Abstract
The Chinese white pine beetle, Dendroctonus armandi Tsai and Li, is considered a serious native pest in the Qinling and Bashan Mountains of China. Relatively few information is available regarding its pheromone characterization, and the functions of its pheromones have not yet been identified. Gas chromatographic and mass spectrometry (GC–MS) analyses of volatiles collected from live D. armandi revealed that (1) virgin female and mated male but not mated female D. armandi produce frontalin and (2) female but not male beetles produce exo-brevicomin. Electroantennography (EAG) and Y-tube laboratory assays indicated that male D. armandi are more sensitive to frontalin and frontalin + α-pinene, whereas female D. armandi are more sensitive to frontalin + α-pinene and exo-brevicomin. These results support frontalin as a virgin female-produced sex pheromone, and frontalin + α-pinene as a virgin female and mated male-produced aggregation pheromone. Furthermore, different concentrations of exo-brevicomin have aggregation and anti-aggregation roles in female D. armandi. This study provides evidence for classifying the two compounds as certain types of pheromones and indicates that these pheromones have clear potential value for monitoring and controlling the outbreak of this serious pest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bark beetles (Curculionidae: Scolytinae) are considered destructive pests in natural and managed coniferous forests and have caused considerable economic losses (Coulson and Stark 1982; Furniss and Carolin 1977). The Chinese white pine beetle (Dendroctonus armandi Tsai and Li) kills mature Pinus armandi Franch, and has caused serious damage to mature P. armandi forests in the Qinling and Bashan Mountains of China (Yin et al. 1984; Chen and Yuan 2000). More than 3 × 108 m3 of Chinese white pines in China have been killed by D. armandi since the 1970s (Xie and Lv 2012). D. armandi mainly attacks healthy Chinese white pines older than 30 years (Chen and Tang 2007), and in recent years, D. armandi has begun to attack younger P. armandi (10–30 years) and another pine species, Pinus tabulaeformis (Chen et al. 2015). Dendroctonus armandi completes its entire lifespan under the bark of P. armandi with the exception of a brief dispersal period, during which adults fly out to find new host trees. Similarly to most Dendroctonus species, D. armandi females primarily attack a tree and use certain semiochemicals to attract both males and females (Wood 1982; Borden et al. 1986; Raffa et al. 1993; Liu et al. 2006; Pureswaran et al. 2008; Xie and Lv 2012). The semiochemical communication of bark beetles enables host and mate location, aggregation, and resource partitioning (Wood 1982; Borden et al. 1986). The aggregation pheromone is required to ensure the successful colonization and reproduction of bark beetles. Therefore, the study of semiochemicals could aid the protection of pine species and lower the primary beetle attack rate (Stephen 2001; Faccoli and Stergulc 2008; Blazenec and Jakus 2009).
The EAG responses of D. armandi to blended volatiles extracted from a host and certain synthetic terpenes showed significant variation with respect to different compound concentrations and the sex of the beetles (Wang et al. 2011a, b; Zhang et al. 2010, 2011). The effects of host volatiles from Chinese white pine, non-host volatiles and hindgut extracts of D. armandi on D. armandi were tested through laboratory olfactometer trials and field trapping experiments (Wu et al. 2012; Xie and Lv 2012; Zhao et al. 2014; Zhang et al. 2015; Chen et al. 2015). Some Dendroctonus species possess common pheromone components, although the sex of the species producing the pheromones and the functions of the components vary (Pitman et al. 1969). The semiochemical frontalin was first identified in male western pine beetles, Dendroctonus brevicomis, and was shown to serve as an aggregation pheromone component of the southern pine beetle, Dendroctonus frontalis, and the Douglas-fir beetle, Dendroctonus pseudotsugae (Silverstein et al. 1968; Bedard et al. 1970; Kinzer et al. 1969; Pitman and Vité 1970). Another semiochemical, exo-brevicomin, is produced by other Dendroctonus species (El Sayed 2013), some wood-boring Curculionidae (Fatzinger 1985; Perez et al. 1996) and Scolytinae (Francke et al. 1979; Camacho et al. 1993; Lindgren and Miller 2002), some Thanasimus predators (Zhou et al. 2001) and even the African elephant (Goodwin et al. 2006).
However, frontalin has not yet been detected in D. armandi, and a research study on frontalin in D. armandi is not found in the literature. Although exo-brevicomin has been detected in the aeration product of a P. armandi log subjected to natural D. armandi attacks and a hindgut extract of D. armandi females (mated and unmated) (Chen et al. 2015), the function of exo-brevicomin in D. armandi remains unclear. Moreover, only information regarding the details of the release and mechanisms of these semiochemicals is currently available. We identified two semiochemicals, frontalin and exo-brevicomin, by GC–MS and studied their biological roles in D. armandi by electrophysiological responses and laboratory olfactometer trials. These tests could provide a basis for future studies, suggesting that these compounds might be useful for beetle biocontrol.
Materials and methods
Insects
Two groups of bark beetles were prepared, and the bark beetles belonging to Group 1 were subjected to GC–MS analysis. The beetles belonging to Group 2 were subjected to electroantennography (EAG) and Y-tube assays in the laboratory.
Group 1: Beetles were collected from newly invaded Chinese white pines from the Qinling Mountains, Shaanxi, China (33°18′–33°28′N, 108°21′–108°39′E, elevation = 1450–1800 m) from June 25 to July 10, 2015 during a search for new invasive beetles from new invasive trees. The intrusion locations were marked, and the time points at which the beetle’s trunk could be observed from outside as the beetles started to invade (but had not completely invaded) the bark of Chinese white pine were recorded. Forty-eight hours after the recording start time, half of these beetles were collected. If only one female beetle was included in the gallery, it was collected and recorded as a virgin female. If the tunnel included two or three beetles, the beetles were not collected. Ninety-six hours after the recording start time, the other half of the beetles were collected from the gallery. If two beetles were found in the gallery and were mating, they were recorded as a mated female beetle and a mated male beetle after their sexes were distinguished by listening for the stimulation produced by males (Xu et al. 2014). If only one female was found in the gallery, the beetle was not collected. The three types of bark beetles (virgin female, mated female and mated male) were divided into eight 50-mL glass vials. Each glass vial accommodated three bark beetles, and the heads of the glass vials were covered with plastic wrap, which was perforated to allow the bark beetles to breathe. The glass vials were immediately transported to the laboratory, and GC–MS tests were performed on the same day. Moreover, the frass at the entrance of the gallery was collected and divided into six glass vials (each vial contained 0.1 g).
Group 2: Logs of Chinese white pine infested with D. armandi from the southern slope of the middle Qinling Mountains (33°26′53.0″N, 108°28′48.3″E, at a mean altitude of 1500 m) were collected in November 2015. These logs were placed in a thin stainless-steel net (bore diameter ≤0.8 mm) in a greenhouse to allow the emergence of adult beetles and were watered daily to keep the log surfaces moist. Upon emergence, adult beetles were collected daily from these logs, transported to the laboratory, sorted by sex and stored at 4 °C on moist paper. Active beetles with intact antennae and legs were subjected to electrophysiological and Y-tube assays (Light 1983; Wood 1982).
Chemicals
The chemicals used for the EAG and laboratory bioassays were (±)-exo-brevicomin (>95% chemical purity) and frontalin (>95% c. p.), which were obtained from Contech Enterprises Inc., Delta, BC, Canada, and hexane (HPLC-certified), which was obtained from Sigma-Aldrich Co.
Collection and identification of volatiles
According to the procedures described by Pureswaran and Borden (2003) and Liu et al. (2013), the following three sets of beetles were sampled for pheromone analysis: (1) virgin females, (2) mated females, and (3) mated males. The volatiles released from these beetles were passively collected by headspace solid-phase microextraction (HS–SPME), which is the technique used for the extraction and concentration of volatile compounds (Chai et al. 2012; Keenan et al. 2012). Three beetles in a 50-mL vial were transported to the laboratory for the collection of volatile compounds. A SPME fiber coated with a 75-μm film thickness of divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) obtained from Supelco (Sigma-Aldrich, Bellefonte, PA, USA) was exposed to the headspace of each vial for 10 min. This fiber was selected for its suitability for gasses and compounds with a low molecular mass. Prior to use, the fiber was preconditioned in the injection port of the gas chromatography at 270 °C for 60 min according to the manufacturer’s instructions.
Extracts were analyzed using a DB-5 MS column (30 m × 0.25 mm × 0.25 μm) (Thermo Fisher Scientific Company). The SPME fiber was injected directly into the injection port at a temperature of 250 °C for 3 min. The temperature of the GC-oven was maintained at 40 °C for 2.5 min, increased to 240 °C at a rate of 6 °C/min and maintained at 240 °C for 10 min. The flow of helium (carrier gas) was 1.0 mL/min. Compounds were identified by a comparison of their retention times and mass spectra to those in the NIST and Varian libraries. Furthermore, the retention times and mass spectra of exo-brevicomin and frontalin detected from the GC–MS experiment were also compared with the purchased standards.
EAG assays
The test method used was that described by Zhang et al. (2010). Antennae were excised from the heads of D. armandi and mounted between two electrodes. The recording electrode was inserted into the distal edge of the club, and the indifferent electrode was inserted into the scape of the antenna. In the experiments, 20 μL of each test solution was absorbed onto a filter paper strip (5 × 50 mm) and placed into a Pasteur pipette (with a diameter of 10 mm and a length of 15 cm) to serve as an odor cartridge after the solvent (hexane) had completely evaporated from the paper. Controls consisting of a filter paper strip treated with 20 μL of hexane alone were prepared. A Pasteur pipette with a test compound was inserted into a small hole in the wall of a steel tube (with a diameter of 15 mm diameter and a length of 15 cm), which was connected to a stimulus air controller (model CS-05b, Syntech, the Netherlands) to deliver a constant flow of humidified air at a rate of 40 mL/min. The open end of the steel tube was positioned 10 mm from the antenna. During the stimulation, air at a rate of 20 mL/min was applied through the pipette into the main airflow for 0.2 s. An interval of at least 1 min between puffs was used to ensure complete antenna recovery. Serial dilutions of each compound from the lowest to the highest concentration were included (Delorme and Payne 1990; Den Otter et al. 1996). A hexane-only control was tested before and after each test compound. A male or female antenna was only tested with one type of compound (Zhang et al. 2010). A hexane-only control and a standard solution (1-hexanol at 1 ng/μL in hexane) were tested before and after each test pheromone. Each pheromone was tested on five male and five female beetles, and the trials with each EAG preparation were repeated at least five times.
Laboratory olfactometer trials
Bioassays were conducted in a glass Y-tube olfactometer (with a diameter of 35 mm, a length of 40 cm, and an inside angle of 120°) with airflow at a rate of 200 mL/min through each branch using the method described by Liu et al. (2013). Four sets of tests were performed. In the first set of tests, one chamber contained a filter paper that had been treated with hexane, and the other chamber contained a filter paper that had been treated with frontalin diluted in hexane (0.0025, 0.05, 0.5, or 5 ng/μL, 10 μL). In the second set of tests, one chamber contained a filter paper that had been treated with hexane, and the other chamber contained a filter paper that had been treated with exo-brevicomin diluted in hexane (0.0025, 0.05, 0.5, or 5 ng/μL, 10 μL). The third set of tests investigated the attraction of beetles to frontalin in hexane (0.05 or 0.5 ng/μL, 10 μL) compared with α-pinene, to frontalin in hexane (0.05 ng/μL, 10 μL) compared with frontalin in α-pinene (0.05 ng/μL, 10 μL), to frontalin in hexane (0.5 ng/μL, 10 μL) compared with frontalin in α-pinene (0.5 ng/μL, 10 μL), and to frontalin in α-pinene (0.05 or 0.5 ng/μL, 10 μL) compared with α-pinene. The above-mentioned α-pinene compound was used without prior dilution. The fourth set of tests investigated the attraction of beetles to exo-brevicomin in hexane (0.05 or 0.5 ng/μL, 10 μL) compared with α-pinene, to exo-brevicomin in hexane (0.05 ng/μL, 10 μL) compared with exo-brevicomin in α-pinene (0.05 ng/μL, 10 μL), to exo-brevicomin in hexane (0.5 ng/μL, 10 μL) compared with exo-brevicomin in α-pinene (0.5 ng/μL, 10 μL), and to exo-brevicomin in α-pinene (0.05 or 0.5 ng/μL, 10 μL) compared with α-pinene. The above-mentioned α-pinene compound was used without dilution, and as the control at the dose of 10 μL. Approximately 30 min before each trial, adult beetles were introduced into a separate holding tube and exposed to the test odors before being released. Moreover, 10 μL of each test chemical was placed on a filter paper strip (5 × 50 mm) and allowed to evaporate for 20 s, and the filter paper was then placed into a holding chamber. In each trial, a solitary beetle was released into the stem of the Y-tube and was given 10 min to respond. When the beetle walked 5 cm past the Y junction, its selection of the left or right branch of the olfactometer was noted. To eliminate directional bias, the treatments associated with the right and left branches of the olfactometer were exchanged after every trial, and the Y tubes were cleaned with 100% alcohol before reuse. The olfactometer was maintained at a temperature of 25 °C and a relative humidity of 70% during the trials. At least 30 females and 30 males were tested in each trial.
Statistical analysis
The EAG responses were corrected for solvent and other background effects by subtracting the mean response to the solvent-only controls before and after exposure to each sample from the response to the test compound. To compensate for the decline in the sensitivity of the antennae during the experiment and individual differences in the test insects, the EAG data were standardized by calculating the EAGs as percentages relative to the response to the standard solution, which allowed direct comparisons of the responses obtained from different preparations. Mann–Whitney tests were performed using SPSS (1999) to determine the significance of differences in the relative EAG responses between the sexes. Chi square tests were performed using SPSS (1999) to assess the differences in the responses to the different compounds measured through the olfactometer bioassays.
Results
Collection and identification of volatiles
As identified by GC–MS, eight major volatile compounds (α-pinene, frontalin, exo-brevicomin, trans-verbenol, verbenone, myrtenal, myrtenol, and myrtanol) were released by D. armandi (Table 1). The experimental results showed that the presence/absence of frontalin, exo-brevicomin and myrtenal significantly differed between the sexes. Frontalin was detected in three of eight groups of virgin females and in all eight tested groups of mated males. Frontalin was not detected in any of the eight groups of mated females tested. exo-Brevicomin was detected in six of eight groups of virgin females and in seven of eight groups of mated females. exo-Brevicomin was not detected in any of the eight groups of mated males tested. Myrtenal was detected in three of eight groups of virgin females and in one of eight groups of mated females. Myrtenal was not detected in any of the eight groups of mated males tested. In addition, α-pinene, trans-verbenol, verbenone, myrtenol and myrtenol were emitted by both female and male beetles might function as pheromones.
EAG assays
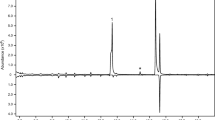
The EAG results revealed that frontalin and exo-brevicomin elicited olfactory responses from D. armandi as follows (Fig. 1). The antennae of male adult D. armandi were more sensitive to frontalin at dose of 0.05, 1, 10 and 100 ng (0.0025, 0.05, 0.5 and 5 ng/μL, 20 μL). Mann–Whitney tests showed significant differences (p ≤ 0.01) between the sexes in the antennal responses to 10 ng frontalin (0.5 ng/μL, 20 μL) (P 0.0025 ng/μL = 0.117, P 0.05 ng/μL = 0.076, P 0.5 ng/μL = 0.009, P 5 ng/μL = 0.117). The antennae of female adult D. armandi were more sensitive to exo-brevicomin at dose of 1, 10 and 100 ng (0.05, 0.5 and 5 ng/μL, 20 μL). (p ≤ 0.01) (P 0.0025 ng/μL = 0.175, P 0.05 ng/μL = 0.009, P 0.5 ng/μL = 0.009, P 5 ng/μL = 0.009).
Laboratory olfactometer trials
Y-tube olfactometer assays with frontalin showed that males were more attracted to frontalin at certain concentrations (0.05 and 0.5 ng/μL, 10 μL) than to the hexane control (Table 2). Instead, females were not attracted to frontalin at any concentration tested. The comparison of α-pinene and frontalin as stimuli in the choice assay revealed that males were significantly more attracted to frontalin than α-pinene (Table 3), and females were not. The mixture of frontalin with α-pinene showed that both females and males preferred the frontalin + α-pinene blend over α-pinene alone.
The Y-tube olfactometer assays with various concentrations of exo-brevicomin showed that females were more attracted to certain concentrations of exo-brevicomin (0.05 ng/μL, 10 μL) and repelled it when the concentration of exo-brevicomin raised to 0.5 ng/μL, 10 μL (Table 4), whereas males were not attracted to any concentration of exo-brevicomin tested. The comparison of α-pinene and exo-brevicomin as stimuli in the choice assay showed that females reacted significantly more to exo-brevicomin (0.05 and 0.5 ng/μL, 10 μL) than α-pinene (Table 5), males were more attracted to exo-brevicomin at 0.5 ng/μL, 10 μL. The mixture of exo-brevicomin with α-pinene revealed that the reactions of the males to the exo-brevicomin + α-pinene blend (0.05 and 0.5 ng/μL, 10 μL) and α-pinene were not significantly different, whereas females preferred α-pinene compared with the exo-brevicomin + α-pinene blend at a concentration of 0.5 ng/μL, 10 μL.
Discussion
We identified frontalin and exo-brevicomin in the volatile compounds produced by male and female D. armandi by GC–MS and demonstrated that the antennae of both sexes showed an electrophysiological response to frontalin and exo-brevicomin. We also confirmed that males exhibited a dose-dependent behavioral response to frontalin and frontalin + α-pinene, and females to frontalin + α-pinene and exo-brevicomin in olfactometer bioassays. Dendroctonus spp. are known to use semiochemicals and host volatiles to colonize hosts and attract mates (Wood 1982; Schlyter and Birgersson 1999; Byers and Zhang 2012). Semiochemicals might be a key characteristic trait that allows beetles to overcome the defense system of host trees, making it a necessary trait for the success of mass attacks by Dendroctonus spp, such as the synergism between semiochemicals and host volatiles in D. valens, D. ponderosae and D. brevicomis (Chen et al. 2015). The bicyclic acetals frontalin, exo- and endo-brevicomin and the hydroxylation products of α-pinene are often identified as pheromones of Dendroctonus spp. (Gary et al. 2010).
Recent GC–MS analyses have mainly focused on the hindgut, fat body, insect odor collection and host logs odor collection (Chen et al. 2015; Xie and Lv 2012; Liu et al. 2013; Song et al. 2014), and the beetles are usually collected after long-term indoor feeding; as a result, the experimental results might yield differences compared with beetles collected from the field. This manuscript describes the first experimental GC–MS and HS-SPME analysis of invaded Dendroctonus spp. collected from the field to more accurately reveal the pheromones released by D. armandi in nature. The GC–MS experiment aimed to compare the differences in the presence or absence of pheromones between the sexes. The results of the GC–MS analysis of D. armandi pheromones show that the release of frontalin and exo-brevicomin differs between sexes and over time. Frontalin and exo-brevicomin might play a significant role in aiding the invasion of D. armandi. What is more, because frontalin and exo-brevicomin have similar material structures and play an important role as pheromones in Dendroctonus spp., so this study focused on exploring the functions and roles of frontalin and exo-brevicomin in D. armandi. The identity and function of trans-verbenol and verbenone were characterized by EAG, Y-tube assays and field trapping (Zhao et al. 2017). The properties and functions of myrtenal, myrtenol and myrtanol need to be further studied.
Frontalin was first identified in a virgin female and was not identified in mated females. After mating, females constructed egg galleries, and males were found to be responsible for the release of frontalin + α-pinene to attract more beetles (Table 1). For convenience, we divided the process of semiochemical release into two stages: (1) after a successful attack, females released semiochemicals to attract more female and male beetles, and (2) after mating, females and males released semiochemicals to adjust the population density. The EAG dose–response curve and laboratory olfactometer trials of D. armandi to frontalin showed that male beetles were more sensitive to frontalin than female beetles (Fig. 1; Tables 2, 3). Although females were not attracted to frontalin at all tested concentrations (Table 2), the response of females and the control group to frontalin supplemented with α-pinene presented significant differences (p ≤ 0.05; Table 3). Females were attracted to the mixture of frontalin and α-pinene. α-Pinene was the main volatile (accounting for 63.18%) in host Chinese white pine (Chen et al. 2015), which was also accounting for >30% in the volatile of D. armandi and accounting for >90% in the frass (Table 1). So frontalin will have chance to co-work with α-pinene in a natural environment. The Y-tube experimental results (Table 3) revealed that frontalin + α-pinene played an important role in attracting females and males. Collectively, these lines of evidence supported our conclusion that frontalin worked together with α-pinene as an aggregation pheromone released by virgin females to attract females and males during the first stage. Males were more attracted to low concentration frontalin (Table 2) and no response to high concentration frontalin. Virgin females released low concentration frontalin (2.18 ± 2.54%) (Table 1), low concentration frontalin single might play a role as a sex pheromone to attract males during the first stage. Still frontalin + α-pinene became an aggregation pheromone released by mated males to attract more females and males in the second stage. High concentration frontalin (5.31 ± 5.18%) (Table 1) was released by mated males, which lost its attraction for males at this time. Frontalin has been identified as an anti-aggregation pheromone in certain Dendroctonus species and an aggregation pheromone in others (Borden 1985). In D. valens, frontalin is only produced by females as an aggregation and sex pheromone (Liu et al. 2013), and frontalin is an anti-aggregation pheromone in D. ponderosae (Conn et al. 1984; Hunt et al. 1986; Greis et al. 1990; Pureswaran et al. 2000). The experimental results reveal that the role of frontalin in D. armandi is adverse to that in D. ponderosae. In contrast with D. valens, mated male D. armandi also release frontalin during the second stage. D. armandi beetles are the pioneering invaders of healthy Chinese white pine and might, therefore, need more companions to overcome the defense of host trees than D. valens. D. armandi males might release the aggregation pheromone frontalin + α-pinene to attract more males and females, which might help them overcome the defense reactions of the host. The biggest difference maybe was that frontalin worked together with α-pinene as an aggregation pheromone in D. armandi, while frontalin alone played its role in the D. valens and D. ponderosae. The function and presence/absence of frontalin in D. armandi, D. valens and D. ponderosae are different, and these differences might be related to the physiological adaptations of these species to different habitats, as well as their specific life cycles and specific behavior habits.
The release of semiochemicals by bark beetles usually occurs through frass, as has been observed for D. brevicomis, Dendroctonus micans, D. ponderosae, D. valens and Dendroctonus rufipennis (Silverstein et al. 1968; Meurisse et al. 2008; Nancy et al. 2006; Borden et al. 1996). The present experimental results, frontalin was detected in frass collected from galleries of D. armandi (Table 1), supported frontalin as one semiochemical of D. armandi.
The collection and identification of volatiles revealed that exo-brevicomin was released by females. endo-Brevicomin is produced by male D. ponderosae following a pattern parallel to that found for exo-brevicomin (Pureswaran et al. 2000), but GC–MS experiments have not detected endo-brevicomin in the volatiles from D. armandi. The similar results were detected in Chen’s experiment (Chen et al. 2015). Moreover, α-pinene, a host monoterpene, preferred over exo-brevicomin. α-Pinene has been found to be attractive for female and male D. armandi, as determined through electroantennography, and has been used as a lure pheromone for trapping D. armandi (Zhang et al. 2010; Xie and Lv 2012). The response of both sexes to exo-brevicomin determined through olfactometer bioassays and EAG experiments revealed that females were more sensitive to exo-brevicomin. Our research study provides evidence that exo-brevicomin plays a role as an aggregation pheromone in females or as spacing factor signaling that “the tree is blank” or “the tree is full” to other females to adjust the population numbers. In addition, exo-brevicomin is an aggregation pheromone component in D. ponderosae (Conn et al. 1984; Hunt et al. 1986; Greis et al. 1990; Pureswaran et al. 2000). Prior to our study, exo-brevicomin was detected in the aeration product of P. armandi logs subjected to natural D. armandi attacks and the hindgut extract of females (Chen et al. 2015). However, the mating status of females was not distinguished, and the hindgut extract of males was not investigated, resulting in an inexplicit role of exo-brevicomin. This conclusion could be inferred because only female D. armandi were thought to produce volatiles as pheromone candidates, and the mating status of female beetles were not considered. However, male beetles could also release the pheromone, and the difference due to mating status was found to be significant. A similar pattern was found for frontalin, which is released by males and not released by mated females. Moreover, field-trapping experiments of D. armandi were performed (Chen et al. 2015), and the role of exo-brevicomin in un-distinguished sexes remains unclear. Further field-trapping experiments are needed to determine the role of exo-brevicomin.
In summary, this manuscript describes the first detection and behavioral analysis of frontalin and exo-brevicomin as possible semiochemicals of D. armandi, shedding light on the biological activities and roles of frontalin and exo-brevicomin through electrophysiological and laboratory olfactometer trials. Our results demonstrate that frontalin might play a role as a sex semiochemical and work together with α-pinene as aggregation pheromone, and provide evidence that exo-brevicomin serves as an aggregation and anti-aggregation pheromone in female D. armandi. Further studies should be performed to confirm the behavioral activity of both sexes of D. armandi to these two semiochemicals.
References
Bedard WD, Silverstein RM, Wood DL (1970) Bark beetle pheromones. Science 167:1638–1639
Blazenec M, Jakus R (2009) Effect of (+)-limonene and 1-methoxy-2-propanol on Ips typographus response to pheromone blends. J For Res 20:37–44
Borden JH (1985) Aggregation pheromones. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology, biochemistry and pharmacology. vol. 9, University of Texas Press, Austin, Texas, pp 257–285
Borden JH, Hunt DWA, Miller DR, Slessor KN (1986) Orientation in forest coleoptera: an uncertain outcome of responses by individual beetles to variable stimuli. In: Payne TL, Birch MC, Kennedy CEJ (eds) Mechanisms in insect olfaction. Oxford University Press, New York, pp 97–110
Borden JH, Gries G, Chong LJ, Werner RA, Holsten EH, Wieser H, Dixon EA, Cerezke HF (1996) Regionally-specific bioactivity of two new pheromones for Dendroctonus rufipennis (Kirby) (Col, Scolytidae). J Appl Entomol 120:321–326
Byers JA, Zhang QH (2012) Chemical ecology of bark beetles in regard to search and selection of host trees. In: Liu TX, Le K (eds) Recent advances in entomological research. Springer, New York, pp 150–190
Camacho A, Pierce JH, Borden J (1993) Geometrical and optical isomerism of pheromones in two sympatric Dryocoetes species (Coleoptera: Scolytidae), mediates species specificity and response level. J Chem Ecol 19:2169–2182
Chai Q, Wu B, Liu W, Wang L, Yang C, Wang Y, Li S (2012) Volatiles of plums evaluated by HS-SPME with GC–MS at the germplasm level. Food Chem 130:432–440
Chen H, Tang M (2007) Spatial and temporal dynamics of bark beetles in Chinese white pine in Qinling Mountains of Shaanxi Province, China. Environ Entomol 36:1124–1130
Chen H, Yuan F (2000) Chinese white pine bark beetle ecosystem and integrated pest management in Qinling Mountain. China Forestry Publishing House, Beijing
Chen GF, Song YS, Wang PX, Chen JY, Zhang Z, Wang SM, Huang XB, Zhang QH (2015) Semiochemistry of Dendroctonus armandi Tsai and Li (Coleoptera: Curculionidae: Scolytinae): both female-produced aggregation pheromone and host tree kairomone are critically important. Chemoecology 25:135–145
Conn JE, Borden JH, Hunt DWA, Holman J, Whitney HS, Spanier OJ, Pierce HD, Oehlschlager AC (1984) Pheromone production by axenically reared Dendroctonus ponderosae and Ips paraconfusus (Coleoptera: Scolytidae). J Chem Ecol 10:281–290
Coulson RN, Stark RW (1982) Integrated management of bark beetles. In: Mitton JB, Sturgeon KB (eds) Bark beetles in North American conifers. University of Texas Press, Austin, pp 315–350
Delorme JD, Payne TL (1990) Antennal olfactory responses of black turpentine beetle, Dendroctonus terebrans (Olivier), to bark beetle pheromones and host terpenes. J Chem Ecol 16:1321–1329
Den Otter CJ, De Cristofaro A, Voskamp KE, Rotundo G (1996) Electrophysiological and behavioural responses of chestnut moths, Cydia fagiglandana and C. splendana (Lep., Tortricidae), to sex attractants and odours of host plants. J Appl Entomol 120:413–421
El Sayed AM (2013) The pherobase: database of insect pheromones and semiochemicals. http://www.pherobase.com/database/compound/compoundsdetail-1R5S7R-exo-brevicomin.php. Accessed 27 Sept 2013
Faccoli M, Stergulc F (2008) Damage reduction and performance of mass trapping devices for forest protection against the spruce bark beetle, Ips typographus (Coleoptera Curculionidae Scolytinae). Ann For Sci 65:309
Fatzinger CW (1985) Attraction of the black turpentine beetle (Coleoptera: scolytidae) and other forest Coleoptera to turpentine-baited traps. Environ Entomol 14:768–775
Francke W, Hindorf G, Reith W (1979) Alkyl-1,6-dioxaspiro[4.5]-decanes—a new class of pheromones. Sci Nat 66:618–619
Furniss RL, Carolin VM (1977) Western forest insects USDA forest service, miscellaneous publications no. 1339, US Government Printing Office, Washington, DC
Gary JB, Rubi FT, Mory AW, Song MM, Andrew G, Nicole L, Abbott EC, Claus T (2010) Pheromone production in bark beetles. Insect Biochem Mol Biol 40:699–712
Goodwin T, Eggert M, House S, Weddell M, Schulte B, Rasmussen LEL (2006) Insect pheromones and precursors in female African elephant urine. J Chem Ecol 32:1849–1853
Greis G, Leufven A, LaFontaine JP, Pierce HD, Borden JH, Vanderwel D, Oehlschlager AC (1990) New metabolites of α-pinene produced by the mountain pine beetle, Dendroctonus ponderosae (Coleoptera: Scolytidae). Insect Biochem 20:365–371
Hunt DWA, Borden JH, Pierce JHD, Slessor KN, King GGS, Czyzewska EK (1986) Sex-specific production of ipsdienol and myrcenol by Dendroctonus ponderosae (Coleoptera: Scolytidae) exposed to myrcene vapors. J Chem Ecol 12:1579–1586
Keenan DF, Brunton NP, Mitchell M, Gormley R, Butler F (2012) Flavour profiling of fresh and processed fruit smoothies by instrumental and sensory analysis. Food Res Int 45:17–25
Kinzer GW, Fentiman AGJ, Page TFJ, Foltz RL, Vitéitoltz RL, Viti JP (1969) Bark beetle attractants: identification, synthesis and field bioassay of a new compound isolated from Dendroctonus. Nature 211:477–478
Light DM (1983) Sensitivity of antennae of male and female Ips paraconfusus (Coleoptera: Scolytidae) to its pheromone and other behavior-modifying chemicals. J Chem Ecol 9:585–606
Lindgren BS, Miller DR (2002) Effect of verbenone on five species of bark beetles (Colopetera: Scolytidae) in lodgepole pine forests. Environ Entomol 31:759–765
Liu ZD, Zhang LW, Sun JH (2006) Attacking behavior and behavioral responses to dust volatiles from holes bored by the red turpentine beetle, Dendroctonus valens (Coleoptera: Scolytidae). Environ Entomol 35:1030–1039
Liu ZD, Xu BB, Miao ZW, Sun JH (2013) The pheromone frontalin and its dual function in the invasive bark beetle Dendroctonus valens. Chem Senses 38:485–495
Meurisse N, Couillien D, Grégoire JC (2008) Kairomone traps: a tool for monitoring the invasive spruce bark beetle Dendroctonus micans (Coleoptera: Scolytinae) and its specific predator, Rhizophagus grandis (Coleoptera: Monotomidae). J Appl Ecol 45:537–548
Nancy EG, John DS, Donald RO, Jeffrey NW, Gary OF, Sylvia RM, David LW (2006) Verbenone-releasing flakes protect individual Pinus contorta trees from attack by Dendroctonus ponderosae and Dendroctonus valens (Coleoptera: Curculionidae, Scolytinae). Agric For Entomol 8:243–251
Perez AL, Gries R, Gries G, Oehlschlager AC (1996) Transformation of presumptive precursors to frontalin and exo-brevicomin by bark beetles and the west Indian sugarcane weevil (Coleoptera). Bioorg Med Chem 4:445–450
Pitman GB, Vité JP (1970) Field response of Dendroctonus pseudotsugae (Coleoptera: Scolytidae) to synthetic frontalin. Ann Entomol Soc Am 63:661–664
Pitman GB, Vité JP, Kinzerb GW, Fentiman AF (1969) Specificity of population-aggregating pheromones in Dendroctonus. J Insect Physiol 15:363–366
Pureswaran DS, Borden JH (2003) Test of semiochemical mediated host specificity in four species of tree killing bark beetles. Environ Entomol 32:963–969
Pureswaran DS, Gries R, Borden JH, Pierce HD (2000) Dynamics of pheromone production and communication in the mountain pine beetle, Dendroctonus ponderosae Hopkins and the pine engraver, Ips pini (Say) (Coleoptera: Scolytidae). Chemoecology 10:153–168
Pureswaran DS, Hofstetter RW, Sullivan BT (2008) Attraction of the southern pine beetle, Dendroctonus frontalis, to pheromone components of the western pine beetle, Dendroctonus brevicomis (Coleoptera: Curculionidae: Scolytinae), in an allopatric zone. Environ Entomol 37:70–78
Raffa KF, Phillips TW, Salom SM (1993) Strategies and mechanisms of host colonization by bark beetles. In: Schowalter TD, Filip GM (eds) Beetle-pathogen interactions in conifer forests. Academic, New York
Schlyter F, Birgersson G (1999) Forest beetles. In: Hardie J, Minks AK (eds) Pheromones in non-lepidopteran insects associated with agricultural plants. CAB International, Oxford, pp 113–148
Silverstein RM, Brownlee RG, Bellas TE, Wood DL, Browne LE (1968) Brevicomin: principal sex attractant in the frass of the female western pine beetle. Science 159:889–891
Song MM, Gorzalski A, Nguyen TT, Liu XB, Jeffrey C, Blomquist GJ, Tittiger C (2014) exo-Brevicomin biosynthesis in the fat body of the mountain pine beetle, Dendroctonus ponderosae. J Chem Ecol 40:181–189
Stephen C (2001) Review of the operational IPM program for the southern pine beetle. Integr Pest Manag Rev 6:293–336
Wang RL, Yang W, Yang ZZ, Chen XP, Yang CP, Li Q, Li F, Chen CM (2011a) Electroantennographic and behavioral responses of Dendroctonus armandi (Coleoptera: Ipidae) to host plant volatiles. Chin J Ecol 30:724–729
Wang RL, Yang W, Yang ZZ, Chen X, Wang XW, Yang CP (2011b) Electroantennographic and behavioral responses of Dendroctonus armandi (Tsai et Li) to 9 plant volatiles. For Pest Dis 30:23–26
Wood DL (1982) The role of pheromones, kairomones and allomones in the host selection and colonization of bark beetles. Annu Rev Entomol 27:411–446
Wu SP, Chen H, Wu Q (2012) Volatile compounds in the frass of adult Chinese white pine beetle. J Northwest For Univ 27:111–116
Xie SA, Lv SJ (2012) An improved lure for trapping the bark beetle Dendroctonus armandi (Coleoptera: Scolytinae). Eur J Entomol 109:569–577
Xu BB, Liu ZD, Sun JH (2014) The effects of α-pineneinene on the feeding performance and pheromone production of Dendroctonus valens. Entomol Exp Appl 150:269–278
Yin HF, Huang FS, Li ZL (1984) Coleoptera: Scolytidae. Economic insect fauna of China, vol 29. Science Press, Beijing, pp 26–35
Zhang LL, Chen H, Ma C, Tian ZQ (2010) Electrophysiological responses of Dendroctonus armandi (Coleoptera: Curculionidae: Scolytinae) to volatiles of Chinese white pine as well as to pure enantiomers and racemates of some monoterpenes. Chemoecology 20:265–275
Zhang LL, Chen H, Chen X (2011) Effect of Dendroctonus armandi infection on the volatile constituents of Pinus armandi in Qinling Mountains. J Northwest For Univ 26:114–118
Zhang WP, Chen ZH, Xie SA, Lv SJ, Liu SP, Li H (2015) Electroantennographic and behavioral responses of Dendroctonus armandi to nonhost volatiles. J Northwest For Univ 30:147–152
Zhao MZ, Chen H, MA C (2014) Behavioral responses of Dendroctonus armandi (Coleopteran: Curculionidae: Scolytidae) to volatiles of Chinese white pine. J Northwest For Univ 29:115–119
Zhao MZ, Dai LL, Sun YY, Fu DY, Chen H (2017) The pheromone verbenone and its function in Dendroctonus armandi (Coleoptera: Curculionidae: Scolytinae). Eur J Entomol 114:53–60
Zhou J, Ross DW, Niwa CG (2001) Kairomonal response of Thanasimus undatulus, Enoclerus sphegeus (Coleoptera: Cleridae), and Temnochila chlorodia (Coleoptera: Trogositidae) to bark beetle semiochemicals in eastern Oregon. Environ Entomol 30:993–998
Acknowledgements
We acknowledge the financial support provided by the National Natural Science Foundation of China (31670658) and the Program for Changjiang Scholars and Innovative Research Team at the University of China (IRT1035).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Michael Heethoff.
Rights and permissions
About this article
Cite this article
Zhao, M., Dai, L., Fu, D. et al. Electrophysiological and behavioral responses of Dendroctonus armandi (Coleoptera: Curculionidae: Scolytinae) to two candidate pheromone components: frontalin and exo-brevicomin. Chemoecology 27, 91–99 (2017). https://doi.org/10.1007/s00049-017-0235-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-017-0235-3