Abstract
In social insects, juvenile hormone (JH) affects the degree of ovarian development, reproductive status, and temporal polyethism in workers. JH also contributes to determining the epicuticular chemical composition, which differentiates the castes of queens and workers. However, a few studies have evaluated the action of JH on behavioral ontogeny, cuticular chemical profile, and oocyte length and width, especially in social wasps of independent foundation. Therefore, the following hypotheses were tested: (i) topical application of JH changes the behavioral ontogeny of newly emerged workers; and (ii) changes might be detected in the cuticular chemical composition and oocyte length and width of newly emerged females receiving topical application of JH. The treatment consisted of application of JH, at a concentration of 25 µg.µL−1 in acetone, to 1-day-old Mischocyttarus consimilis workers. The application of JH to newly emerged M. consimilis females significantly altered oocyte length and width, with effects on behavioral ontogeny and the cuticular chemical compounds signaling these parameters in the colony. No effects of the solvent on female physiology were observed, reinforcing that the observed changes were due to the specific effects of JH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The reproductive division of labor is one of the remarkable features among social insects. In this model of division, one or a few females may be responsible for laying eggs, while the rest of the colony works to maintain the resources necessary for the success of the group, involving feeding, cleaning, caring for the immature, and colony defense (Wilson 1971). Species of independent foundation build simple nests without a protective envelope, founded by one or a few females (Gadagkar 1991; Jeane 1991). A feature shared among these species is slight or absent morphological differentiation between queens and workers (Robinson and Vargo 1997). Furthermore, the workers do not lose the ability to reproduce (Gadagkar et al. 1991). However, due to greater access to food and more aggressive behavior, the queens maintain a high level of ovarian development and avoid high-risk tasks that have high energy costs (Queller and Strassmann 1989; Röseler 1991; O’Donnell 1998; Torres et al. 2012).
In social insects, the division of labor is mediated, among other factors, by juvenile hormone (JH), which modulates the physiology and behavior of queens and workers (Robinson and Vargo 1997). In Apis mellifera bees, JH is known to modulate caste determination, with its level being higher in critical steps during the development of queen larvae (Rembold et al. 1992). The JH level also modulates extranidal activities in Apis workers (Robinson 1992). In the newly emerged individuals, the level of JH in the hemolymph is low, while its synthesis by the corpora allata gradually increases with age, leading to changes in the development of intranidal tasks, culminating in foraging (Huang et al. 1991).
The topical application of JH alters its level in the hemolymph, affecting the age polyethism. This mechanism of task specialization has been observed for the leaf-cutting ant Acromyrmex octospinosus (Reich 1793) (Norman et al. 2016) and A. mellifera (Sullivan et al. 2000), where the application of JH in young workers advanced the foraging age. In social wasps, although the effects of JH have been less investigated, studies with wasps of the genus Polybia have indicated that JH is responsible for the production of new foragers, accelerating temporal polyethism, defined as the modification of tasks with increasing age (Wilson 1985; O’Donnell and Jeanne 1993; O’Donnell 1998). Even in species of independent foundation, such as Polistes canadensis, increase of the JH titer anticipates implementation of the guard task and increases the number of foragers (Giray et al. 2005). In colonies of Polistes fuscatus, increase of the JH titer was found to modulate sexual receptivity, promoting the development of ovaries, but negatively affected female life expectancy (Walton et al. 2020). However, there is a need for further studies concerning the effects of JH in social wasps. In Ropalidia marginata, the Indian paper wasp, changes in the JH titer did not lead to altered temporal polyethism (Agrahari and Gadagkar 2003). Evidence suggests that the JH titer is equally important for social wasps and bees. The work of Montagna et al. (2015) evidenced that the JH titer may even be decisive in the determination of castes in social wasps of independent foundation, in this case being, at least in part, pre-imaginal.
In addition to JH, cuticular hydrocarbons (CHCs) are also important in modulating and mediating behaviors in colonies of social insects, constituting part of their chemical communication (Hölldobler and Wilson 1990; Billen 2006). The qualitative and quantitative variation of these compounds present in the epicuticle of insects signals a variety of information to other individuals, enabling cohesion of the colonies (Blomquist and Bagnères 2010). Studies have shown a relationship between the JH titer and the CHCs’ composition, reflected in differential abundances of these compounds between workers and queens (Bonavita-Cougourdan et al. 1991; Sledge et al. 2001). Studies including those of Torres (2014); Soares et al. (2014); and Oi et al. (2019) have shown that for females of different species of social wasps, there is a relationship between the CHCs’ composition and physiological condition. Oliveira et al. (2017) found a correlation among reproductive status, JH titer, and CHC composition in females of Vespula vulgaris. Studies with colonies of Polistes dominula (Sledge et al. 2001, 2004) and Polistes metricus (Judd et al. 2010) also showed a relationship between JH levels and the CHCs’ composition.
However, although several studies have identified a relationship between JH levels and CHCs composition, only a single study has investigated the effects of JH on behavior, physiology, and CHCs’ composition (Kelstrup et al. 2017). The latter study was carried out with a species of social wasp in a temperate climate, reinforcing the need for further studies elsewhere. Therefore, the present work investigates the effect of topical application of JH on behavioral ontogeny, oocyte length and width, and CHCs in newly emerged workers of the species Mischocyttarus consimilis, a social wasp from a tropical climate region. Two hypotheses were tested: (i) there is significant alteration of the oocyte length and width and behavioral ontogeny of newly emerged workers receiving topical application of JH; and (ii) these modifications lead to a significant change in the CHCs.
Materials and methods
Collection of individuals and treatments
The study involved the experimental manipulation of 11 colonies of the eusocial wasp Mischocyttarus consimilis, employing a total of 98 females in the phase of production of workers (Keeping 2002), under natural conditions in rural areas of the municipality of Dourados, Mato Grosso do Sul State, Brazil (22º13′16″ S, 54º48′20″ W).
Newly emerged 1-day-old females were collected from their nests using pincers, marked with nontoxic ink on a right leg and then subjected to the different procedures. Thirty-five workers received application of JH (Hormone III Code J2000, Sigma-Aldrich, São Paulo, Brazil), using acetone as solvent, while 35 workers received application of acetone alone, enabling evaluation of the effects of the application of JH, in relation to the solvent. Twenty-eight workers were used as controls, without receiving any type of treatment, and were submitted to the same analyses employed for the treatment groups.
Females that received JH treatment were subjected to a single application of JH at a concentration of 25 µg.µL−1, following the methodology proposed by Montagna et al. (2015). The application was performed with 1-day-old females, because at this age, the wasps of this species have not yet acquired the colonial signature (Neves et al. 2012). In the treatment with the solvent, the females were subjected to a single application of 1 µL of acetone, at the same age. For the behavioral observations, the females submitted to the treatments were divided into two groups. The first group, with ages from 1 to 5 days, was considered young, while the second group consisted of females with ages between 6 and 12 days, considered to be older individuals (Torres et al. 2011).
Effects of topical application of JH on oocyte length and width and behavior
Following the treatment with JH, the death/disappearance rates were recorded during the entire period of the experiment, noting the number of wasps that exited the colony every day after the applications. All the marked females were monitored for 12 days, 4 h per day (9:00–11:00 am and 2:00–4:00 pm), between September 2013 and September 2015. The period of 12 days was chosen, because it allowed the M. consimilis workers to develop all the intranidal and extranidal behaviors of the ontogeny of their behavioral repertoire (Torres et al. 2011). The behavioral repertoires were evaluated using the focal animal method (Del Claro 2010), based on the behavioral acts described by Torres et al. (2011). The observations were divided into sessions of 1 h, during the peak of activities described for M. consimilis (Torres et al. 2011), recording the number of behaviors exhibited by each marked individual.
For both young and old females, the behaviors quantified during 12 days were grouped into three categories, based on the study of Bruyndonckx et al. (2006): 1—aggressive acts (butt given, butt received, dispute for food, and request for food); 2—inactivity (remaining still); 3—foraging (foraging for nectar, water, and wood pulp, and unsuccessful foraging). The inactivity behavior was observed as a response to the application of JH, due to changes in oocyte length and width. Larger ovaries reduce the foraging behavior, so the wasp becomes more immobile and leaves the nest less frequently (Montagna et al. 2015). Regarding foraging, JH might alter the temporal polyethism, leading females submitted to JH treatment to begin foraging earlier (Sullivan et al. 2000; Giray et al. 2005).
Since the JH treatment led to physiological changes, the level of aggressiveness was evaluated. Alterations in ovary development may cause wasps to become more or less aggressive, compared to control wasps (Röseler et al. 1984; Giray et al. 2005). The foraging frequencies of the females were also recorded. Work with social insects has shown that application of JH promotes earlier initiation of foraging, with the insects presenting anticipating behavioral ontogeny (O’Donnel and Jeanne 1993). For the same reason, the frequency of inactivity was noted, since changes in oocyte length and width affect the performance of this behavior. Twelve days after the JH treatment, the wasps were collected using pincers.
For analysis of ovarian development, the gasters were dissected in a Petri dish containing saline solution. Determination of the average length of the six largest oocytes was used to obtain the individual ovarian development index (Giray et al. 2005), with measurements made using a stereomicroscope (Stemi 2000C, Carl Zeiss, Oberkochen, Germany) fitted with a micrometric eyepiece.
Effect of topical application of JH on cuticular hydrocarbons determined by gas chromatography–mass spectrometry
Gas chromatography coupled with mass spectrometry was used to analyze the CHCs of the thorax of each collected female, after dissection of the gaster for evaluation of the ovaries. Each sample was incubated for 2 min in 2 mL of hexane, in a glass flask. The solution resulting from the extraction was dried under an exhaust hood and stored at – 20 ºC for a maximum of 30 days. For chromatographic analysis, the extracts were solubilized in 200 μL of hexane.
The samples (1 µL) were analyzed using a gas chromatograph (GC-2010 Plus, Shimadzu, Kyoto, Japan) coupled to a mass detector (GCMS Ultra 2010, Shimadzu, Kyoto, Japan). The chromatograph was fitted with a fused silica DB-5 capillary column (5% phenyl-dimethylpolysiloxane, 30 m length × 0.25 mm diameter × 0.25 μm film thickness, J & W, Folsom, California, USA). Helium gas (99.999%) was used as the mobile phase, at a flow rate of 1.0 mL.min−1 (in splitless injection mode). The heating program started at 150 ºC, followed by a ramp to 300 ºC, at 3 ºC.min−1, and holding at 300 ºC for 10 min. The injector temperature was set at 250 ºC, while the detector and transfer line temperatures were set at 300 ºC. The mass spectrometer was operated using electron impact ionization voltage of 70 eV, mass range from m/z 45 to 600, and scan interval of 0.3 s.
Identification of the compounds was performed using the calculated retention index (Van den Dool and Kratz 1963), according to the following equation:
LRI: linear retention index.
RtX: component retention time.
RtN: retention time of the N alkane with retention time prior to the X component.
RtN+1: retention time of the N alkane with retention time after the X component.
n: number of carbon atoms of alkane N.
The numbers of carbon atoms in the compounds were determined using a series of linear alkanes (C7-C40, purity ≥ 95%, Sigma-Aldrich) and comparison with the literature (Bonavita-Courgourdan et al. 1991; Howard 2001; Howard et al. 2001; Abdalla et al. 2003; Zhu et al. 2006; Bonckaert et al. 2012; Costanzi et al. 2013; Weiss et al. 2015) or with mass spectral data from the equipment library (NIST 21 and Wiley 229). The major compounds were considered to be those with relative percentage area greater than 4%.
Statistical analysis
The data for the degree of ovarian development in the females were submitted to analysis of variance (ANOVA), using a logarithmic function to homogenize the data variance (Bussab and Morrettin 2002). For analysis of the differences between the groups, the Tukey post hoc test was applied (Hair et al. 2009). The effect of the application of JH on the behavioral ontogeny of the females was analyzed using the nonparametric chi-square test (X2), with a significance level of p < 0.05 (Siegel and Castellan-Junior 2006). The same test was applied to the relative frequencies of performance of aggressive behavior (typical of dominants) and foraging (typical of workers), since foraging frequency is expected to be higher in older females (Torres et al. 2012).
Discriminant analysis was used to analyze the effects of the topical applications of the treatments on CHCs, in comparison to the control group. The relative abundances of the chromatogram peaks were used to identify any significant differences between the CHCs of the females in the acetone, JH, and control groups (Quinn and Keough 2005). In this analysis, p ≤ 0.05 was considered significant.
Results
Effects of topical application of JH on oocyte length and width and behavior
Of the total of 98 females, 32 individuals had their behavioral ontogeny monitored during 12 days. The mortality/disappearance rates were 71% of females treated with JH, 68.5% of females treated with acetone, and 60.7% of females that did not receive any treatment (control).
Table 1 shows the average length and width measurements of the oocytes. The results revealed significant differences among the oocyte lengths for the control, acetone, and JH group females (ANOVA, F = 39.53, p = 0.03). Application of the Tukey test revealed that the JH group females had significantly larger oocytes (p < 0.05), compared to the control group.
The foraging frequencies showed that the wasps treated with JH started to forage within 3 days after emergence, whereas the wasps of the acetone and control groups went out to forage more frequently from the 4th day after emergence (Fig. 1). The chi-square analysis revealed that for the number of foraging events within 3 days following emergence, there were significant differences between the JH and control groups (X2 = 3.90, p = 0.04), as well as between the JH and acetone groups (X2 = 3.90; p = 0.04). No difference was observed between the control and acetone groups (p > 0.05). For the older wasps, there were no significant differences in foraging frequency among the groups (p > 0.05).
Figure 2 shows the behavioral categories that were analyzed. Chi-square analysis showed no significant differences in the frequency of aggressive behavior between the JH and control groups (X2 = 1.14, p = 0.19), the acetone and control groups (X2 = 0.40, p = 0.53), and the JH and acetone groups (X2 = 0.19, p = 0.66). Furthermore, there were no significant differences in the level of inactivity between the JH and control groups (X2 = 0.32, p = 0.57), the acetone and control groups (X2 = 0.17, p = 0.68), and the JH and acetone groups (X2 = 0.02, p = 0.89). A similar trend was observed for foraging frequency, comparing the JH and control groups (X2 = 0.02, p = 0.89), the acetone and control groups (X2 = 0.04, p = 0.85), and the JH and acetone groups (X2 = 0.11, p = 0.74).
Figure 3 shows the frequencies with which the wasps performed foraging in search of resources. Significant differences between the JH and control wasps were observed for foraging prey (X2 = 16.58, p < 0.01), nectar (X2 = 6.84, p < 0.01), and water (X2 = 8.72, p < 0.01). The acetone and control groups showed no significant differences in the frequencies of foraging for any resource (prey: X2 = 0.45, p = 0.50; nectar: X2 = 0.00, p = 1.00; water: X2 = 1.10, p = 0.29; wood pulp: X2 = 0.00, p = 1.00). With the exception of wood pulp (X2 = 0.43, p = 0.51), significant differences were found between the foraging frequencies of the JH and acetone groups (prey: X2 = 22.06, p < 0.01; nectar: X2 = 6.84, p < 0.01; water: X2 = 15.51, p < 0.01).
Analysis of the effect of topical application of JH on cuticular hydrocarbons using gas chromatography-mass spectrometry
The different treatments resulted in qualitative and quantitative variations of the CHCs (Table 2, Fig. 4). The percentage of branched alkanes was higher than that of linear alkanes, with the treatments using JH and acetone causing no change in the percentages of compounds of the two categories, compared to the control. However, the application of JH reduced the number of branched alkanes. About 80% of the compounds were found in all three groups of females. Four compounds were unique to the JH treatment, two were only found in samples from the acetone treatment, and two were exclusive to the control samples (Table 2).
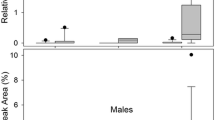
Major compounds found in the three groups were 3-methylheneicosane, heptacosane, 3-methylheptacosane, 3-methylnonacosane, triacontane, and 13, 17-dimethylhentriacontane. Nonacosane was a major compound for the JH treatment (Table 2). Discriminant analysis revealed differences in the CHCs profiles among the three treatments (Wilks’ lambda = 0.000, F = 49.029, p = 0.000). The first canonical root explained 99% of the results, while the first and second canonical roots together explained 100% of the results (Fig. 5). The statistical analysis indicated ten peaks as being most significant for separation of the treatments (Fig. 5, Table 3).
Discussion
The results demonstrated that topical application of JH to newly emerged M. consimilis females acted to modify oocyte length and width and ontogeny of the behavioral repertoire, in agreement with previous work (Giray et al. 2005). The treatment with JH also altered the chemical composition of the epicuticle of M. consimilis. These findings corroborated the observation that application of JH in workers of V. vulgaris led to the occurrence of CHCs’ signals similar to those produced by the queen, suggesting that these signals were under the control of JH (Oliveira et al. 2017).
The oocytes from the females treated with JH were larger than those from the control females, leading to significant behavioral alterations. Previous studies found that topical application of JH could induce physiological modification, accompanied by behavioral changes, in the wasp species Belonogaster longitarsus (Kelstrup et al. 2017) and Polistes fuscatus (Walton et al. 2020). In the ant species Dinoponera quadriceps, Norman et al. (2019) observed alteration of reproductive physiology after application of JH, although no change in dominance was noted. In Vespula vulgaris, application of JH did not alter ovary development, but it was demonstrated that V. vulgaris queen fertility and signals production were both under the control of JH (Oliveira et al. 2017).
Aggressiveness, inactivity, and foraging are behaviors that can indicate behavioral changes due to physiological modifications, since females with larger oocytes tend to have higher hierarchical rank, becoming more aggressive and inactive, and performing less foraging. In B. longitarsus, increase of the hemolymph JH titer was not related to dominance behaviors Kelstrup et al. (2017), similar to the present findings. The application of JH was more important for modulating ovarian development and the CHCs profile. In M. consimilis colonies, subordinate females foraged more frequently than dominant females that displayed higher aggression (Torres et al. 2012).
Analysis of the items foraged by M. consimilis showed that the wasps treated with JH reduced foraging for prey, presenting preference for the foraging of water and nectar. This behavior might reflect changes in their physiology, since larger oocytes influence the hierarchical rank in colonies (O’Donnell 1998). Dominant females forage less for prey, avoiding higher risks and energy-intensive tasks (Ross and Matthews 1991; O’Donnell 1998; Torres et al. 2011, 2012), corroborating the present findings, since the M. consimilis females treated with JH foraged for materials that were less costly in terms of energy and risk.
The application of JH also changed the ontogeny of the behavioral repertoire. Newly emerged females treated with JH began foraging earlier and displayed a higher frequency of foraging during the first 3 days following emergence (Fig. 1). Similar features were described for newly emerged A. mellifera bees (Robinson and Ratnieks 1987) and P. canadensis wasps (Giray et al. 2005), where the application of JH advanced the foraging activities among the workers. Therefore, the effect of JH in modulating and advancing foraging varies among different species of social Hymenoptera, based on different levels of sociability.
Following the treatments with JH and acetone, a high percentage of the females disappeared from the colonies. A possible explanation was death due to handling, marking, or toxic effects of the solvent. An additional possibility was related to changes in oocyte length and width and behavior of the females, with reduced tolerance of other queen-like individuals. Some individuals may have been expelled for not fitting in the hierarchical ranking, or may have left their colonies to create new ones (Gianotti and Machado 1999; Zara and Balestieri 2000).
Acetone, the solvent used in this study, did not have any effects on oocyte length and width or behavior, as reported previously (Tibbetts et al. 2018; c; Jatsch and Ruther 2021). Therefore, the observed effects could be attributed to the treatment with JH. Nonetheless, other studies suggested that acetone might affect the performance of A. mellifera (Paes de Oliveira and Cruz Landim 2001; Pinto et al. 2002), indicating the need for further investigations concerning the effects of solvents in social insects. The JH treatment altered the cuticular chemical signature (Fig. 5), which could be explained, at least in part, by changes in oocyte length and width and/or behavior (Oliveira et al. 2017), since these compounds mediate interactions within the colony, signaling the hierarchical rank (Sledge et al. 2004; Monnin et al. 1998). In the ant Myrmicaria eumenoides, treatment with JH also led to modification of the chemical signature of workers performing intranidal tasks, which then resembled the profile for the foragers (Lengyel et al. 2007). Kelstrup et al. (2017) found a clear association among JH, ovarian status, and the proportions of CHCs classes in females of B. longitarsus. Oliveira et al. (2017) demonstrated that JH treatment caused the CHCs profile of V. vulgaris workers to become similar to that of queens (Fig. 6).
Representative chromatograms of cuticular hydrocarbons in samples from Mischocyttarus consimilis wasps, indicating (*) 11 compounds that were identified: (1) unknown; (2) nonadecane; (3) 9-methylnonadecane; (4) 3-methylnonadecane; (5) 1-eicosene; (6) 1-heneicosene; (7) 3-methylheneicosane; (8) tricosane; (9) 3-methylpentacosane; (10) 3-methylheptacosane; (11) octacosane
Methyl-branched compounds were the most abundant, in terms of both percentage and number (Table 2). Branched alkanes have been shown to be present at high percentages in M. consimilis (Soares et al. 2017; Michelutti et al. 2017), Mischocyttarus cassununga (Murakami et al. 2015), Mischocyttarus bertonii and Mischocyttarus latior (Soares et al. 2017), and other species of social wasps such as P. dominula (Bonavita-Cougourdan et al. 1991) and Polybia occidentalis (Singer et al. 1998). The application of JH promoted changes in the epicuticular composition, especially branched alkanes (Table 3), with the number of compounds decreasing, compared to the control treatment. Branched alkanes were found to be associated with development of the ovaries in B. longitarsus, reinforcing that they are the main compounds responsible for modulating female wasp colonies (Kelstrup et al. 2017; Dani et al. 2001).
Nonacosane was the major compound identified in females treated with JH (Table 2). A further four compounds occurred exclusively in females that received JH application, while two compounds (nonadecane and 10, 14-dimethyltriacontane) were absent in these individuals. In other work, it was found that the most abundant compounds in V. vulgaris workers treated with JH were long-chain linear alkanes, 3-methyl branched alkanes, and other monomethyl and dimethyl branched compounds (Oliveira et al. 2017). The compounds nonacosane and 3-methylnonacosane, abundant in M. consimilis treated with JH, are considered queen pheromones that are known to suppress worker reproduction (Oliveira et al. 2017). Nonacosane was also the major epicuticular component identified in queens of the Saxon wasp Dolichovespula saxonica (Van Zweden et al. 2013).
Conclusions
Treatment of newly emerged M. consimilis females with JH led to morphological alteration, which was reflected by changes in both behavioral ontogeny and the chemical signature of the epicuticle, especially in relation to compounds with essential signaling roles in the colony. The findings bring new information concerning a little investigated wasp species from a tropical climate, showing that physiological and behavioral responses were associated with the effect of juvenile hormone. Further studies with different species of tropical wasps are necessary to understand whether the responses observed in the present study may also apply to other species.
References
Abdalla FC, Jones GR, Morgan ED, da Cruz-Landim C (2003) Comparative study of the cuticular hydrocarbon composition of Melipona bicolor Lepeletier, 1836 (Hymenoptera, Meliponini) workers and queens. Genet Mol Res 2:191–199
Agrahari M, Gadagkar R (2003) Juvenile hormone accelerates ovarian development and does not affect age polyethism in the primitively eusocial wasp, Ropalidia marginata. J Insect Physiol 49:217–222. https://doi.org/10.1016/S0022-1910(02)00268-8
Billen J (2006) Signal variety and communication in social insects. Proc of the Sect Exp and Applied Ent 17:9–25
Blomquist G (2010) Structure and analysis of insect hydrocarbons. In: Bonquist GJ, Bagnéres AG (eds) insect hydrocarbons. Cambridge University Press, Cambridge, Pp, pp 19–34
Bonavita-Cougourdan A, Theraulaz G, Bagnères AG, Roux M, Pratte M, Clément PE, JL, (1991) Cuticular hydrocarbons, social organization and ovarian development in a Polistes wasp: Polistes dominulus Christ. Comp Biochem Physiol Part B 100:667–680. https://doi.org/10.1016/0305-0491(91)90272-F
Bonckaert W, Drijfhout FP, D’Ettorre P, Billen J, Wenseleers T (2012) Hydrocarbon signatures of egg maternity, caste membership and reproductive status in the common wasp. J Chem Ecol 38:42–51. https://doi.org/10.1007/s10886-011-0055-9
Bruyndonckx N, Kardile SP, Gadagkar R (2006) Dominance behaviour and regulation of foraging in the primitively eusocial wasp Ropalidia marginata (Lep.) (Hymenoptera: Vespidae). Behav Process 72:100–103. https://doi.org/10.1016/j.beproc.2005.11.013
Bussab WO, Morrettin PA (2002) Estatística Básica. 5, a. Saraiva, São Paulo
Costanzi E, Bagnères A-G, Lorenzi MC (2013) Changes in the hydrocarbon proportions of colony odor and their consequences on nestmate recognition in social wasps. PLoS ONE 8(5):e65107. https://doi.org/10.1371/journal.pone.0065107
Dani FR, Jones GR, Destri S, Spencer SH, Turillazzi S (2001) Deciphering the recognition signature within the cuticular chemical profile of paper wasps. An Beh 62:165–171
Del-Claro K (2010) Introdução à Ecologia Comportamental : um manual para o estudo do comportamento animal / Kleber Del-Claro, 1st edn. Technical Books, Rio de Janeiro, p 128
Gadagkar R (1991) Belonogaster, Mischocyttarus, Parapolybia, and independent founding Ropalidia. In: Ross KG, Matthews RW (eds) The social biology of wasps. Cornell University Press, Ithaca, pp 149–190
Gadagkar R, Bhagavan S, Chandrashekara K, Vinutha C (1991) The role of larval nutrition in pre-imaginal biasing of caste in the primitively eusocial wasp Ropalidia marginata (Hymenoptera, Vespidae). Ecol Entomol 16:435–440. https://doi.org/10.1111/j.1365-2311.1991.tb00236.x
Giannotti E, Machado VLL (1999) Behavioral castes in the primitively eusocial wasp Polistes lanio Fabricius (Hymenoptera:Vespidae). Rev Bras Entomol 43(3–4):185–190
Giray T, Giovanetti M, West-Eberhard MJ (2005) Juvenile hormone, reproduction, and worker behavior in the neotropical social wasp Polistes canadensis. Proceed Nat Acad Sci USA 102:3330–3335. https://doi.org/10.1073/pnas.0409560102
Hair JF, Black WC, Babin BJ, Anderson RE, Tatham RL (2009) Análise multivariada de dados. Trad. Adonai Schlup Sant’Anna. Rev. Maria Aparecida Gouvêa. 6. ed. Porto Alegre, Bookman. 688
Hölldobler B, Wilson E (1990) The Ants. Valenzuela Gonzalez J, Lopez-Mendez A, Garcia-Ballinas A (1994) Activity pattern and foraging habits of Pachycondyla villosa (Hymenoptera, Formicidae) in cacao agroecosystems from Soconusco. Folia Entomol Mex. Chiapas, Mexico, pp 9–21
Howard RW (2001) Cuticular hydrocarbons of adult Pteromalus cerealellae (Hymenoptera: Pteromalidae) and two larval hosts, angoumois grain mothn (Lepidoptera: Gelechiidae) and cowpea weevil (Coleptera: Bruchidae). Ann Entomol Soc Am 94:152–158. https://doi.org/10.1603/0013-8746(2001)094[0152:CHOAPC]2.0.CO;2
Howard RW, Perez-Lachaud G, Lachaud J-P (2001) Cuticular hydrocarbons of Kapala sulcifacies (Hym: Eucharitidae) and its host, the Ponerine ant Ectatomma ruidum (Hym: Formicidae). Ann Entomol Soc Am 94:707–716
Huang Z-Y, Robinson GE, Tobe SS, Yagi KJ, Strambi C, Strambi A, Stay B (1991) Hormonal regulation of behavioural development in the honey bee is based on changes in the rate of juvenile hormone biosynthesis. J Insect Physiol 37(10):733–741. https://doi.org/10.1016/0022-1910(91)90107-b
Jatsch AS, Ruther J (2021) Acetone application for administration of bioactive substances has no negative effects on longevity, fitness, and sexual communication in a parasitic wasp. PLoS ONE 16(1):e0245698. https://doi.org/10.1371/journal.pone.0245698
Jeanne RL (1991) The swarm-founding Polistinae. In: Ross KG, Matthews RW (eds) The social biology of wasps. Cornell University Press, Ithaca, NY, pp 191–231
Judd TM, Magnus RM, Fasnacht MP (2010) A nutritional profile of the social wasp Polistes metricus: differences in nutrient levels between castes and changes within castes during the annual life cycle. J Insect Physiol 56:42–56. https://doi.org/10.1016/j.jinsphys.2009.09.002
Keeping MG (2002) Reproductive and worker castes in the primitively eusocial wasp Belonogaster petiolata (DeGeer) (Hymenoptera: Vespidae): evidence for pre-imaginal differentiation. J Insect Physiol 48:867–879. https://doi.org/10.1016/s0022-1910(02)00156-7
Kelstrup HC, Hartfelder K, Esterhuizen N, Wossler TC (2017) Juvenile hormone titers, ovarian status and epicuticular hydrocarbons in gynes and workers of the paper wasp Belonogaster longitarsus. J Insect Physiol 98:83–92. https://doi.org/10.1016/j.jinsphys.2016.11.014
Lengyel F, Westerlund SA, Kaib M (2007) Juvenile hormone III influences task-specific cuticular hydrocarbon profile changes in the ant Myrmicaria eumenoides. J Chem Ecol 33:167–181. https://doi.org/10.1007/s10886-006-9185-x
Michelutti KB, Cardoso CAL, Antonialli-Junior WF (2017) Evaluation of chemical signatures in the developmental stages of Mischocyttarus consimilis Zikán (Hymenoptera, Vespidae) employing gas chromatography coupled to mass spectrometry. Rev Virtual Quim 9:535–547. https://doi.org/10.21577/1984-6835.20170031
Monnin T, Malosse C, Peeters C (1998) Solid Phase Microextraction and cuticular hydrocarbon differences related to reproductive activity in the queenless ant Dinoponera quadriceps. J Chem Ecol 24:473–490
Montagna TS, Raizer J, Antonialli-Junior WF (2015) Effect of larval topical application of juvenile hormone on caste determination in the independent-founding eusocial wasp Mischocyttarus consimilis (Hymenoptera: Vespidae). Open J Anim Sci 5:174–184. https://doi.org/10.13102/sociobiology.v67i3.4366
Murakami ASN, Nunes TM, Desuó IC, Shima SN, Mateus S (2015) The Cuticular Hydrocarbons Profiles in the Colonial Recognition of the Neotropical Eusocial Wasp Mischocyttarus cassununga (Hymenoptera Vespidae). Sociobiol. https://doi.org/10.13102/sociobiology.v62i1.109-115
Neves EF, Andrade LHC, Súarez YR, Lima SM, Antonialli-Junior WF (2012) Age-related changes in the surface pheromones of the wasp Mischocyttarus consimilis (Hymenoptera: Vespidae). Genet Mol 11(3):1891–1898. https://doi.org/10.4238/2012.July.19.8
Norman VC, Hughes WOH (2016) Behavioural effects of juvenile hormone and their influence on division of labour in leaf-cutting ant societies. J Exp Biol 219(1):8–11. https://doi.org/10.1242/jeb.132803
Norman VC, Pamminger T, Nascimento F, Hughes WOH (2019) The role of juvenile hormone in regulating reproductive physiology and dominance in Dinoponera quadriceps ants. PeerJ 7:e6512. https://doi.org/10.7717/peerj.6512
O’Donnell S (1998) Reproductive caste determination in eussocial wasps (Hymenoptera: Vespidae). Annu Rev Entomol 43:323–346
O’Donnell S, Jeanne RL (1993) Methoprene accelerates age polyethism in workers of a social wasp (Polybia occidentalis). Physiol Entomol 18:189–194. https://doi.org/10.1111/j.1365-3032.1993.tb00467.x
Oi CA, Oliveira RC, van Zweden JS, Mateus S, Millar JG, Nascimento FS, Wenseleers T (2019) Do primitively eusocial wasps use queen pheromones to regulate reproduction? A case study of the paper wasp Polistes satan. Front Ecol Evol 7:199. https://doi.org/10.3389/fevo.2019.00199
Oliveira RC, Vollet-Neto A, Oi CA, van Zweden JS, Nascimento F, Brent CS, Wenseleers T (2017) Hormonal pleiotropy helps maintain queen signal honesty in a highly eusocial wasp. Sci Rep. https://doi.org/10.1038/s41598-017-01794-1
Paes de Oliveira VT, Cruz-Landim C (2001) Experimental control of the extra doses of juvenile hormone on bee development: the case of the wax glands of Apis mellifera (Hymenoptera: Apidae). Soc 38:513–521
Pamminger T, Treanor D, Hughes WOH (2016) Pleiotropic effects of juvenile hormone in ant queens and the escape from the reproduction–immunocompetence trade-off. Proc R Soc B 283:20152409. https://doi.org/10.1098/rspb.2015.2409
Pinto LZ, Hartfelder K, Bitondi MM, Simões ZLP (2002) Ecdysteroid titers in pupae of highly social bees relate to distinct modes of caste development. J Insect Physiol 48:783–790. https://doi.org/10.1016/s0022-1910(02)00103-8
Queller DC, Strassmann JE (1989) Measuring inclusive fitness in social wasps. In: Breed MD, Page RE (eds) The genetics of social evolution. Westview Press, Boulder, Genetics Selection Evolution, pp 103–122
Quinn GP, Keough MJ (2005) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Rembold H, Czoppelt C, Grune M, Lacner B, Pfeffer J, Woker E (1992) Juvenile hormone titers during honey bee embryogenesis and metamorfhosis. In: Research IJH, fundamental and Applied Approaches, (eds) Mauchamp B. Couillaud F, Baehr JC) Institut Nacional de la Recherche Agronomique, Paris, pp 37–43
Reich GC (1793) Kurze Beschreibung neuen, oder noch wenig bekkanten Thiere, welche Herr Le Blond der naturforschenden Gesellschaft zu Paris aus Cayenne als Geschenk überschikt hat. Magazin des Thierreichs 1:128–134
Robinson GE (1992) Regulation of division of labor in insect societies. Annu Rev Entomol 37:637–665. https://doi.org/10.1146/annurev.en.37.010192.003225
Robinson GE, Ratnieks FLW (1987) Induction of premature honey bee (Hymenoptera; Apidae) flight by juvenile hormone analogs administered orally or topically. J Econ Entomol 80:784–787. https://doi.org/10.1093/jee/80.4.784
Robinson GE, Vargo EL (1997) Juvenile hormone in adult eussocial Hymenoptera: gonadotropin and behavioral pacemaker. Arch Insect Biochem Physiol 35:559–583
Röseler PF (1991) Reproductive competition during colony establishment. In: Ross KG, Matthews RW (eds) The social biology of wasps. Cornell University, Ithaca, pp 309–335
Röseler PF, Roseler I, Strambi A, Augier R (1984) Influence of insect hormones on the establishment of dominance hierarchies among foundresses of the paper wasp, Polistes gallicus. Behav Ecol Sociobiol 15:133–142
Ross KG, Matthews RW eds. (1991) The Social Biology of Wasps. Cornell University Press; Ithaca, New York; xvii + 678 pp.
Siegel S, Castellan JR (2006) Estatística não-paramétrica para ciências do comportamento; [Tradução: Carmona SIC], 2ª. Artmed, Porto Alegre
Singer TL (1998) Roles of hydrocarbons in the recognition systems of insects. Am Zool 38:394–405. https://doi.org/10.1093/icb/38.2.394
Singer TL, Espelie KE, Gamboa GJ (1998) Nest and nestmate discrimination in independent-founding wasps. In: Vander Meer RK, Breed MD, Winston ML, Espelie EK editors. Pheromone communication in social insects 104–125. Boulder, Westview Press
Sledge MF, Boscaro F, Turillazzi S (2001) Cuticular hydrocarbons and reproductive status in the social wasp Polistes dominulus. Behav Ecol Sociobiol 49:401–409. https://doi.org/10.1007/s002650000311
Sledge MF, Trinca I, Massolo A, Boscaro F, Turillazzi S (2004) Variation in cuticular hydrocarbon signatures, hormonal correlates and establishment of reproductive dominance in a polistine wasp. J Insect Physiol 50:73–83. https://doi.org/10.1016/j.jinsphys.2003.10.001
Soares ER, Torres VO, Antonialli-Junior WF (2014) Reproductive status of females in the eusocial wasp Polistes ferreri saussure (Hymenoptera: Vespidae). Neotrop Entomol 43(6):500–508. https://doi.org/10.1007/s13744-014-0242-9
Soares ERP, Batista NR, Souza RDS, Torres VDO, Cardoso CAL, Nascimento FS, Antonialli-Junior WF (2017) Variation of cuticular chemical compounds in three species of Mischocyttarus (Hymenoptera: Vespidae) eusocial wasps. Rev Bras Entomol 61(3):224–231. https://doi.org/10.1016/j.rbe.2017.05.001
Sullivan JP, Jassim O, Fahrbach SE, Robinson GE (2000) Juvenile hormone paces behavioral development in the adult worker honey bee. Horm Behav 37:1–14. https://doi.org/10.1006/hbeh.1999.1552
Tibbetts EA, Fearon ML, Wong E, Huang ZY, Tinghitella RM (2018) Rapid juvenile hormone downregulation in subordinate wasp queens facilitates stable cooperation. Proc Royal Soc B. https://doi.org/10.1098/rspb.2017.2645
Torres VO, Montagna TS, Fernandes WD, Antonialli-Junior WF (2011) Colony cycle of the social wasp Mischocyttarus consimilis Zikán (Hymenoptera: Vespidae). Rev Bras Entomol 55(2):247–252. https://doi.org/10.1590/S0085-56262011000200016
Torres VO, Montagna TS, Raizer J, Antonialli-Junior WF (2012) Division of labor in colonies of the eusocial wasp Mischocyttarus consimilis. J Insect Sci 12:21. https://doi.org/10.1673/031.012.2101
Torres VO, Sguarizi-Antonio D, Lima SM, Andrade LHC, Antonialli-Junior WF (2014) Reproductive Status of the social wasp Polistes versicolor (Hymenoptera Vespidae). Sociobiol 61(2): 218–224, https://doi.org/10.13102/sociobiology.v61i2.218-224
Van den Dool H, Kratz PD (1963) A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J Chromatogr 11, 463–471. https://doi.org/10.1016/S0021-9673(01)80947-X
Van Zweden JS, Bonckaert W, Wenseleers T, d’Ettorre P (2013) Queen signaling in social wasps. Evol 68:976–986. https://doi.org/10.1111/evo.12314
Walton A, Tumulty JP, Toth AL, Sheehan MJ (2020) Hormonal modulation of reproduction in Polistes fuscatus social wasps: Dual functions in both ovary development and sexual receptivity. J Insect Physiol 120:103972. https://doi.org/10.1016/j.jinsphys.2019.103972
Weiss I, Hofferberth J, Ruther J, Stokl J (2015) Varying importance of cuticular hydrocarbons and iridoids in the species-specific mate recognition pheromones of three closely related Leptopilina species. Front Ecol Evol 3:1–12. https://doi.org/10.3389/fevo.2015.00019
Wilson EO (1971) The insect societies. Belknap Press, Cambridge, p 548
Wilson EO (1985) The principles of caste evolution. Fortschr Zool 31:307–324
Zara FJ, Balestieri JBP (2000) Behavioural catalogue of Polistes versicolor Oliver (Vespidae: Polistinae) post–emergence colonies. Naturalia 25:301–319
Zhu G, Ye G, Hu C, Xu X, Li K (2006) Development changes of cuticular hydrocarbons in Chrysomya rufifacies larvae: potential for determining larval age. Med Vet Entomol 20:438–444. https://doi.org/10.1111/j.1365-2915.2006.00651.x
Acknowledgements
The authors would like to thank Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (FUNDECT) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for providing doctorate fellowships to the first, second, and third authors. CALC (Grant No. 312671/2021‑0) and WFAJ (Grant No. 308182/2019-7) acknowledge research grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests.
Additional information
Communicated by Günther Raspotnig.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Neves, E.F., dos Santos Montagna, T., Michelutti, K.B. et al. Role of juvenile hormone in oogenesis, chemical profile, and behavior of the wasp Mischocyttarus consimilis (Vespidae: Polistinae). Chemoecology 32, 197–207 (2022). https://doi.org/10.1007/s00049-022-00378-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-022-00378-4