested ANOVA with treatment as the fixed factor since surviving individuals were composed of cohorts from different colonies. STATISTICA 7.1 (StatSoft Inc, Tulsa, OK) was used for analysis.
Results
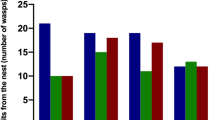
Overall, expression of the 6,9,17-tritriacontatriene peak was significantly affected by treatment and collection time (10, 30 or 50 days) (Table 1). JHA application was consistently associated with expression of the peak (Fig. 1). After 10 days, traces of the reproductive-specific peak were found in 29 % of combined JHA-treated males and females, but in only 4 % of the controls. By day 50, 88 % of JHA-treated individuals expressed the peak, in contrast to the 22 % of control individuals (Table 2). In addition to occurring more frequently, the amount of 6,9,17-tritriacontatriene as a proportion of our four reference peaks also increased over time in the JHA-treated individuals (Fig. 1). While the median percentage at day 10 was 0, by day 50, it had increased to 0.29 and 0.46 % in JHA-treated females and males, respectively. During this same period, the maximum amounts increased more strongly, from 0.51 to 3.87 % in females, and from 0.37 to 10.05 % in males. In contrast, the maximum percentage of this hydrocarbon peak for acetone-treated individuals rose to no higher than 0.17 % by day 50 (Fig. 1).
Relative areas of the main reproductive-specific peak (6,9,17-tritriacontatriene), after standardizing to 100 % the sum of four associated peaks, for female and male primary reproductives of Z. nevadensis sampled 10, 30 and 50 days after being removed from their natal nest and treated with either an acetone solvent control or a juvenile hormone analog (JHA). The medians, interquartile ranges, 90th and 10th percentiles (error bars), and maxima (closed circles) are indicated. Note the twofold larger scale used for males. Samples sizes ranged from 7 to 16
Although JHA enhanced the production of 6,9,17-tritriacontatriene in both sexes, there was no or little effect of the juvenoid on gonad development. After 50 days, treatment did not influence either the number of active ovarioles (Nested ANOVA; F = 2.42; df = 1, 18; P = 0.13) or vitellogenic oocytes (Nested ANOVA; F = 2.48; df = 1, 18; P = 0.14) present in the ovaries (Fig. 2). The median diameters of the testes of JHA-treated males were 25 % larger than those of the control group, but the difference was not statistically significant (Nested ANOVA; F = 3.32; df = 1, 15; P = 0.09; Fig. 3).
Number of ovarioles per ovary and vitellogenic oocytes in Z. nevadensis females 50 days after treatment with either a control solvent (n = 21) or JHA (n = 11). The medians, interquartile ranges, 90th and 10th percentiles (error bars), and maxima (circles) are indicated. There was no difference in the distribution between treatments for either trait (Nested ANOVA, P = 0.14 and P = 0.13)
Testis diameter of Z. nevadensis males 50 days after treatment with either a control solvent (n = 20) or JHA (n = 9). The medians, interquartile ranges, 90th and 10th percentiles (error bars), and maxima (open circles) are indicated. The difference between treatments was not significant (Nested ANOVA, P = 0.09)
Discussion
The increased expression of a reproductive-specific hydrocarbon by both males and females in response to JHA application suggests a causal relationship, where JH may be a key component in driving signal production. The temporal pattern of the production of this hydrocarbon suggests that JH does not directly influence its biosynthesis. JH may rather induce the production of other biosynthesis-affecting factors that are potentially associated with the activation of the gonads. This association is suggested by the long delay observed between JHA application and the expression of the hydrocarbon peak. Even though at 30 days, there was already a significant effect in hydrocarbon expression, the reproductive-associated peak (for 6,9,17-tritriacontatriene) was very small (Fig. 1), especially when compared to those of fully mature and highly fecund reproductives (Liebig et al. 2009). However, the median expression level increased 3–7-fold over the next 20 days. This enhanced expression coincides with the normal start of gonadal activity (Brent and Traniello 2001a, b, c), which in our experiment was evident from the presence of vitellogenic oocytes (Fig. 2) and testes that were of a larger size (Fig. 3) than is normally observed in alates at the colony-founding stage (Brent and Traniello 2001a) in both treatment groups.
There have been few other attempts to characterize the relationship between reproductive physiology and the expression of fertility-related hydrocarbons in social insects. In the epiponine wasp Syneoca surinama, workers treated with a JH-analog increased ovarian development and changed their cuticular hydrocarbon profiles toward those of reproductive workers (Kelstrup et al. 2014). In contrast to this and our own approach, other studies have relied upon physiological manipulations that inhibited the production of a fertility signal. Application of a JHA to reproductives of the ant Streblognathus peetersi, in which non-reproductive individuals have a higher JH titer than reproductives (Brent et al. 2006), reduced the relative quantities of the fecundity-associated hydrocarbons (Cuvillier-Hot et al. 2004). In the termite Cryptotermes secundus, an RNAi-induced knock-down of a reproductive-specific expressed gene in queens resulted in a worker behavioral response that is typically produced only with the loss of queens (Korb et al. 2009b). We now show a relationship between JH and putative signals of reproductive status through induction rather than inhibition, which removes the potential side effects of stress induced by hormone disruption that could inhibit reproductive activity. Despite the different approaches taken, each of these experiments has highlighted the capacity of reproductives to convey information about their status to nestmates as well as a connection between physiological factors and signal production.
Unlike the hydrocarbons, a gonadal response to JHA was completely absent in the females (Fig. 2) and only suggestive in the males (Fig. 3). A tight positive association between JH and vitellogenin has previously been observed in termites (Greenberg and Tobe 1985; Scharf et al. 2005a, b). Increased JH has also been linked with ovarian development and activity in females of the sister species Z. angusticollis (Brent et al. 2005), and a recent study in Z. nevadensis found that JHA did stimulate gonad activity in a reproductive soldier-like caste (Saiki et al. 2014). However, these studies did not elucidate the specific nature of the relationship. In the cockroach Diploptera punctata, JH synthesis is driven by the presence of early vitellogenic oocytes, but inhibited by relatively mature oocytes (Rankin and Stay 1984, 1985; Elliott et al. 2006). A similar pattern has been observed for Reticulitermes flavipes neotenic reproductives (Elliott and Stay 2007) and may be consistent across the termites. Ovarian control of circulating JH would ensure that the fat body produces vitellogenin only when the oocytes are ready to incorporate it. The failure of ovaries of Z. nevadensis females to respond to JHA may simply be an indication that their oocytes were not yet sufficiently mature to uptake vitellogenin. During the period tested, females were undergoing major organizational and behavioral shifts as they transitioned from a stage optimized for dispersal to one focused on egg production. This transition takes several weeks (Brent and Traniello 2001b, c), during which the female’s regulatory system is reorganized so that JH goes from acting as a suppressor of ovarian activity to a facilitator (Brent et al. 2005). The maturing queen also needs this time to acquire the resources necessary to produce eggs. JHA applied prior to this transition in reproductive capability would not produce any substantive effects on the ovaries, and if applied too early could even delay reproductive development. Similar developmental constraints may also limit the male gonadal response, however, more research is needed before any substantive conclusions can be drawn.
The disparity between hydrocarbon expression and gonadal activity in their response to JHA does not rule out the utility of these compounds for signaling individual fertility. Indirect links between gonadal development and signaling of reproductive status have been observed in other social insects. Decoupling of developmental stage and hydrocarbon profile has been found in Diacamma quadriceps (Peeters et al. 1999) and Polistes dominulus (Sledge et al. 2001). Similarly, topical application of a JHA can accelerate changes to the hydrocarbon profile of workers of the ant Myrmicaria eumenoides, but does not change the speed with which individuals transitioned to the behavioral stage associated with the new profile (Lengyel et al. 2007). Alternatively, there may be a more direct link that our experimental approach failed to reveal. While JHAs do mimic the effects of JH, they are not completely interchangeable. Scharf et al. (2003) found that termites exhibit varied developmental responses when treated with different JHAs. The juvenoid used here, pyriproxyfen, has a structure that is quite dissimilar from that of JHIII. Pyriproxyfen may be effective at modulating some changes, such as shifting hydrocarbon expression, but may fail to induce others, such as promoting vitellogenesis. In addition, the quantity of pyriproxyfen applied may have been insufficient to influence gonadal development, given that the effects of this JHA on reproduction are frequently dosage-dependent (Cusson et al. 1994; Pinto et al. 2000; Singh and Kumar 2015; Xu et al. 2015).
Collectively, the evidence that JH can induce the expression of reproductive-specific hydrocarbons, and the association of that hormone with termite gonadal activity (Vieau and Lebrun 1981; Greenberg and Tobe 1985; Brent et al. 2005; Elliott and Stay 2007), supports the idea that these hydrocarbons inform nestmates of the potential fecundity of a reproductive. However, definitive proof that these hydrocarbons can influence the reproductive development of nestmates does not yet exist and alternative mechanisms cannot be excluded. Recently, Matsuura et al. (2010) and Matsuura and Yamamoto (2011) found a volatile primer pheromone in Reticulitermes speratus which was able to inhibit the differentiation of replacement reproductives. In addition, reproductive-specific external expression of proteinaceous secretions which might serve in fertility signaling has been observed in Kalotermes flavicollis, Prorhinotermes simplex and Reticulitermes santonensis (Hanus et al. 2010). In addition to testing for these possible factors, future research will need to clarify the role of JH in hydrocarbon expression and gonadal activity before we can truly understand how these compounds might work in conjunction to produce an authentic fertility signal.
References
Baayan R (2008) Analyzing linguistic data. Cambridge University Press, Cambridge
Bagnères AG, Hanus R (2015) Communication and social regulation in termites. In: Aquiloni L, Tricarico E (eds) Social recognition in invertebrates. Springer International Publishing, Switzerland, pp 193–248
Bates D, Maechler M (2010) lme4: Linear mixed-effects model using S4 classes. R package version 0.999375-35
Bordereau C, Lacey MJ, Sémon E, Braekman JC, Ghostin J, Alain R, Shellman-Sherman J, Sillam-Dussès D (2010) Sex pheromones and trail-following pheromone in the basal termites Zootermopsis nevadensis (Hagen) and Z. angusticollis (Hagen) (Isoptera: Termopsidae: Termopsinae). Biol J Linn Soc 100:519–530
Brent CS, Traniello JFA (2001a) Social regulation of testicular development in primary and secondary males of the dampwood termite Zootermopsis angusticollis Hagen. Insectes Soc 48:384–391
Brent CS, Traniello JFA (2001b) Influence of sex-specific stimuli on ovarian maturation in both primary and secondary reproductives of the dampwood termite Zootermopsis angusticollis. Physiol Entomol 26:239–247
Brent CS, Traniello JFA (2001c) Social influence of larvae on ovarian maturation in primary and secondary reproductives of the dampwood termite Zootermopsis angusticollis. Physiol Entomol 26:78–85
Brent CS, Schal C, Vargo EL (2005) Endocrine changes in maturing primary queens of Zootermopsis angusticollis. J Insect Physiol 51:1200–1209
Brent CS, Peeters C, Dietmann V, Crewe R, Vargo EL (2006) Hormonal correlates of reproductive status in the queenless ponerine ant, Streblognathus peetersi. J Comp Physiol A 192:315–320
Castle GB (1934) The damp-wood termites of western United States, genus Zootermopsis. In: Kofoid C (ed) Termites and termite control. University of California Press, Berkeley, pp 264–282
Cornette R, Gotoh H, Koshikawa S, Miura T (2008) Juvenile hormone titers and caste differentiation in the damp-wood termite Hodotermopsis sjostedti (Isoptera, Termopsidae). J Insect Physiol 54:922–930
Cusson M, Yu CG, Carruthers K, Wyatt GR, Tobe SS, McNeil JN (1994) Regulation of vitellogenin production in armyworm moths, Pseudaletia unipuncta. J Insect Physiol 40:129–136
Cuvillier-Hot V, Lenoir A, Peeters C (2004) Reproductive monopoly enforced by sterile police workers in a queenless ant. Behav Ecol 15:970–975
Darrouzet E, Labédan M, Landré X, Perdereau E, Christides JP, Bagnères A (2014) Endocrine control of cuticular hydrocarbon profiles during worker-to-soldier differentiation in the termite Reticulitermes flavipes. J Insect Physiol 61:25–33
Elliott KL, Stay B (2007) Juvenile hormone synthesis as related to egg development in neotenic reproductives of the termite Reticulitermes flavipes, with observations on urates in the fat body. Gen Comp Endocrinol 152:102–110
Elliott KL, Stay B (2008) Changes in juvenile hormone synthesis in the termite Reticulitermes flavipes during development of soldiers and neotenic reproductives from groups of isolated workers. J Insect Physiol 54:492–500
Elliott KL, Woodhead AP, Stay B (2006) A stage-specific ovarian factor with stable stimulation of juvenile hormone synthesis in corpora allata of the cockroach Diploptera punctata. J Insect Physiol 52:929–935
Endler A, Liebig J, Schmitt T, Parker JE, Jones GR, Schreier P, Hölldobler B (2004) Surface hydrocarbons of queen eggs regulate worker reproduction in a social insect. Proc Nat Acad Sci USA 101:2945–2950
Engelmann F (1959) The control of reproduction in Diploptera punctata (Blattaria). Biol Bull 116:406–419
Fan Y, Chase J, Sevala VL, Schal C (2002) Lipophorin-facilitated hydrocarbon uptake by oocytes in the German cockroach Blattella germanica (L.). J Exp Biol 205:781–790
Fox J, Wiesberg S (2011) An R Companion to Applied Regression, 2nd edn. Sage Publications, Thousand Oaks
Gadagkar R (1994) Why the definition of eusociality is not helpful to understand its evolution and what should we do about it. Oikos 70:485–488
Greenberg SLW, Stuart AM (1979) The influence of group size on ovarian development in adult and neotenic reproductives of the termite Zootermopsis angusticollis Hagen (Hododtermitidae). Int J Invertebr Reprod 1:99–108
Greenberg SLW, Tobe SS (1985) Adaptation of a radiochemical assay for juvenile hormone biosynthesis to study caste differentiation in a primitive termite. J Insect Physiol 31:347–352
Hanus R, Vrkoslav V, Hrdy I, Cvacka J, Sobotnik J (2010) Beyond cuticular hydrocarbons: evidence of proteinaceous secretion specific to termite kings and queens. Proc R Soc B 277:995–1002
Haverty MI, Thorne BL (1989) Agonistic behavior correlated with hydrocarbon phenotypes in dampwood termites, Zootermopsis (Isoptera, Termopsidae). J Insect Behav 2:523–543
Haverty MI, Page M, Nelson LJ, Blomquist GJ (1988) Cuticular hydrocarbons of dampwood termites, Zootermopsis: intracolony and intercolony variation and potential as taxonomic characters. J Chem Ecol 14:1035–1058
Heath H (1903) The habits of California termites. Biol Bull 4:47–63
Heinze J (2004) Reproductive conflict in insect societies. Adv Study Behav 34:1–57
Hewitt PH, Watson JAL, Nel JJC, Schoeman I (1972) Control of the change from group to pair behaviour by Hodotermes mossambicus reproductives. J Insect Physiol 18:143–150
Holman L, Jorgensen CG, Nielsen J, D’Ettorre P (2010) Identification of an ant queen pheromone regulating worker sterility. Proc R Soc B 277:3793–3800
Howard RW, Blomquist GJ (2005) Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu Rev Entomol 50:371–393
Howard RW, McDaniel CA, Nelson DR, Blomquist GJ, Gelbaum LT, Zalkow LH (1982) Cuticular hydrocarbons of Reticulitermes virginicus (Banks) and their role as potential species and caste-recognition cues. J Chem Ecol 8:1227–1239
Inward D, Beccaloni G, Eggleton P (2007) Death of an order: a comprehensive molecular phylogenetic study confirms that termites are eusocial cockroaches. Biol Lett 3:331–335
Kambhampati S (1995) A phylogeny of cockroaches and related insects based on DNA sequence of mitochondrial ribosomal RNA genes. Proc Natl Acad Sci USA 92:2017–2020
Keller L, Nonacs P (1993) The role of queen pheromones in social insects: queen control or queen signal? Anim Behav 45:787–794
Kelstrup HC, Hartfelder K, Nascimento FS, Riddiford LM (2014) The role of juvenile hormone in dominance behavior, reproduction and cuticular pheromone signaling in the caste-flexible epiponine wasp, Synoeca surinama. Front Zool 11:78
Klochkov SG, Kozlovskii VI, Belaeva NV (2005) Caste and population specificity of termite cuticular hydrocarbons. Chem Nat Comp 41:1–6
Koeppe JK (1981) Juvenile hormone regulation of ovarian maturation in Leucophaea maderae. In: Zabza SF, Menn JJ, Cymborowski B (eds) Regulation of insect development and behavior. Wroclaw Technical University Press, Wroclaw, pp 505–522
Koeppe JK, Fuchs M, Chen TT, Hunt LM, Kovalick GE, Briers T (1985) The role of juvenile hormone in reproduction. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology, biochemistry and pharmacology. Elsevier, New York, pp 165–203
Korb J, Hoffmann K, Hartfelder K (2009a) Endocrine signatures underlying plasticity in postembryonic development of a lower termite, Cryptotermes secundus (Kalotermitidae). Evol Dev 11:269–277
Korb J, Weil T, Hoffmann K, Foster KR, Rehli M (2009b) A gene necessary for reproductive suppression in termites. Science 324:758
Le Conte Y, Hefetz A (2008) Primer pheromones in social hymenoptera. Annu Rev Entomol 53:523–542
Lengyel F, Westerlund SA, Kaib M (2007) Juvenile Hormone III influences task-specific cuticular hydrocarbon profile changes in the ant Myrmicaria eumenoides. J Chem Ecol 33:167–181
Leniaud L, Darrouzet E, Dedeine F, Ahn K, Huang Z, Bagneres AG (2011) Ontogenic potentialities of the worker caste in two sympatric subterranean termites in France. Evol Dev 13:138–148
Lenz M (1976) The dependence of hormone effects in termite caste determination on external factors. In: Lüscher M (ed) Phase and caste determination in insects. Pergamon Press, Oxford, pp 73–89
Liebig J (2010) The evolution of hydrocarbon profiles as dominance and fertility signals in social insects. In: Blomquist GJ, Bagnères AG (eds) Insect hydrocarbons: biology, biochemistry, and chemical ecology. Cambridge University Press, Cambridge, pp 254–281
Liebig J, Eliyahu D, Brent CS (2009) Cuticular hydrocarbon profiles indicate reproductive status in the termite Zootermopsis nevadensis. Behav Ecol Sociobiol 63:1799–1807
Liebig J, Eliyahu D, Brent CS (2012) Erratum to: cuticular hydrocarbon profiles indicate reproductive status in the termite Zootermopsis nevadensis. Behav Ecol Sociobiol 66:1095
Lüscher M (1972) Environmental control of juvenile hormone (JH) secretion and caste differentiation in termites. Gen Comp Endocrinol Supp 3:509–514
Matsuura K, Yamamoto Y (2011) Workers do not mediate the inhibitory power of queens in a termite, Reticulitermes speratus (Isoptera, Rhinotermitidae). Insectes Soc 58:513–518
Matsuura K, Himuro C, Yokoi T, Yamamoto Y, Vargo EL, Keller L (2010) Identification of a pheromone regulating caste differentiation in termites. Proc Natl Acad Sci USA 107:12963–12968
Meyer DR, Lanzrein B, Lüscher M, Nakanishi K (1976) Isolation and identification of a juvenile hormone (JH) in termites. Experientia 32:773
Miura T, Koshikawa S, Matsumoto T (2003) Winged presoldiers induced by a juvenile hormone analog in Zootermopsis nevadensis: implications for plasticity and evolution of caste differentiation in termites. J Morphol 257:22–32
Monnin T (2006) Chemical recognition of reproductive status in social insects. Ann Zool Fenn 43:515–530
Ogino K, Hirono Y, Matsumoto T, Ishikawa H (1993) Juvenile hormone analogue, S-31183, causes a high level induction of presoldier differentiation in the Japanese damp-wood termite. Zool Sci 10:361–366
Okot-Kotber BM (1982) Correlation between larval weights, endocrine gland activities and competence period during differentiation of workers and soldiers in Macrotermes michaelseni (Isoptera: Termitidae). J Insect Physiol 28:905–910
Okot-Kotber BM, Prestwich GD (1991a) Identification of a juvenile hormone binding protein in the castes of the termite, Reticulitermes flavipes, by photoaffinity labeling. Insect Biochem 21:775–784
Okot-Kotber BM, Prestwich GD (1991b) Juvenile hormone binding proteins of termites detected by photoaffinity labeling: comparison of Zootermopsis nevadensis with two Rhinotermitids. Arch Insect Biochem Physiol 17:119–128
Peeters C, Liebig J (2009) Fertility signaling as a general mechanism of regulating reproductive division of labor in ants. In: Fewell J, Gadau J (eds) Organization of insect societies: from genome to socio-complexity. Harvard University Press, Cambridge, pp 220–242
Peeters C, Monnin T, Malosse C (1999) Cuticular hydrocarbons correlated with reproductive status in a queenless ant. Proc R Soc Lond 266:1323–1327
Penick C, Trobaugh B, Brent CS, Liebig J (2012) Head butting as an early indicator of reproductive disinhibition in the termite Zootermopsis nevadensis. J Insect Behav 26:23–34
Pinto LZ, Bitondi M, Simões ZLP (2000) Inhibition of vitellogenin synthesis in Apis mellifera workers by a juvenile hormone analogue, pyriproxyfen. J Insect Physiol 46:153–160
R Development Core Team (2009) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rankin SM, Stay B (1984) The changing effect of the ovary on rates of juvenile hormone synthesis in Diploptera punctata. Gen Comp Endocrinol 54:382–388
Rankin SM, Stay B (1985) Ovarian inhibition of juvenile hormone synthesis in the viviparous cockroach, Diploptera punctata. Gen Comp Endocrinol 59:230–237
Saiki R, Yaguchi H, Hashimoto Y, Kawamura S, Maekawa K (2014) Reproductive soldier-like individuals induced by juvenile hormone analog treatment in Zootermopsis nevadensis (Isoptera, Archotermopsidae). Zool Sci 31:573–581
Saiki R, Gotoh H, Toga K, Miura T, Maekawa K (2015) High juvenile hormone titre and abdominal activation of JH signalling may induce reproduction of termite neotenics. Insect Mol Biol 24:432–441
Schal C, Gu XP, Burns EL, Blomquist GJ (1994) Patterns of biosynthesis and accumulation of hydrocarbons and contact sex-pheromone in the female german-cockroach, Blattella germanica. Arch Insect Biochem Physiol 25:375–391
Schal C, Holbrook GL, Bachmann JAS, Veeresh LS (1997) Reproductive biology of the German cockroach, Blattella germanica: juvenile hormone as a pleiotropic master regulator. Arch Insect Biochem Physiol 35:405–426
Schal C, Fan Y, Blomquist GJ (2003) Regulation of pheromone biosynthesis, transport, and emission in cockroaches. In: Blomquist GJ, Vogt R (eds) Insect pheromones-biochemistry and molecular biology. Academic Press, New York, pp 283–322
Scharf ME, Ratliff CR, Hoteling JT, Pittendrigh BR, Bennett GW (2003) Caste differentiation responses of two sympatric Reticulitermes termite species to juvenile hormone homologs and synthetic juvenoids in two laboratory assays. Insectes Soc 50:346–354
Scharf ME, Ratliff CR, Wu-Scharf D, Zhou X, Pittendrigh BR, Bennett GW (2005a) Effects of juvenile hormone III on Reticulitermes flavipes: changes in haemolymph protein composition and gene expression. Insect Biochem Mol Biol 35:207–215
Scharf M, Wu-Scharf D, Zhou X, Pittendrigh B, Bennett G (2005b) Gene expression profiles among immature and adult reproductive castes of the termite Reticulitermes flavipes. Insect Mol Biol 14:31–44
Seeley TD (1979) Queen substance dispersal by messenger workers in honeybee colonies. Behav Ecol Sociobiol 5:391–415
Seeley TD (1985) Honeybee ecology: a study of adaptation in social life. Princeton University Press, Princeton
Sevala VL, Bagneres A-G, Kuenzli M, Blomquist GJ, Schal C (2000) Cuticular hydrocarbons of the dampwood termite, Zootermopsis nevadensis: caste differences and role of lipophorin in transport of hydrocarbons and hydrocarbon metabolites. J Chem Ecol 26:765–789
Sherman PW, Lacey EA, Reeve HK, Keller L (1995) The eusociality continuum. Behav Ecol 6:102–108
Singh S, Kumar K (2015) Effects of juvenoid Pyriproxyfen on reproduction and F1 progeny in myiasis causing flesh fly Sarcophaga ruficornis L. (Sarcophagidae: Diptera). Parasitol Res 114:2325–2331
Sledge MF, Boscaro F, Turilazzi S (2001) Cuticular hydrocarbons and reproductive status in the social wasp Polistes dominulus. Behav Ecol Sociobiol 49:401–409
Smith A, Hölldobler B, Liebig J (2009) Cuticular hydrocarbons reliably identify cheaters and allow enforcement of altruism in a social insect. Curr Biol 19:78–81
Smith AA, Millar JG, Hanks LM, Suarez AV (2013) A conserved fertility signal despite population variation in the cuticular chemical profile of the trap-jaw ant Odontomachus brunneus. J Exp Biol 216:3917–3924
Stay B, Friedel T, Tobe SS, Mundall EC (1980) Feedback control of juvenile hormone synthesis in cockroaches: possible role of ecdysterone. Science 207:898–900
Terrapon N, Li C, Robertson HM, Ji L, Meng XH, Booth W, Chen ZS, Childers CP, Glastad KM, Gokhale K, Gowin J, Gronenberg W, Hermansen RA, Hu HF, Hunt BG, Huylmans AK, Khalil SMS, Mitchell RD, Munoz-Torres MC, Mustard JA, Pan HL, Reese JT, Scharf ME, Sun FM, Vogel H, Xiao J, Yang W, Yang ZK, Yang ZQ, Zhou JJ, Zhu JW, Brent CS, Elsik CG, Goodisman MAD, Liberles DA, Roe RM, Vargo EL, Vilcinskas A, Wang J, Bornberg-Bauer E, Korb J, Zhang GJ, Liebig J (2014) Molecular traces of alternative social organization in a termite genome. Nat Comm 5:3636
Thorne BL (1997) Evolution of eusociality in termites. Annu Rev Ecol Syst 28:27–54
Tillman JA, Seybold SJ, Jurenka RA, Blomquist GJ (1999) Insect pheromones—an overview of biosynthesis and endocrine regulation. Insect Biochem Mol Biol 29:481–514
Van Oystaeyen A, Oliveira RC, Holman L, van Zweden JS, Romero C, Oi CA, d’Ettorre P, Khalesi M, Billen J, Wäckers F (2014) Conserved class of queen pheromones stops social insect workers from reproducing. Science 343:287–290
Vieau F, Lebrun D (1981) Juvenile hormone, vitellogenesis and egg laying in termite Kalotermes flavicollis. CR Acad Sci Ser III 293:399–402
Wanyonyi K (1974) The influence of the juvenile hormone analogue ZR512 (Zoecon) on caste development in Zootermopsis nevadensis (Hagen) (Isoptera). Insectes Soc 21:35–44
Weil T, Hoffmann K, Kroiss J, Strohm E, Korb J (2009) Scent of a queen-cuticular hydrocarbons specific for female reproductives in lower termites. Naturwissenschaften 96:315–319
Wilson EO (1971) The insect societies. Harvard University Press, Cambridge
Xu Q, Tang B, Zou Q, Zheng H, Liu X, Wang S (2015) Effects of pyriproxyfen on female reproduction in the common cutworm, Spodoptera litura (F.) (Lepidoptera: Noctuidae). PLoS One 10:e0138171
Yin CM, Gillot C (1975) Endocrine activity during caste differentiation in Zootermopsis angusticollis Hagen (Isoptera): a morphometric and autoradiographic study. Can J Zool 53:1690–1700
Acknowledgments
We thank the administrators of the Pebble Beach Company for permission to collect termites. We would also like to thank Kevin Haight for assisting with colony collection and maintenance, and Steven Prager for assisting with colony maintenance and JHA treatments. All experiments were conducted in accordance with American statutes governing research. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer. This project was supported by the Agriculture and Food Research Initiative Competitive Grant No. 2007-35302-18172 from the United States Department of Agriculture National Institute of Food and Agriculture.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Stephen J. Martin.
Rights and permissions
About this article
Cite this article
Brent, C.S., Penick, C.A., Trobaugh, B. et al. Induction of a reproductive-specific cuticular hydrocarbon profile by a juvenile hormone analog in the termite Zootermopsis nevadensis . Chemoecology 26, 195–203 (2016). https://doi.org/10.1007/s00049-016-0219-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-016-0219-8