Abstract
The olive fruit fly, Bactrocera oleae, has a peculiar sexual chemoecology, guided by both male- and female-borne olfactory cues, mostly produced in rectal glands. Despite the research on B. oleae female pheromones has a long history, only few components (mainly 1,7-dioxaspiro[5.5]undecane) have been deeply investigated. Detailed evidences about the chemical identity and bioactivity of several others C10–C18 molecules produced in female rectal glands are lacking. We conducted GC and GC/EI–MS, identifying nine sex-specific chemicals and an additional compound [ethyl(Z)-9-octadecenoate], less abundant in females over males. Age-related production of all compounds raised over time. In 21-day-old females, it reached amounts from a minimum of 8.08 ng/fly (n-butyl dodecanoate) to a maximum of 87.19 ng/fly (ethyl hexadecanoate). In EAG experiments, all chemicals were perceived by both sexes. Methyl hexadecanoate and ethyl decanoate attracted males and females, respectively. This is the first report on a female-borne compound attracting conspecific females in Tephritidae. Our study sheds light on the bioactivity of female-borne pheromones involved in the B. oleae chemoecology. Further research is ongoing to test methyl hexadecanoate and ethyl decanoate as lures to enhance sex pheromone blends used in IPM programmes against B. oleae, thus improving control tools against this key pest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The family Tephritidae (Diptera), also known as “true fruit flies”, contains over 4,000 species, many of which constitute enormous threats to fruit and vegetable production throughout the world (Benelli et al. 2014a, b). The olive fruit fly is a tephritid carpophagous on fruits of a few Olea species, including Olea europaea L. Nowadays, it is considered the major pest of commercial olives worldwide, affecting almost all the world olive production with few exceptions in isolated areas where low temperatures limit its occurrence (Daane and Johnson 2010). The control of B. oleae is based mainly on the use of chemical insecticides, posing serious threats to human health and environmental safety (Stark et al. 2004; Thomas and Mangan 2005). Some plant-borne compounds have been proposed as potentially useful chemicals against B. oleae (Canale et al. 2013a). However, they are toxic against non-target arthropods, such as olive fruit fly parasitoids (Benelli et al. 2013a). Concerning natural enemies, several braconid species were proposed to improve IPM programmes against the olive fruit fly (Wang et al. 2011; Benelli and Canale 2012), but no consistent results were obtained in the control of B. oleae populations (Yokoyama et al. 2008; Canale and Benelli 2012). Regarding the use of semiochemicals in integrated pest management programmes against B. oleae, the major known component of the female sex pheromone, 1,7-dioxaspiro[5.5]undecane (DSU), has been extensively used for monitoring and mass-trapping (Haniotakis et al. 1977). Also lure and kill strategies have been proposed in different olive-grove countries by formulating food baits plus DSU, with patchy results (Daane and Johnson 2010).
However, these semiochemicals-based control approaches rely only to the major component of the female sex pheromone (DSU), while the B. oleae sexual chemoecology is driven by a higher number of compounds, largely unknown (Canale et al. 2013b). Indeed, in the olive fruit fly the perception of sex-specific olfactory cues from both sexes is crucial during courtship and mating (Benelli et al. 2012; Benelli 2014). The role of sex attractants produced by males has been recently investigated. Young B. oleae males produce DSU in the rectal glands (Levi-Zada et al. 2012). The presence of DSU in young males cannot be considered a case of chemical mimicry (sensu Ruther and Steiner 2008), since the mating performance of young males is not superior to that of adults (Benelli et al. 2013b). When olive fruit fly males become sexually mature, they significantly increase the production of (Z)-9-tricosene, a compound unique to males, which is able to selectively attract females during the close-range phase only (Carpita et al. 2012; Canale et al. 2013a). On the other hand, knowledge about sex-specific chemicals produced by B. oleae females has a long research history. Virgin females produce a multi-component sex pheromone containing four major constituents with a synergistic action: DSU and methyl dodecanoate are produced in the rectal glands, while α-pinene and nonanal are produced elsewhere in the body (Baker et al. 1980; Mazomenos and Haniotakis 1981, 1985). Among these compounds, DSU is described as the most abundant component and exhibits the highest biological activity towards males (Mazomenos and Haniotakis 1981, 1985). Recently, Gerofotis et al. (2013) highlighted that the exposure of sexually mature male and female olive flies to the aroma of α-pinene increases subsequent mating success compared to non-exposed individuals. Overall, the olive fruit fly sexual chemoecology is far from a full understanding. In addition to the four well-known pheromone components, several researches claimed that other compounds are produced in the female rectal glands (Rossi et al. 1978; Gariboldi et al. 1982, 1983). Among them, there are a number of C6–C18 fatty acid esters identified by Gariboldi et al. (1983), but no data are available on sex-specificity and bioactivity of such molecules. More recently Carpita et al. (2011) provided preliminary results about the presence of at least eight sex-specific compounds (C10–18 fatty acid esters) produced by B. oleae females in their rectal glands.
In this research, we conducted gas chromatography (GC) and gas chromatography–electron impact mass spectrometry (GC/EI–MS) analyses searching for sex-specific compounds in rectal glands of olive fruit fly females. We identified nine sex-specific chemicals, typical of sexually mature virgin females and a further compound, less abundant in females over males. All chemicals were subjected to absolute quantification in relation to age. Furthermore, we evaluated the behavioural and electrophysiological responses of both sexes to the ten synthetic chemicals.
Materials and methods
Olive fruit fly rearing
Insects used in this study were obtained from pupae collected in a Tuscan olive-mill during late December 2013. Pupae were then maintained in a laboratory in Pisa, under controlled conditions (22 °C ± 1, 50–60 % RH and natural photoperiod) to wait for adult emergence. To obtain coeval virgin specimens, within 24 h of emergence flies were separated according to sex and singly placed in clean glass vials (diameter: 10 mm, length: 50 mm). Olive fruit fly adults were fed on a dry diet (yeast extract and sucrose mixture, at ratio 1:10 w/w), while water was provided separately on a cotton wick (Canale and Benelli 2012; Canale et al. 2013b).
Rectal glands extraction
Prior to glandular dissection, individuals were anaesthetised using CO2 and maintained at −18 °C for 5–10 min. Rectal glands will be extracted from both virgin males and females of 1, 4, 9, 13, 17 and 21-day-old. Using a stereomicroscope (Leica, Germany), in each specimen the rectal ampulla was dissected out by pulling off the Terminalia with forceps (Canale et al. 2013b). To obtain glandular extracts, rectal glands from ten specimens were immediately immersed in 140 µL hexane for 2 h. Extracts were stored at −20 °C until needed.
Chemical analyses, identifications and quantifications
Extracts from rectal glands of virgin males and females at 1, 4, 9, 13, 17 and 21 days after emergence (starting from early December 2013) were analysed by GC and GC/EI–MS. For each age, three extracts (each from ten virgin females or males) were analysed. GC analyses were performed using a Dani GC 1000 instrument with PTV injectors, equipped with a Dani DDS 1000 data station and two-bonded FSOT column (Dani DN-5 and Dani DN-20, both 30 m × 0.25 mm i.d.). GC/EI–MS analyses were performed with an Agilent apparatus: mass selective detector 5973 Network, 6890N Network GC system and HP-5MS bonded FSOT column (30 m × 0.25 mm i.d.). Both GC and GC/EI–MS analyses of extracts were carried out under splitless conditions, injecting 3 µL of hexane extract and using helium as carrier gas (1 mL/min); the oven temperature was programmed as follows: 1 min at 40 °C, to 250 °C at 10 °C/min, 5 min at 250 °C, to 280 °C at 20 °C/min, 30 min at 280 °C. To compare retention times, a slower temperature programme was also used (1 min at 40 °C, to 200 at 10 °C/min, to 280 at 6 °C/min, 30 min at 280 °C). Compounds were identified comparing their mass spectra and retention times (on two columns of different polarity) to those of commercial standards (purchased from Sigma-Aldrich). Retention indices were calculated using retention times of n-alkanes standard (C7–C40) homologous series, injected after the extracts at the same chromatographic conditions (above mentioned, on HP-5MS column), according to Van den Dool and Kratz (1963). Quantifications of identified compounds were performed by GC using absolute calibration curves, obtained by injecting pure commercial standards (three replicates) at five concentrations ranging from 0.5 to 8.6 µg/mL. The same pure compounds were used in bioassays.
Differences in the age-related production of chemicals were analysed using a General Linear Model with two factors: fly’s age and identified chemical: \(y_{j} = \mu + A_{j} + C_{j} + A_{j} \times C_{j} + e_{j}\), in which y j is the observation, μ is the overall mean, A j the fly’s age (j = 1–6), C the identified chemical (j = 1–10), A j × C the interaction fly’s age × chemical and e j the residual error. Averages were separated by the Tukey's HSD test. A probability level of P < 0.05 was used to test significance of differences between means.
Electrophysiological experiments
The antennal response of 5–10-day-old B. oleae males and females to the ten synthetic chemicals was evaluated according to the electroantennography technique (EAG) described in previous studies (Germinara et al. 2011; Canale et al. 2013b). A male or female fly was inserted in a plastic pipette tip (0.1 mL) whose end was properly cut to allow the insect head to protrude. Two glass electrodes filled with 0.1 M KCl saline solution were used. The indifferent electrode was inserted into the head at the base of the antennae and the recording electrode was put in contact with the tip of an antenna. AgCl-coated silver wires were used to maintain the electrical continuity between the antennal preparation and an AC/DC UN-6 amplifier in DC mode connected to a PC equipped with the EAG 2.0 programme (Syntech Laboratories, Hilversum, The Netherlands).
Stimuli were hexane solutions of pure chemicals applied to a filter paper (Whatman No. 1, Brentford, UK) strip (1 cm2) inserted into Pasteur pipettes (150 mm long). For each compound, five dosages from 0.001 to 10 µM were tested. Stimuli were blown by a disposable syringe into a constant stream of charcoal-filtered humidified air (500 mL/min) flowing in a stainless steel delivery tube (1 cm diameter) with the outlet positioned at ~1 cm from the antenna. During 1 s, 2.5 cm3 of vapour from an odour cartridge were added. Control (10 µL of hexane) and standard (10 µL of a 100 µg/µL hexanal solution) stimuli were applied at the beginning of the experiment and after each group of five test stimuli. Intervals between stimuli were 30 s. Stimuli were applied in ascending doses. The amplitude (mV) of the EAG response to each test stimulus was adjusted to compensate for solvent and/or mechanosensory artefacts by subtracting the mean EAG response of the two nearest hexane controls (Raguso and Light 1998). To compensate for the decrease in the antennal responsiveness during the experiment, the resulting EAG amplitude was corrected according to the reduction in the EAG response to the standard stimulus (Den Otter et al. 1991). Each test solution of a pure compound was tested on five antennae of different males and females. In dose–response curves, the activation threshold was considered to be the lowest dose at which the lower limit of the standard error of the mean response was greater than the upper limit of the standard error for the lowest dilution tested (Sant’ana and Dickens 1998). Saturation level was taken as the lowest dose at which the mean response was equal to or less than the previous dose (Germinara et al. 2009).
Behavioural experiments
The attractiveness of the ten chemicals identified in the female rectal glands was evaluated testing pure synthetic chemicals vs. hexane in a two-choice bioassay conducted in a still-air tested arena, described below. The compounds proved as attractive towards males (methyl hexadecanoate and methyl tetradecanoate) or females (ethyl decanoate) were evaluated for attractiveness in Y-tube experiments, using one and five 21-day-old female’s equivalents (Table 1).
Still-air arena experiments: we used the Plexiglas unit (150 × 150 × 15 mm) described in Carpita et al. (2012) as a still-air arena. In the centre of this unit, there was a circular chamber (i.e. the specimen release chamber, diameter 40 mm) connected to two other identical chambers by means of two linear paths (20 mm in length, 10 mm in width), forming a 90° angle. The top of the arena was covered with of a removable panel of glass. At the beginning of the tests, an individual was gently transferred to the release chamber using a glass vial and released on the floor of the chamber. The choice for a given cue was recorded if the fly moved to the cue within 3 min after being released and if it engaged in searching behaviours on the chosen cue for at least 30 s (Carpita et al. 2012; Canale et al. 2013b). The attractiveness of the ten synthetic chemicals was evaluated both on virgin males and females (age 10–16-day-old), applying one or five 21-day-old female’s equivalents (Table 1) on a filter-paper dish (diameter 15 mm) then placed in a side chamber of the arena. An equal clean filter paper dish was placed in the other side chamber, and a single individual of B. oleae was exposed in the release chamber. In all bioassays, with each new fly, the arena was rotated clockwise 90° to avoid positional effects. Moreover, the relative position of the sources was randomised, to avoid that one source was always on the right and the other on the left (Benelli and Canale 2013; Benelli et al. 2013b). Between each replicate, the odour-cleaning procedure was as follows: the Plexiglas arena (and the glass lid) was first washed for about 30 s with hexane, then with warm water at 35–40 °C, thus cleaned in a water bath with mild soap for about 5 min, rinsed with hot water for about 30 s, and finally rinsed with distilled water at room temperature (Carpita et al. 2012). Tested chemicals were renewed at each replicate. Flies that did not make any choice (i.e. the fly remains in the release chamber and does not show any particular behaviour) were excluded. For each bioassay, 40 replicates with responsive flies were performed.
Y-tube olfactometer experiments: the attractiveness of the chemicals identified in the female rectal glands and proved as attractive to at least one B. oleae sex (i.e. ethyl decanoate, methyl tetradecanoate and methyl hexadecanoate) in the previously described still-air arena, was evaluated towards both B. oleae sexes testing pure synthetic chemicals vs. hexane in a Y-tube olfactometer. The system consists of a Plexiglas unit (200 × 190 × 15 mm) consisting of a central tube (90 mm long, 15 mm large) and two lateral arms (75 mm long, 15 mm large). A sieve inlay in the lateral arms and extending glass tube 5.25 cm away from the connection prevents escape of insects and serves as an end point of each lateral arm. The top of the unit was covered with a removable panel of glass. Humidified and purified air was passed into the extending glass tube through a teflon connection at 1 mL/min. The Y-tube olfactometer was positioned horizontally, at a height of 80 cm to the ground. Illumination was provided by vertically hanging cold light lamp (20 W, 250 lux) above (height 60 cm) the olfactometer unit. At the beginning of the tests, a fly was gently introduced individually into the central arm of the Y-tube using a glass vial. The choice for a given cue was recorded if the fly moved to the cue within 3 min after being released and if it engaged in searching behaviour on the chosen Y-tube arm for at least 30 s (Carpita et al. 2012; Canale et al. 2013b). The attractiveness of the ten synthetic chemicals was evaluated towards virgin males and females (age 10–16-day-old). Each compound was tested at a dosage of one and five 21-day-old female equivalents. The sample was placed on a filter paper dish (diameter 10 mm, Whatman no. 1). After allowing for solvent evaporation (i.e. 20 s, the filter paper was inserted into a designated arm of the olfactometer). A similar filter paper dish treated with the same quantity of pure hexane was inserted into the second arm and served as clean air control. Only first choices in which the tested fly responded by walking into one of the two arms and remained there at least 30 s were considered for data analysis. If a fly did not make a choice (i.e. the fly remains in the release chamber and does not show any particular behaviour) within 3 min of being released, it was removed and discarded. Flies that did not walk into any of the arms were not counted (Carpita et al. 2012; Canale et al. 2013b). At each replicate, the olfactometer arms were flipped around (180°) to minimise positional effect. At each replicate, the olfactometer set up was washed as described above for the still-air arena (Carpita et al. 2012), then air-dried and chemicals were renewed. For each bioassay, 30 replicates carried out with responsive flies were performed.
All behavioural assays were carried out over a period of several weeks to account for any daily variability. For each replicate, each fly was replaced by a new one of the same age. Both sexes were tested daily using a random order (Ngumbi et al. 2012). All experiments were performed between 15.30 and 19.30 and conducted in a room uniformly lit with daylight fluorescent tubes (Philips 30 W/33). Light intensity was approximately, 1,000 lux (estimated over the 300–1,100 nm waveband using a LI-1800 spectroradiometer LI-COR Inc., Lincoln, NE, USA equipped with a remote cosine receptor). The temperature was set at 22 °C ± 1, whereas the relative humidity was kept at 45 % ± 5.
Both for still-air arena and Y-tube experiments, for each choice test, a likelihood χ 2 test with Yates correction was used to compare the number of flies choosing the tested chemical over the control. A probability level of P < 0.05 was used for the significance of differences between fly choices.
Results
Gas chromatography (GC) and gas chromatography–electron impact mass spectrometry (GC/EI–MS)
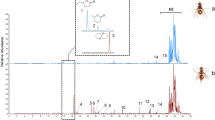
GC and GC/EI–MS analyses searching for sex-specific compounds in rectal glands of B. oleae females, confirms the presence of DSU (1, whose retention time under the mentioned conditions is 9.59 min), of several carboxylic esters (A), typical of mature females, and of high-boiling hydrocarbons (B) present also in male rectal gland extracts. Figure 1 shows the compounds identified in hexane extracts of 21-day-old rectal glands of females. Among the ester compounds, we identified ten sex-specific chemicals (Fig. 1, chemicals 2–10) by comparing its mass spectra and retention times to those of commercial standards. We detected the presence of ethyl decanoate (2, C10Et), methyl dodecanoate (3, C12Me), ethyl dodecanoate (4, C12Et), methyl tetradecanoate (5, C14Me), n-butyl dodecanoate (6, C12Bun), ethyl tetradecanoate (7, C14Et), methyl(Z)-9-hexadecenoate (8, (Z)-9-C16Me), methyl hexadecanoate (9, C16Me) and ethyl hexadecanoate (10, C16Et). All compounds are present only in the female rectal gland extracts. We also identified ethyl(Z)-9-octadecenoate (11, (Z)-9-C18Et), a compound produced in higher quantities by male rectal glands over female ones (about seven times more, 230.15 ± 2.73 ng in rectal glands of 21-day-old males).
Typical GC/MS chromatogram obtained from hexane extracts of 21-day-old Bactrocera oleae female (lower line, red) and male (upper line, black) rectal glands, showing: 1 = 1,7-dioxaspiro[5.5]undecane (absent in 21-day-old male rectal gland extracts). (A) compounds unique to the female rectal gland extracts: 2 = ethyl decanoate (C10Et); 3 = methyl dodecanoate (C12Me); 4 = ethyl dodecanoate (C12Et); 5 = methyl tetradecanoate (C14Me); 6 = n-butyl dodecanoate (C12Bun); 7 = ethyl tetradecanoate (C14Et); 8 = methyl (Z)-9-hexadecenoate [(Z)-9-C16Me]; 9 = methyl hexadecanoate (C16Me); 10 = ethyl hexadecanoate (C16Et); with the exception of 11 = ethyl (Z)-9-octadecenoate [(Z)-9-C18Et)], that is present in minimal quantities in female rectal glands, if compared to male ones. (B) high-boiling hydrocarbons (present also in male rectal gland extracts, see Carpita et al. 2012)
The nine female-specific chemicals (2–10) and 11 were subjected to absolute quantification in relation to age (Fig. 2). We found significant differences in the compounds’ production over time as function of the fly’s age (F 5,146 = 2,093.212, P < 0.001), the identified chemical (F 9,140 = 347.668, P < 0.001) and the interaction fly’s age × identified chemical (F 45,103 = 117.048, P < 0.001). The production of the ten chemicals in female rectal glands reached a maximum at 21 days from emergence (Fig. 2). At that time, they contained a mean of 8.45 ng of 2 (SD ± 0.14), 13.64 ng of 3 (SD ± 0.80), 32.42 ng of 4 (SD ± 1.15), 56.52 ng of 5 (SD ± 3.19), 8.08 ng of 6 (SD ± 0.05), 77.90 ng of 7 (SD ± 2.36), 21.00 ng of 8 (SD ± 0.18), 74.55 ng of 9 (SD ± 3.03), 87.19 ng of 10 (SD ± 1.49), 32.45 ng of 11 (SD ± 0.40).
Electrophysiological experiments
The sensitivity of male and female B. oleae antennae towards increasing concentrations of authentic standards of compounds identified in the female rectal glands are reported in Fig. 3. In the dose range tested, all compounds elicited measurable EAG responses in both sexes. For all compounds, the amplitude of male and female EAG responses increased with dose. The lowest activation thresholds were recorded for C10Et, C12Et, C12Bun, C14 Et, (Z)-9-C18Et in both sexes and for C14Me in males at the 0.01 µM dose. The mean amplitude of male EAG response decreased from 1 to 10 µM dose for C12Me, C12Et, C14Me, C12Bun, C14Et, (Z)-9-C12Me, and C14Me suggesting saturation of receptors at the lowest dose. In females, a similar pattern was observed only for C14Et (Fig. 3).
Behavioural experiments
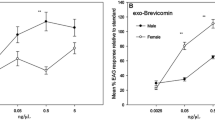
In still-air arena experiments, methyl hexadecanoate tested at five 21-day-old female equivalents evoked attraction towards males (χ 2 = 4.225, df = 1, P = 0.039). Also methyl tetradecanoate tested at one 21-day-old female equivalent attracted males (χ 2 = 4.225, df = 1, P = 0.039) (Fig. 4a). Furthermore, ethyl decanoate was attractive to females, at both tested dosages (one 21-day-old female equivalent: χ 2 = 4.225, df = 1, P = 0.039; five 21-day-old female equivalents: χ 2 = 4.225, df = 1, P = 0.039) (Fig. 4a).
Number of choices made by Bactrocera oleae virgin males and females on the components (except 1,7-dioxaspiro[5.5]undecane) of female sex pheromones, tested vs. hexane in two-choice bioassays conducted in a still-air arena, and b Y-tube olfactometer (only chemicals proved as attractive in the still-air arena were tested in the latter). fe fly equivalents. Forty flies were tested in each still-air arena experiment. Thirty flies were tested in each Y-tube experiment. For each experiment, asterisks indicate significant differences in the number of flies landing on the two given cues; ns not significant (χ 2 test with Yates correction, P < 0.05)
Y-tube experiments confirmed the attraction towards males of methyl hexadecanoate tested at five 21-day-old female equivalents (χ 2 = 5.630, df = 1, P = 0.018), while methyl tetradecanoate resulted unattractive (Fig. 4b). Moreover, the attractiveness of ethyl decanoate towards females was confirmed for both tested dosages (one and five 21-day-old female equivalents: χ 2 = 4.030, df = 1, P = 0.045) (Fig. 4b).
Discussion
Results highlighted the presence of nine sex-specific compounds produced in the rectal glands of olive fruit fly virgin females. Furthermore, an additional component [ethyl(Z)-9-octadecenoate], produced in lower quantities by females than males, was also identified. Our findings confirmed earlier records of methyl dodecanoate (Mazomenos and Haniotakis 1981, 1985), ethyl dodecanoate, methyl tetradecanoate, ethyl tetradecanoate, methyl(Z)-9-hexadecenoate, methyl hexadecanoate and ethyl hexadecanoate (Gariboldi et al. 1983) by previous researchers. Furthermore, we reported three new compounds present in the rectal glands of olive fruit fly females: ethyl decanoate, n-butyl dodecanoate and ethyl(Z)-9-octadecenoate. Interestingly, the three new compounds we found in B. oleae female rectal glands have never been detected as semiochemicals of other Tephritidae species (Benelli et al. 2014a, b; El-Sayed 2014). On the other hand, several other compounds reported by previous research have been not found in our analyses of rectal glands of olive fruit fly females [e.g. n-butyl hexanoate, ethyl hexadecenoate, n-butyl tetradecenoate, n-butyl hexadecanoate, n-butyl hexadecenoate, ethyl octadecanoate, methyl(Z)-9-octadecenoate and n-butyl octadecenoate] (Gariboldi et al. 1983). Previous identification of these chemicals in extracts of B. oleae female rectal glands may be due to misleading mass-spectra interpretation or sample contamination with foreign chemicals.
In our experiments, we observed that the age-related production of all compounds raised over time and in 21-day-old females reached amounts ranging from a minimum of 8.08 ng/fly (n-butyl dodecanoate) to a maximum of 87.19 ng/fly (ethyl hexadecanoate). These quantities are lower if compared with the DSU production in B. oleae females of comparable age (ranging from 3,000 to 4,000 ng/fly) (Canale and Benelli 2012), while they are close to the mean production of the main male sex pheromone component, (Z)-9-tricosene (about 50 ng/fly for sexually mature males) (Canale et al. 2013b). Noticeably, this latter compound is involved in the sexual communication of B. oleae through transfer on male urotergal glands. Indeed, it has been demonstrated that its amount on male urotergal glands is due to the transfer of rectal content to urotergal glands through a peculiar leg rubbing behaviour (Canale et al. 2013b, see also Webb et al. 1976; Shelly and Kaneshiro 1991; Briceño et al. 1996). Conversely, no leg rubbing behaviour has been observed in B. oleae females (Canale et al. 2013b). The role of urotergal glands in females is still unclear and deserves further efforts (Raspi et al. 1997), as well as additional research is also needed about their chemical characterization.
All identified compounds were proved as active in EAG experiments and evoked EAG dose-dependent responses, even if with different activation thresholds (ranging from 0.01 to 1 µM). Similar patterns of EAG responses have been observed testing DSU and (Z)-9-tricosene with the same experimental apparatus. Male flies showed an activation threshold to both DSU and (Z)-9-tricosene of 0.1 µM, while female flies had an activation threshold of 0.1 to DSU and 0.01 to (Z)-9-tricosene (Canale et al. 2013a, b). Behavioural assays highlighted that only two molecules evoked attraction towards conspecific flies. Methyl hexadecanoate showed dose-dependent attraction towards virgin males, while ethyl decanoate was attractive towards virgin females. Dose-dependent attraction is not rare in Tephritidae flies (Benelli et al. 2014a, b). Good examples about the olive fruit fly are the male urotergal glands containing (Z)-9-tricosene, attractive towards virgin females only when tested at groups of ten (Canale et al. 2013b) as well as the synthetic (Z)-9-tricosene, attractive to virgin females only when tested at 1.5 or 3 male rectal gland equivalents (Carpita et al. 2012). The finding concerning the attractiveness evoked by ethyl decanoate towards other virgin females is surprising. Indeed, to the best of our knowledge, no female-borne chemicals attractive to conspecific females have been reported for tephritid flies (El-Sayed 2014). In addition, the lekking sex in B. oleae is the male (Benelli 2014), thus the chemoecological role of this molecule is unclear. Ethyl decanoate may be involved in female-female aggressions for single oviposition sites, recently described for the olive fruit fly (Benelli 2014, 2015a, b). Further research on this point is strongly encouraged.
Overall, our research sheds light on the age-related production and bioactivity of some female-borne overlooked chemicals involved in the olive fruit fly chemical ecology, adding valuable information to understand the mating system of this fly. Furthermore, our findings have pivotal potential applications in B. oleae control strategies. Methyl hexadecanoate and ethyl decanoate can be suitable lures (for males and females, respectively) to enhance sex pheromone blends tested in long lasting pheromone dispensers (Gil-Ortiz 2012). This can improve the success of IPM programmes against the olive fruit fly, improving the success of monitoring, mass-trapping and “lure and kill” tools against this key tephritid pest. Further research is ongoing to test blends of DSU plus the two mentioned esters in long lasting dispensers.
References
Baker R, Herbert RH, Howse PE, Jones OT, Francke W, Reith W (1980) Identification and synthesis of the major sex pheromone of the olive fly, Dacus oleae. J Chem Soc Chem Comm 1:52–54
Benelli G (2014) Aggressive behavior and territoriality in the olive fruit fly, Bactrocera oleae (Rossi) (Diptera: Tephritidae): role of residence and time of day. J Ins Behav 27:145–161
Benelli G (2015a) Aggression in Tephritidae flies: where, when, why? Future directions for research in Integrated Pest Management. Insects (in press)
Benelli G (2015b) Should I fight or should I flight? How studying insect aggression can help Integrated Pest Management. Pest Manag Sci (in press)
Benelli G, Canale A (2012) Learning of visual cues in the fruit fly parasitoid Psyttalia concolor (Szépligeti) (Hymenoptera: Braconidae). Biocontrol 57:767–777
Benelli G, Canale A (2013) Do tephritid-induced fruit volatiles attract males of the fruit flies parasitoid Psyttalia concolor (Szépligeti) (Hymenoptera: Braconidae)? Chemoecology 23:191–199
Benelli G, Canale A, Bonsignori G, Ragni G, Stefanini C, Raspi A (2012) Male wing vibration in the mating behavior of the olive fruit fly Bactrocera oleae (Rossi) (Diptera: Tephritidae). J Ins Behav 25:590–603
Benelli G, Canale A, Flamini G, Cioni PL, Demi F, Ceccarini L, Macchia M, Conti B (2013a) Biotoxicity of Melaleuca alternifolia (Myrtaceae) essential oil against the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae), and its parasitoid Psyttalia concolor (Hymenoptera: Braconidae). Ind Crops Prod 50:596–603
Benelli G, Bonsignori G, Stefanini C, Raspi A, Canale A (2013b) The production of female sex pheromone in Bactrocera oleae (Rossi) young males does not influence their mating chances. Entomol Sci 16:47–53
Benelli G, Daane KM, Canale A, Niu CY, Messing RH, Vargas RI (2014a) Sexual communication and related behaviours in Tephritidae: current knowledge and potential applications for Integrated Pest Management. J Pest Sci 87:385–405
Benelli G, Giunti G, Canale A, Messing RH (2014b) Lek dynamics and cues evoking mating behavior in tephritid flies infesting soft fruits: implications for behavior-based control tools. Appl Entomol Zool 49:363–373
Briceño D, Ramos D, Eberhard W (1996) Courtship behaviour of male Ceratitis capitata (Diptera: Tephritidae) in captivity. Fla Entomol 79:130–143
Canale A, Benelli G (2012) Impact of mass-rearing on the host-seeking behaviour and parasitism by the fruit fly parasitoid Psyttalia concolor (Szépligeti) (Hymenoptera: Braconidae). J Pest Sci 85:65–74
Canale A, Benelli G, Conti B, Lenzi G, Flamini G, Francini A, Cioni PL (2013a) Ingestion toxicity of three Lamiaceae essential oils incorporated in protein baits against the olive fruit fly, Bactrocera oleae (Rossi) (Diptera Tephritidae). Nat Prod Res 27:2091–2099
Canale A, Germinara SG, Carpita A, Benelli G, Bonsignori G, Stefanini C, Raspi A, Rotundo G (2013b) Behavioural and electrophysiological responses of the olive fruit fly, Bactrocera oleae (Rossi) (Diptera: Tephritidae), to male- and female-borne sex attractants. Chemoecology 23:155–164
Carpita A, Canale A, Benelli G, Conti B, Raspi A (2011) Componenti secondari presenti nelle ghiandole associate all’ampolla rettale delle femmine di Bactrocera oleae (Rossi). XXIII Congresso Nazionale Italiano di Entomologia. Genova, Italy, 13–16 Giugno 2011, Atti p 169
Carpita A, Canale A, Raffaelli A, Saba A, Benelli G, Raspi A (2012) (Z)-9-tricosene identified in rectal gland extracts of Bactrocera oleae males: first evidence of a male-produced female attractant in olive fruit fly. Naturwissenschaften 99:77–81
Daane KM, Johnson MW (2010) Olive fruit fly: managing an ancient pest in modern times. Annu Rev Entomol 55:151–169
Den Otter CJ, Tchicaya T, Schutte AM (1991) Effects of age, sex and hunger on the antennal olfactory sensitivity of tsetse flies. Phys Entomol 16:173–182
El-Sayed AM (2014) The pherobase: database of insect pheromones and semiochemicals, http://pherobase.com
Gariboldi P, Jommi G, Rossi R, Vita G (1982) Studies on the chemical constitution and sex pheromone activity of volatile substances emitted by Dacus oleae. Experientia 38:441–444
Gariboldi P, Verotta L, Fanelli R (1983) Studies on the sex pheromone of Dacus oleae. Analysis of the substances contained in the rectal glands. Experientia 39:502–505
Germinara GS, De Cristofaro A, Rotundo G (2009) Antennal olfactory responses to individual cereal volatiles in Theocolax elegans (Westwood) (Hymenoptera: Pteromalidae). J Stored Prod Res 45:195–200
Germinara GS, De Cristofaro A, Rotundo G (2011) Chemical cues for host location by the chestnut gall wasp, Dryocosmus kuriphilus. J Chem Ecol 37:49–56
Gerofotis CD, Ioannou CS, Papadopoulos NT (2013) Aromatized to find mates: α-pinene aroma boosts the mating success of adult olive fruit flies. PLoS One 8:e81336
Gil-Ortiz R (2012) Development of new ecological long-lasting dispensers of semiochemicals for the control of Bactrocera oleae (Rossi). Pest Manag Sci. doi:10.1002/ps.3415
Haniotakis GE, Mazomenos BE, Tumlinson IH (1977) A sex attractant of the olive fruit fly, Dacus oleae, and its biological activity under laboratory and field conditions. Entomol Exp Appl 21:81–87
Levi-Zada A, Nestel D, Fefer D, Nemni-Lavy E, Deloya-Kahane I, David M (2012) Analyzing diurnal and age-related pheromone emission of the olive fruit fly, Bactrocera oleae by sequential SPME-GCMS analysis. J Chem Ecol 38:1036–1041
Mazomenos BE, Haniotakis GE (1981) A multicomponent female sex pheromone of Dacus oleae Gmelin: isolation and bioassay. J Chem Ecol 7:1561–1573
Mazomenos BE, Haniotakis GE (1985) Male olive fruit fly attraction to synthetic sex pheromone components in laboratory and field tests. J Chem Ecol 11:397–405
Ngumbi E, Jordan M, Fadamiro H (2012) Comparison of associative learning of host-related plant volatiles in two parasitoids with different degrees of host specificity, Cotesia marginiventris and Microplitis croceipes. Chemoecology 22:207–215
Raguso RA, Light DM (1998) Electroantennogram responses of male Sphinx perelegans hawkmoths to floral and ‘green leaf volatiles’. Entomol Exp Appl 86:287–293
Raspi A, Canale A, Lucchi A (1997) Preliminary observations on integumentary urotergal glands in Bactrocera oleae (Gmelin) (Diptera Tephritidae). Frustula Entomol 20:193–202
Rossi R, Carpita A, Vita G (1978) Z)-6-Nonen-l-ol and related compounds as attractants of the olive fruit fly, Dacus oleae (Gmelin) (Diptera: Tephritidae. Gazz Chim Ital 108:709–712
Ruther J, Steiner S (2008) Costs of female odour in males of the parasitic wasp Lariophagus distinguendus (Hymenoptera: Pteromalidae). Naturwissenschaften 95:547–552
Sant’ana J, Dickens JC (1998) Comparative electrophysiological studies of olfaction in predaceous bugs, Podisus maculiventris and P. nigrispinus. J Chem Ecol 24:965–984
Shelly TE, Kaneshiro KY (1991) Lek behavior of the oriental fruit fly, Dacus dorsalis, in Hawaii (Diptera: Tephritidae). J Ins Behav 4:235–241
Stark JD, Vargas R, Miller N (2004) Toxicity of spinosad in protein bait to three economically important tephritid fruit fly species (Diptera: Tephritidae) and their parasitoids (Hymenoptera: Braconidae). J Econ Entomol 97:911–915
Thomas DB, Mangan RL (2005) Nontarget impact of spinosad GF-120 bait sprays for control of the Mexican fruit fly (Diptera: Tephritidae) in Texas citrus. J Econ Entomol 98:1950–1956
Van den Dool H, Kratz PD (1963) A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J Chromatogr 11:463–471
Wang XG, Johnson MW, Yokoyama VY, Pickett CH, Daane KM (2011) Comparative evaluation of two olive fruit fly parasitoids under varying abiotic conditions. Biocontrol 56:283–293
Webb JC, Sharp L, Chambers DL, McDow JJ, Benner JC (1976) Analysis and identification of sounds produced by the male Caribbean fruit fly, Anastrepha suspensa. Ann Entomol Soc Am 69:415–420
Yokoyama VY, Rendon PA, Sivinski J (2008) Psyttalia cf. concolor (Hymenoptera: Braconidae) for biological control of olive fruit fly (Diptera: Tephritidae) in California. Environ Entomol 37:764–773
Acknowledgments
We are grateful to two anonymous reviewers for their comments on an earlier version of the manuscript. We would like to thank Roberto Canovai and Giulia Giunti for assistance in the olive fruit fly rearing. This research was financially supported by MIUR (Ministry of Education, University and Research, Italy)—PRIN 2007. Giovanni Benelli is supported by a MIS. 124 MODOLIVI Grant. Funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Paulo H. G. Zarbin.
A. Canale and G. Benelli contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Canale, A., Benelli, G., Germinara, G.S. et al. Behavioural and electrophysiological responses to overlooked female pheromone components in the olive fruit fly, Bactrocera oleae (Diptera: Tephritidae). Chemoecology 25, 147–157 (2015). https://doi.org/10.1007/s00049-014-0183-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-014-0183-0