Abstract
In lower termites, functionally sterile larval helpers are totipotent—capable of becoming reproductively active with the loss of their colony’s king or queen. Full reproductive development may take several weeks, but initiation of this developmental response most likely occurs shortly after colony members detect when a reproductive-specific signal is missing. We investigated the early response of termite helpers to the removal of their king and queen in the basal termite species Zootermopsis nevadensis. Within 6–12 h after reproductives were removed, helpers displayed an increase in head-butting—a behavior associated with dominance in other termite species as well as in closely related roaches. The loss of just one reproductive, either the king or queen, was also sufficient to cause an increase in head-butting. We did not find evidence, however, that this response was sex-specific: males and females were equally likely to increase head-butting independent of the sex of the reproductive that was removed. Finally, we discovered that reproductive-specific compounds present on the cuticle of king and queen termites were also present in their feces, but the presence of the feces did not seem sufficient to inhibit the increased head-butting after the reproductives were removed. Collectively, these results indicate that termite workers readily detect the loss of reproductives in their colony and that they at least initially respond in a non sex-specific manner.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The maintenance of a reproductive division of labor within eusocial insect societies requires that reproductives continuously signal their presence (Seeley 1985; Keller and Nonacs 1993). When this signal is absent in a colony, non-reproductive individuals of many species are capable of becoming reproductively active. In the lower termites, functionally sterile larval helpers (sensu Thorne 1996) display a high degree of developmental plasticity and can molt into replacement reproductives, known as neotenics (Myles 1999). Like their predecessors, neotenics must assert their dominance over potential competitors to maintain colony reproductive dynamics. Those that do so first have the best chance of becoming the new king or queen. In order for this system to work stably, reproductives must produce a signal to which their nestmates are highly sensitive, and the response of those nestmates to any changes in the signal should be rapid. However, for most termite species the dynamics of this signaling system have been poorly, if at all, characterized.
In the dampwood termite Zootermopsis nevadensis, cuticular hydrocarbons that are specific to the reproductive caste have been identified as a putative fertility signal (Liebig et al. 2009), and sex-specific pheromones have also been identified (Bordereau et al. 2010). How these compounds influence non-reproductive nestmates has yet to be determined. It is known that in Zootermopsis, established reproductives are capable of inhibiting the differentiation of neotenics, and this inhibition generally appears to target members of the same gender (Heath 1903; Light 1943; Light and Weesner 1951). Measures of the responses of potential replacements to removal from the inhibitory stimuli of functional reproductives have been limited to long-term physiological surveys that tracked the number of weeks before functional neotenics were present in a colony and producing eggs (Light and Weesner 1951; Greenberg and Stuart 1982). However, the initiation of this response is likely to occur much sooner as the candidates race to be the first to differentiate into reproductives before competing nestmates.
One possible indicator that might facilitate measurement of how quickly individuals can respond to a change in colony dynamics is a shift in behavior. While overt displays of aggression are rare among termite nestmates, at least one basal species exhibits a characteristic head-butting behavior that is displayed more frequently by individuals that later become replacement reproductives (Korb et al. 2009). This behavior involves one termite lunging towards another termite and usually striking it with its head. A similar behavior has been associated with dominance interactions in cockroaches (Ewing 1967; Bell et al. 1979; Clark and Moore 1994), which are closely related to termites (Inward et al., 2007). Although this specific behavior has never been before reported in Z. nevadensis, members of this genus are known to produce an alarm response by shaking their head up and down (Stuart 1963; Kirchner et al. 1994; Rosengaus et al. 1999), so there is a precedent for such physical signaling. We monitored the frequency of head-butting before and after reproductive removal in order to quantify the timing and extent of worker response to the loss of their king and queen. Furthermore, we analyzed whether this response was sex-specific (i.e. only females respond when the queen is removed and only males respond when the king is removed). In addition to studying the behavioral response of termite workers to king and queen removal, we conducted a separate experiment to address how a chemical fertility signal may be distributed throughout the colony. There has been previous speculation that reproductive-specific compounds are distributed throughout termite colonies via king and queen feces (Lüscher 1955; Hanus et al. 2010). We addressed this issue by analyzing whether reproductive-specific hydrocarbon compounds found on the cuticle of kings and queens were also present in their feces. We combine results from our chemical analyses and behavioral experiments to interpret how chemical fertility signaling may function in this and related species.
Methods
Study Species and Laboratory Conditions
Stock colonies of Z. nevadensis were collected from the Del Monte Forest in Pebble Beach, CA, USA during August 2010. In the laboratory, colonies were maintained at 20°C and kept in plastic boxes that contained nests with pre-cavitated chambers constructed from moistened slats of spruce pine (Pinus glabra) held together by plastic bolts. In order to monitor behavior, observation nests were constructed with an 8 x 6 x 1 cm chamber bored into a block of spruce pine. This chamber was covered with a glass plate so that colonies could be filmed. Colonies were held in constant light for the duration of behavioral experiments so that they could become acclimated to the conditions necessary for filming. Observation nests were opened daily to moisten the wood with a spray mister. At this time, we would clean the glass cover and plug newly excavated holes with tissue paper to ensure the termites were always visible. Our stock colonies ranged in size from 200–500 individuals and included all larval stages, nymphs, soldiers and reproductives.
Behavioral Experiments
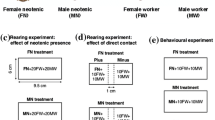
To test the response of larval helpers to the removal of one or both reproductives, colony fragments were produced from 10 independent stock colonies to avoid pseudoreplication. These fragments consisted of 30 helpers (largest instar) and a single pair of neotenic reproductives (these were mature reproductives that could be distinguished from helpers and immature neotenics by having a dark coloration (Heath 1903) and displaying reproductive-specific cuticular compounds (Liebig et al. 2009)). Before behavioral observations were carried out, we allowed colony fragments to acclimate to their new nest conditions for at least 3 days. Colonies were filmed for 30 min intervals under three conditions: with the king and queen present, 6–8 h after both reproductives were removed, and 30–32 h after both reproductives were removed. The cuticular hydrocarbon profile of each king and queen was examined upon their removal from the observation nest to ensure that they were expressing a reproductive-specific profile (methods described below). The number of head-butting events that occurred among larval helpers and/or reproductives was recorded. Head-butting was defined as a clear lateral lunge by one individual in the direction of another, and this often resulted in direct physical contact (Online resource, Video 1). Head-butting could be distinguished from head-banging, which is an alarm response that is generally not directed toward another individual and consists of repeated beating of the head on the wood substrate in an up and down motion (Video 1; Stuart 1963; Kirchner et al. 1994; Rosengaus et al. 1999). Recordings were analyzed using a semi-blind method; while the presence/absence of reproductives was visible to the observer, high levels of inter-colonial differences in head-butting prevented observer bias when videos were analyzed in a randomized order without knowledge of colony identity. A control experiment was conducted in a similar manner, but instead of removing the king and queen, two larval helpers were removed (N = 10 independent colonies).
To determine if larval helpers were more responsive to the loss of a reproductive of the same sex, we examined their behavior after removal of just the king or just the queen (N = 9 independent stock colonies per treatment). This experiment was conducted as described above, except each larval helper was individually identifiable by paint marking the thorax (Testors Pactra® enamel, Rockford, IL, USA). The gender of each helper was identified by dissection at the end of the experiment. Colonies were filmed intact and then 6–8 h after removing the king or queen.
Fertility Signaling in Feces
Because the behavioral responses of larval helpers could have been muted to some extent by the continued presence of reproductive-specific hydrocarbon in the feces left behind by the king or queen, fecal composition was examined. We isolated a pair of secondary reproductives (king and queen) with two larval helpers. Ten such groupings were produced, each from a separate stock colony. Helpers were included to care for the king and queen during isolation. These groups were placed into clear plastic nests (diameter 50 mm) that contained a pre-soaked ring (diameter 45 mm, height 6 mm) of spruce pine with a central hole (25 mm) that served as a nesting chamber. Nests were provided with a paper towel, which was moistened three times per week. As a control, 10 additional nests were established as described above, and these contained four workers and no reproductives. At the end of 2 weeks, 30 fecal pellets were collected from each group, and these were extracted in 100 μl of hexane (Sigma-Aldrich, St. Louis, MO) for 2 min. For comparison to the source animals, cuticular hydrocarbons were extracted for analysis from reproductives using solid-phase microextraction (SPME), and from larval helpers by shaking them in 100 μl of hexane for 2 min.
Hydrocarbon Analysis
Hydrocarbon composition of the cuticular profiles of reproductives, larval helpers and their feces were determined by gas-chromatography mass spectrometry (GC-MS). 1 μl aliquots of the hexane extracts were injected into an Agilent 6890 N (GC) (Agilent, Santa Clara, CA, USA) coupled with an Agilent 5975 mass selective detector, operated in the electron impact ionization mode. The GC was operated in splitless injection mode with helium as carrier gas at 1 ml/min flow rate. It was fitted with a 30 m × 0.25 mm (ID) × 0.25 μm DB-1MS non-polar column (Agilent). The oven temperature was programmed to rise from 60°C to 200°C at 40°C min-1 after an initial delay of 2 min, including a splitless time of 0.5 min. Subsequently, the temperature rose from 200°C to 320°C at 5°C min-1. Injector temperature was 260°C, MS quad 150°C, MS source 230°C, and transfer line 300°C.
For SPME analysis, a fiber (Supelco Inc., PA, USA) coated with a 30 μm polydimethylsiloxane film, was brushed across the abdomen of each individual termite for a period of 2 min. The fiber was inserted into an injection port of an Agilent 6890 N gas-chromatograph (GC), and the same settings were used as described above. For comparisons of peak areas, we standardized to 100 % the sum of four prominent peaks (C21, C23:2, C23, and C25), and calculated the relative amount of 6,9,17-tritriacontatriene in each sample as a percentage. Tritriacontatriene was previously identified as being one of four reproductive-specific hydrocarbons, and it is typically the most robust peak (Liebig et al. 2009).
Statistical Analyses
All comparisons were conducted using Statistica version 7 (StatSoft, Tulsa, OK, USA). Due to a lack of normality, non-parametric Friedman ANOVAs followed by the Wilcoxon matched pairs test for multiple comparisons were used to determine differences in head-butting rates when the king and queen were removed. P-values for multiple comparisons were adjusted using the Bonferroni correction to prevent alpha inflation. Wilcoxon matched pairs tests were also used to analyze the change in head-butting associated with the removal of only one reproductive. For analysis of a sex-specific response to king or queen removal, we used chi-square analysis based on a 2 × 2 contingency table controlling for treatment (king vs. queen removal) and gender of the individual observed head-butting at the highest frequency. Pearson correlation was used to determine the relationship between the relative amount of reproductive-specific compounds on the cuticle of kings and queens and the relative amount present in their feces.
Results
Response to Removal of Reproductives
After both reproductives were removed from treatment colonies, head-butting increased by an average of 114 % per colony 6–8 h after removal and stayed elevated through 30–32 h after removal (Friedman ANOVA, N = 10, p = 0.0004; multiple comparisons: Wilcoxon matched pairs test, [0 h vs. 6–8 h, p = 0.015], [0 h vs. 30–32 h, p = 0.015], [6–8 h vs. 30–32 h, p = 0.46], Fig. 1a). In contrast, head-butting gradually decreased from starting values in control colonies where two larval helpers were removed rather than the reproductives, but this decrease was not significant until 30–32 h after helper removal. (Friedman ANOVA, N = 10, p = 0.006; multiple comparisons: Wilcoxon matched pairs test, [0 h vs. 6–8 h, p = 0.51], [0 h vs. 30–32 h, p = 0.043], [6–8 h vs. 30–32 h, p = 0.14], Fig. 1b). Prior to removal of the reproductives in treatment colonies, a disproportionate amount of butting (42 %) was directed at the king and queen (compared to 6.25 % expected if butting was equally distributed across all colony members (Chi-square test, df = 9, p = 0.0124)).
Frequency of head-butting by larval helpers (Median, 25–75 % Confidence Interval, and Range) before and 6–12 h or 30–32 h after the removal of either (a) the king and queen (treatment) or (b) two random larval helpers (control). Letters indicate significant differences between groups (p < 0.05). N = 10 independent colony replicates for each treatment
In all cases, the king and queen were found to display the reproductive-specific cuticular compound 6,9,17-tritriacontatriene (N = 20). However, the average proportion of reproductive-specific compounds displayed on the cuticle was not correlated with the change in head-butting rate by larval helpers after the reproductives were removed (Spearman rank correlation, N = 10, p = 0.176).
Sex-Specific Response
We tested whether head-butting would increase after just the king or just the queen were removed and we found that head-butting increased in both cases among mixed-gender groups of larval helpers (average increase in head-butting: king removal, 104 %; queen removal, 146 %) (Wilcoxon matched pairs test, N = 9 [king removal, p = 0.015], [queen removal, p = 0.008], Fig. 2). Larval helpers significantly increased head-butting regardless whether the reproductive removed was of the same or the opposite sex (Wilcoxon matched pairs test, N = 9 [king removal: (males, p = 0.012), (females, p = 0.038)]; [queen removal: (males, p = 0.017), (females, p = 0.0077)], Fig. 2). Additionally, helpers of both genders increased head-butting by similar amounts regardless of removal treatment (Wilcoxon matched pairs test, N = 9, [king removal, p = 0.14], [queen removal, p = 0.59], Fig. 2). There was also no gender difference in the total proportion of individuals butting versus non-butting after the king or queen was removed (Wilcoxon matched pairs test of ratio males to females head-butting before and after king/queen removal, N = 9, [king removal, p = 0.67], [queen removal, p = 0.67]). Among the 18 colonies examined, the highest frequency of head-butting by a single individual ranged from 3–11 head-butts over 30 min. The individual displaying the most butting was male in 5 out of 9 trials when the king was removed, and was female in 4 out of 9 trials when the queen was removed. Thus, the gender of the individual observed butting at the highest frequency was not related to the gender of the reproductive removed (Chi-square test, df = 1, p = 0.614).
Percentage change in head-butting by larval helpers after either their king or their queen was removed (Median, 25–75 % Confidence Interval, and Range). The plot shows the response of both sexes, the response by males only, and the response by females only. Asterisks indicate values significantly different from zero (p < 0.05), and positive values indicate an increase in head-butting. Differences in the degree of increased head-butting between males and females in each treatment were non-significant
Reproductive-Specific Hydrocarbons in Feces
We furthermore investigated whether reproductive-specific hydrocarbons are found in the feces of the reproductives, and if so, would it have any inhibiting effect despite the absence of the reproductives. The reproductive-specific hydrocarbon tritriacontatriene was found in feces from 10 out of 10 colonies where a king and queen were present and in 0 out of 10 colonies when only workers were present (Fisher’s exact test: p < 0.001; Fig. 3a). In confirmation of previous results, all reproductive individuals displayed tritriacontatriene on their cuticle and all larval helpers lacked this peak. The relative amount of tritriacontatriene present in feces of reproductives was positively correlated with the amount present on their cuticle (Pearson correlation, r = 0.84, p = 0.002; Fig. 3b). However, the correlation was not well resolved in the lower concentration range, although this is likely due to the mixture of reproductive feces with feces from two larval helpers. Despite the presence of the reproductive-specific hydrocarbon in the feces that is produced by reproductives, it had no discernable effect in preventing the changes in head-butting rates after the removal of the reproductives.
Reproductive-specific hydrocarbons on cuticle and in feces. (a) Total ion chromatograms of lipid extracts from fecal pellets of secondary reproductives and worker-like larvae. The upper chromatogram is based on the cumulative feces from a queen, a king and 2 final instar larvae, and the lower inverted chromatogram represents feces from 4 final instar larvae. Typical reproductive compounds are represented by 6,9 hentriacontadiene (C31:2) and 6,9,17 tritriacontatriene (C33:3). Reference compounds are heneiscosane (C21), tricosane (C23), tricosadiene (C23:2), and pentacosane (C25). Compounds next to the reproductive peaks vary across chromatograms. The amount of tritricontatriene relative to the four reference peaks is 34 % (b) Correlation between the relative amount of tritriacontatriene present on the cuticle of secondary reproductives and their feces mixture (see text for further details)
Discussion
Larval helpers of Z. nevadensis rapidly detect the absence of their colony’s reproductives and display a clear behavioral response. While it may take several weeks before new neotenics emerge, an increase in head-butting was present within 6–8 h after king and queen removal. This increase was maintained over at least 30–32 h, and was readily induced with the removal of one or both reproductives. In contrast, when larval helpers were removed from control colonies, head-butting gradually decreased. These results indicate that larval helpers can detect and quickly respond to the loss of their colony’s reproductives, and similar to another termite species (Korb et al. 2009), head-butting may serve as a behavioral component of the competition among workers to become replacement reproductives.
The increase in head-butting we observed among helpers was robust and occurred in all trials when just one or both reproductives were removed. One surprising result, however, was that head-butting decreased in our control treatment when we would have expected no change in head-butting. Because this pattern was opposite from what we observed in our trials where reproductives were removed, it does not confound our results; instead, head-butting may have decreased in control colonies as helpers gradually acclimated to their new nests. This may have allowed the social structure of the colony to stabilize and head-butting to decrease. When head-butting was observed in colonies where the reproductives were present, the king and queen received a higher frequency of head-butting compared to helpers. One explanation for this pattern may be that helpers use head-butting as a form of challenge or test directed towards existing reproductives to probe for an opportunity when helpers can attempt to develop into replacement reproductives. Alternatively, helpers that engaged in head-butting tended to aggregate around the king and queen, and the increased frequency of head-butting received by the king and queen may have simply been a result of this spatial arrangement.
The response to the absence of a king or queen was not sex-specific: total observations of head-butting increased in both males and females no matter the sex of the reproductive that was removed, and the proportion of individuals observed head-butting after the removal of a single reproductive was not biased by gender (Fig. 2). Some authors have reported finding a sex-specific difference in neotenic development when only one reproductive was removed (Castle 1934; Light and Weesner 1951; Mensa-Bonsu 1976; Matsuura et al. 2010), but in other cases no clear evidence of a sex-specific response has been observed (Nagin 1972; Costa-Leonardo et al. 2004). Unlike previous studies, we examined whether a sex-specific pattern was evident during the initial response to reproductive removal rather than the final development of neotenics. Our results support the hypothesis that reproductive-specific signaling can inhibit initiation of worker reproduction but that information about gender, if present, may not be used by workers during this stage of their response.
There are several reasons that may explain why a sex-specific response wasn’t observed. First, workers were observed in colony fragments, an environment that may have unduly influenced the expression of their natural behavior. However, experiments using whole colonies of Cryptotermes secundus also found the same head-butting response when reproductives were removed (Korb et al. 2009), suggesting this may be a conserved behavior among the basal termites which is unaffected by the number of individuals present. A second reason why Z. nevadensis may not display a sex-specific response is that when reproductives are replaced in the wild they are superseded by multiple neotenic individuals, suggesting a tolerance for supernumeraries that would render a sex-specific response at this stage unnecessary. A third possibility is that although the initial behavioral response to the loss of a reproductive is not gender-biased, the subsequent physiological differentiation to produce replacements could still be sex-specific, evidence of which has been found for Zootermopsis (Castle 1934; Light and Weesner 1951). The behavioral response we measured occurs within hours of the removal of reproductives, but the physiological changes that cause helpers to develop into replacement neotenics occurs over several weeks (Heath 1903; Light 1943) and may be attuned to separate reproductive cues.
The likeliest means by which Z. nevadensis reproductives signal their presence to nestmates is through the expression of a characteristic blend of cuticular hydrocarbons that strongly correlate with fertility (Liebig et al. 2009). Cuticular hydrocarbons serve as fertility signals in numerous species of the eusocial Hymenoptera (reviewed in Monnin 2006; Le Conte and Hefetz 2008; Peeters and Liebig 2009; Liebig 2010), and the association of these compounds with reproduction in termites may represent convergence (Liebig et al. 2009; Weil et al. 2009). However, these reproductive-specific cuticular hydrocarbons do not differ between sexes. Our results for Z. nevadensis show that workers do not necessarily respond to a sex-specific component of the reproductive signal if it is present. In Neotermes jouteli two reproductives are necessary to fully suppress neotenic development, but the gender of the reproductives does not matter (Nagin 1972). This indicates that the reproductive signal in N. jouteli may have an additive affect that does not require a sex-specific component. We see similar evidence for this in Z. nevadensis, where larval helpers exhibit a gender-independent response to changes in the strength of a reproductive signal.
There is evidence that compounds in addition to hydrocarbons signal individual fertility or gender. Reproductives of Kalotermes flavicollis, Prorhinotermes simplex and Reticulitermes santonensis produce a proteinaceous secretion that may serve as reproductive signals, and unlike hydrocarbons, these signals are sex-specific (Hanus et al. 2010). Recently, a volatile pheromone was identified in Reticulitermes speratus that suppresses neotenic development through contact with the queen or her eggs (Matsuura et al. 2010, Matsuura and Yamamoto 2011). Primary reproductives of Z. nevadensis are known to produce volatile pheromones that are sex-specific and used in courtship (Pasteels 1972; Bordereau et al. 2010), and adult reproductive development in the sister species Z. angusticollis is affected by sex-specific stimuli (Brent and Traniello 2001; Brent et al. 2007). However, based on our experiments a sex-specific signal is not necessary to explain the behavioral response of Z. nevadensis workers to removal of a single reproductive. Therefore, we do not rule out cuticular hydrocarbons as a potential reproductive signal, but hydrocarbons may represent only one component of a complex signal that potentially includes several other compounds.
One concern in conducting this experiment was that feces of reproductives might contain the same hydrocarbon blend as is found on their cuticle, which is common for termites (Haverty et al. 2005). We found this to be the case (Fig. 3a), but apparently the putative fertility-signaling hydrocarbons contained in the feces were insufficient to suppress the behavioral response of workers when the reproductives were removed even though the relative proportions of these hydrocarbons are up to 10 times larger in the feces compared to cuticle (Fig. 3b). Lüscher (1955) hypothesized that reproductives may inhibit neotenic development via a compound present in their feces that may be distributed throughout the colony by proctodeal trophallaxis or by some other mechanism. Because we did not remove feces in our trials and we did not find that feces prevented disinhibition, it is possible that either the feces alone cannot inhibit head-butting by larval helpers or that the short duration of the experiment prevented the accumulation of a sufficient quantity of fecal hydrocarbons to have an effect. In a previous experiment, we did remove feces when we removed the reproductives, but the increase in worker head-butting was no different from when only the reproductives were removed (unpublished data). It is also possible that the fecal hydrocarbons lose their potency over time, but experiments testing the efficacy of transferring fresh feces from reproductives to larval helpers failed to inhibit neotenic development in other termite species (Nagin 1972; Stuart 1979).
These results combined indicate that head-butting behavior can be used as a reliable indicator of the sensitivity of Z. nevadensis larval helpers to changes in the reproductive hierarchy. Additionally, this response, unlike the weeks-long differentiation needed for full neotenic development, occurs within hours, providing a useful tool for characterizing the mechanism regulating colony reproductive dynamics. Cryptotermes secundus shows a similar behavior in this context (Korb et al. 2009), suggesting this response may be wide-spread in the basal termites.
References
Bell W, Gorton R, Tourtellot M, Breed M (1979) Comparison of male agonistic behavior in five species of cockroaches. Insectes Soc 26:252–263
Bordereau C, Lacey MJ, Sémon E, Braekman JC, Ghostin J, Robert A, Shellman Sherman J, Sillam-Dussès D (2010) Sex pheromones and trail-following pheromone in the basal termites Zootermopsis nevadensis (Hagen) and Z. angusticollis (Hagen) (Isoptera: Termopsidae: Termopsinae). Biol J Linn Soc 100:519–530
Brent CS, Traniello JFA (2001) Influence of sex specific stimuli on ovarian maturation in primary and secondary reproductives of the dampwood termite Zootermopsis angusticollis. Physiol Entomol 26:239–247
Brent CS, Schal C, Vargo EL (2007) Endocrine effects of social stimuli on maturing queens of the dampwood termite Zootermopsis angusticollis. Physiol Entomol 32:26–33
Castle G (1934) The damp-wood termites of western United States, genus Zootermopsis (formerly, Termopsis). In: Kofoid C (ed) Termites and termite control. University of California Press, Berkeley, pp 264–282
Clark DC, Moore AJ (1994) Social interactions and aggression among male Madagascar hissing cockroaches (Gromphadorhina portentosa) in groups (Dictyoptera: Blaberidae). J Insect Behav 7:199–215
Costa-Leonardo AM, Arab A, Casarin FE (2004) Neotenic formation in laboratory colonies of the termite Coptotermes gestroi after orphaning. J Insect Sci 4:10
Ewing L (1967) Fighting and death from stress in a cockroach. Science 155:1035–1036
Greenberg SLW, Stuart A (1982) Precocious reproductive development (neoteny) by larvae of a primitive termite Zootermopsis angusticollis (Hagen). Insectes Soc 29:535–547
Hanus R, Vrkoslav V, Hrdy I, Cvacka J, Sobotník J (2010) Beyond cuticular hydrocarbons: evidence of proteinaceous secretion specific to termite kings and queens. Proc Roy Soc B 277:995–1002
Haverty MI, Woodrow RJ, Nelson LJ, Grace JK (2005) Identification of termite species by the hydrocarbons in their feces. J Chem Ecol 31:2119–2151
Heath H (1903) The habits of California termites. Biol Bull 4:47–63
Inward D, Beccaloni G, Eggleton P (2007) Death of an order: a comprehensive molecular phylogenetic study confirms that termites are eusocial cockroaches. Biol Let 3:331–335
Keller L, Nonacs P (1993) The role of queen pheromones in social insects: queen control or queen signal? Anim Behav 45:787–794
Kirchner WH, Broecker I, Tautz J (1994) Vibrational alarm communication in the damp-wood termite Zootermopsis nevadensis. Physiol Entomol 19:187–190
Korb J, Weil T, Hoffmann K, Foster KR, Rehli M (2009) A gene necessary for reproductive suppression in termites. Science 324:758
Le Conte Y, Hefetz A (2008) Primer pheromones in social hymenoptera. Ann Rev Entomol 53:523–542
Liebig J (2010) Hydrocarbon profiles indicate fertility and dominance status in ant, bee, and wasp colonies. In: Blomquist GJ, Bagnères AG (eds) Insect hydrocarbons: biology, biochemistry, and chemical ecology. Cambridge University Press, New York, pp 254–281
Liebig J, Eliyahu D, Brent C (2009) Cuticular hydrocarbon profiles indicate reproductive status in the termite Zootermopsis nevadensis. Behav Ecol Sociobiol 63:1799–1807
Light SF (1943) The determination of the castes of social insects (concluded). Quart Rev Biol 18:46–63
Light SF, Weesner FM (1951) Further studies on the production of supplementary reproductives in Zootermopsis (Isoptera). J Exper Zool 117:397–414
Lüscher M (1955) Zur Frage der Übertragung sozialer Wirkstoffe bei Termiten. Naturwissenschaften 42:186–186
Matsuura K, Yamamoto Y (2011) Workers do not mediate the inhibitory power of queens in a termite, Reticulitermes speratus (Isoptera, Rhinotermitidae). Insect Soc 58:513–518
Matsuura K, Himuro C, Yokoi T, Yamamoto Y, Vargo E, Keller L (2010) Identification of a pheromone regulating caste differentiation in termites. Proc Nat Acad Sci USA 107:12963–12968
Mensa-Bonsu A (1976) The production and elimination of supplementary reproductives in Porotermes adamsoni (Froggatt). Insectes Soc 23:133–154
Monnin T (2006) Chemical recognition of reproductive status in social insects. Ann Zool Fennici 43:515–530
Myles TG (1999) Review of secondary reproduction in termites (Insecta: Isoptera) with comments on its role in termite ecology and social evolution. Sociobiol 33:1–94
Nagin R (1972) Caste determination in Neotermes jouteli (Banks). Insectes Soc 19:39–61
Pasteels JM (1972) Sex-specific pheromones in a termite. Cell Molec Life Sci 28:105–106
Peeters C, Liebig J (2009) Fertility signaling as a general mechanism of regulating reproductive division of labor in ants. In: Gadau J, Fewell J (eds.) Organization of Insect Societies: From Genome to Socio-Complexity. Harvard University Press, pp 220–242
Rosengaus RB, Jordan C, Lefebvre ML, Traniello JFA (1999) Pathogen alarm behavior in a termite: a new form of communication in social insects. Naturwissenschaften 86:544–548
Seeley TD (1985) Honeybee ecology: a study of adaptation in social life. Princeton University Press, Princeton
Stuart A (1963) Studies on the communication of alarm in the termite Zootermopsis nevadensis (Hagen), Isoptera. Physiol Zool 36:85–96
Stuart A (1979) The determination and regulation of the neotenic reproductive caste in the lower termites (Isoptera): with special reference to the genus Zootermopsis (Hagen). Sociobiol 4:223–237
Thorne BL (1996) Termite terminology. Sociobiol 28:253–263
Weil T, Hoffmann K, Kroiss J, Strohm E, Korb J (2009) Scent of a queen: cuticular hydrocarbons specific for female reproductives in lower termites. Naturwissenschaften 96:315–319
Acknowledgements
We would like to thank Kevin Haight for assistance with colony collection and the administrators of the Pebble Beach Company for permission to collect termites. Additionally, we would like to thank Steve Prager and Barbara Birtcil for assistance with colony maintenance and experiments. All experiments were conducted in accordance with American statutes governing research. This project was supported by the Agriculture and Food Research Initiative Competitive Grant no. 2007-35302-18172 from the USDA National Institute of Food and Agriculture.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Esm 1
Footage providing examples of head-butting behavior and head-banging (alarm) behavior. Head-butting consists of a single forward motion directed towards another individual while head-banging is a repeated up and down motion of the head that is not usually directed at another individual. (M1V 2545 kb)
Rights and permissions
About this article
Cite this article
Penick, C.A., Trobaugh, B., Brent, C.S. et al. Head-butting as an Early Indicator of Reproductive Disinhibition in the Termite Zootermopsis nevadensis . J Insect Behav 26, 23–34 (2013). https://doi.org/10.1007/s10905-012-9332-x
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10905-012-9332-x