Abstract
In the olive fruit fly, Bactrocera oleae, females attract males by producing 1,7-dioxaspiro[5.5]undecane (olean), the main component of the sex pheromone secreted by rectal glands. It has been recently demonstrated that males are able to produce (Z)-9-tricosene (muscalure) in rectal glands, a compound that selectively attracts females. In this study, a male grooming reaction that may transfer the male-borne compounds from rectal to urotergal glands was observed, suggesting that urotergal glands could be involved in B. oleae sexual communication. GC/MS, EAG, GC/EAD analyses and behavioural assays were carried out to compare the role of male rectal and urotergal glands during courtship. In both male glands, olean and muscalure amounts were age dependent. Extracts of rectal glands contained higher amounts of olean and/or muscalure than urotergal ones. Extracts of rectal and urotergal glands of males and females elicited EAG responses in both sexes. GC/EAD showed that female EAG response to male rectal extracts was mainly due to olean and muscalure. Synthetic compounds evoked EAG dose-dependent responses in both sexes, and the EAG response to muscalure was higher as compared to olean. Rectal and urotergal glands from old males were able to attract females, while urotergal glands from young males attracted only males. Overall, our results add knowledge to the mating system of B. oleae, giving first evidences on the electrophysiological activity of muscalure towards both sexes, as well as on the involvement of male urotergal glands in the chemical sexual communication of this pest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The olive fruit fly, Bactrocera oleae (Rossi), is a key pest of olives in many countries of the Mediterranean basin (Daane and Johnson 2010). In recent years, this species was discovered in Southern California and then spread widely throughout the State, posing a serious threat to the olive industry (Rice et al. 2003). For table olives, the oviposition punctures lead to a serious reduction in crop value. In olives cultivated for oil production, the damage consists mainly of premature fruit drop and a lower quality of the oil (Tzanakakis 2006; Gucci et al. 2012). B. oleae females are oligogamous and mate 1–3 times during their lifetime (Cavalloro and Delrio 1970; Zouros and Krimbas 1970) while males are polygamous and mate daily if receptive females are available (Zervas 1982). In laboratory and field conditions, matings occur in the late afternoon or at dusk, indicating that there is a daily rhythm in sexual activities (Mazomenos 1989). The sequence of events leading to copulation can be divided into three main phases: first, a mate-searching phase during which visual and olfactory cues play an important role, which culminates with the male’s arrestment near the female; secondly, a close-range phase, in which the female mating decision is affected by the quality of male wing vibrations; and thirdly a final contact phase, with male copulation attempts and leg scrubs, in which tactile cues probably dominate (Benelli et al. 2012).
Olfactory cues from both sexes are known to be crucial in B. oleae courtship and mating. Schultz and Boush (1971) have described glands associated with the rectal ampulla of olive fruit fly males and it has been suggested that these glands are involved in the production and storage of the male sexual pheromone (De Marzo et al. 1978). This gland-like complex is also present in olive fruit fly females (Economopoulos et al. 1971). It is commonly named as “rectal gland” and consists of a reservoir occupying the right lateral posterior of the rectal sac and a small bulbar secretory sac opening into the reservoir at its base. The whole gland complex is lined with large prominent columnar epithelial cells, which are continuous with the epithelial lining of the anterior portion of the rectal sac. Both sexes store the glandular secretion in a special gland reservoir (i.e. a modification of the epithelial wall of the rectum) prior to releasing them outside through the anal opening (Economopoulos et al. 1971). In B. oleae females, the main component of the secretion produced by glands associated with the rectal ampulla was identified as 1,7-dioxaspiro[5.5]undecane (olean, hereafter) by Baker et al. (1980). Furthermore, it has been reported that B. oleae females produce a multi-component sex pheromone containing four constituents with a synergistic action: olean and methyl dodecanoate, produced in the rectal glands, and α-pinene and nonanal, produced elsewhere in the insect body (Mazomenos and Haniotakis 1981, 1985). Anyway, olean has been reported as the most abundant component and exhibited the highest biological activity toward males in laboratory and field experiments (Mazomenos and Haniotakis 1981, 1985). In contrast, in the majority of Tephritidae, sex attractants are produced exclusively by males (Kitching et al. 1989; Fletcher and Kitching 1995; Wicker-Thomas 2007). Interestingly, olean has been also isolated from glands associated with the rectal ampulla of B. oleae males and it does not attract the females, whereas it is able to attract sexually mature males (Mazomenos and Pomonis 1983; Mazomenos 1989). Canale et al. (2012) have shown that both sexes of B. oleae are able to produce olean, from the 1st day after emergence and this production has been found as a function of age. In females, olean content is greater than in males, and it is unceasingly detectable at least until the 45th day of life. For males, the amount of olean reaches the maximum when gonad maturation was complete (5–8 days old), thereafter decreasing to 0 by the 11th day of life (Canale et al. 2012). Levi-Zada et al. (2012) have confirmed the age-related olean emission in both sexes, also showing a characteristic diurnal release pattern.
Recently, olfactometer assays have highlighted that extracts of B. oleae male bodies are highly attractive to virgin females (Mavraganis et al. 2010). A further research on rectal gland extracts of B. oleae adult males lead to the first identification of a substance unique to males and able to attract females (Z)-9-tricosene (muscalure, hereafter) (Carpita et al. 2012). In laboratory bioassays, the pure compound, as well as the male rectal gland extracts, is able to selectively attract the females, giving the first evidence of a male-produced female attractant in B. oleae and pointing out the need to deeply examine the role of female attraction to male-borne odours. Furthermore, preliminary ethological observations conducted on B. oleae have suggested that the urotergal glands, two clearly visible glandular areas in the fifth urotergite of both sexes, could play a role in the olfactory communication of the olive fruit fly during courtship and mating. In B. oleae, these glandular structures produce a secretion accumulating at the base of the bristles that are associated with the glands themselves (Raspi et al. 1997). However, the whole chemical composition of the urotergal glands secretion is currently unknown. In 15 days old B. oleae males a peculiar grooming reaction has been observed during courtship in connection with secretions from both rectal and urotergal glands. The males periodically rub a hind tarsus (1st tarsomere) on the distal tip of the abdomen, in close proximity of the anus. Subsequently, the male rubs the hind tarsus over the urotergal glands area. We hypothesize that this behaviour could be functional to transfer the rectal glandular content on the urotergal area.
Here, we present results from our research aimed at investigating the role of B. oleae male-borne compounds in mating behaviour and testing the hypothesis of an involvement of the urotergal glands in the sexual communication system of this species. In this study, we investigated: (1) the electroantennographic (EAG) activity of rectal and urotergal gland extracts towards both sexes, (2) the gaschromatographic–electroantennographic detection (GC/EAD) response of B. oleae female to male rectal gland extracts, (3) the presence of (Z)-9-tricosene and 1,7-dioxaspiro[5.5]undecane in rectal and urotergal glands of B. oleae males, as a function of age, (4) the EAG activity of pure muscalure and olean towards both sexes of olive fruit fly, (5) the bioactivity of male urotergal and rectal glands in vivo towards both sexes, (6) the above-mentioned male grooming reaction, through high-speed video recordings.
Materials and methods
Insect rearing
Insects used in this study were obtained from pupae collected in a Tuscan olive-mill during late December 2010. Pupae were then maintained in a laboratory in Pisa, under controlled conditions (22 °C ± 1, 50–60 % R.H. and natural photoperiod) to wait for adult emergence. To obtain coeval virgin specimens, within 24 h of emergence flies were separated according to sex and singly placed in clean glass vials (diameter: 10 mm, length: 50 mm). Olive fruit fly adults were fed on a dry diet (yeast extract and sucrose mixture, at ratio 1:10 w/w), while water was provided separately on a cotton wick (Canale and Benelli 2012).
Glands extraction
Prior to glandular dissection, individuals were anaesthetized using CO2 and maintained at −18 °C for 5–10 min. To remove both rectal and urotergal glands, we proceeded as follow. First, using a stereomicroscope, in each specimen the rectal ampulla was dissected out by pulling off the terminalia with forceps. Subsequently, the urotergal glands were removed in toto by micro-dissection of the glandular area in question, thereby avoiding contamination with the rectal content. To obtain glandular extracts, rectal and urotergal glands from 10 specimens were immediately immersed in 140 μL hexane for at least 2 h. Both extracts were stored at −20 °C until needed.
Electroantennography (EAG)
The antennal response of virgin male and female B. oleae (7 days old) to extracts from rectal or urotergal glands of virgin males and females (7 days old) was evaluated according to the technique described in the previous studies (Rotundo et al. 2001; Germinara et al. 2009). Extracts were prepared as described above and then concentrated to a volume of 100 μl. To obtain dose–response curves from both sexes, decimal hexane solutions from 0.001 to 10 μM of muscalure or olean were also prepared and stored at −20 °C until needed. A male or female fly was inserted in a plastic pipette tip (0.1 ml) whose end was properly cut to allow the insect head to protrude. Two glass electrodes filled with 0.1 M KCl saline solution were used. The indifferent electrode was inserted into the head at the base of the antennae and the recording electrode was put in contact with the tip of an antenna. AgCl-coated silver wires were used to maintain the electrical continuity between the antennal preparation and an AC/DC UN-6 amplifier in DC mode connected to a PC equipped with the EAG 2.0 program (Syntech Laboratories, Hilversum, The Netherlands). Stimuli were 10 μl (1 insect equivalent) of a gland extract or a hexane solution of a pure compound applied to a filter paper (Whatman no. 1, Brentford, UK) strip (1 cm2) inserted into Pasteur pipettes (15 cm long). Stimuli were blown by a disposable syringe into a constant stream of charcoal-filtered humidified air (500 ml/min) flowing in a stainless steel delivery tube (1 cm diameter) with the outlet positioned at approximately 1 cm from the antenna. During 1 s, 2.5 cm3 of vapour from an odour cartridge were added.
Control (10 μl of hexane) and standard (10 μl of a 100 μg/μl hexanal solution) stimuli were applied at the beginning of the experiment and after each group of 4 test stimuli. Intervals between stimuli were 30 s. In dose–response experiments, stimuli were applied in ascending doses, whereas in the other experiments they were randomly sequenced. The amplitude (mV) of the EAG response to each test stimulus was adjusted to compensate for solvent and/or mechanosensory artefacts by subtracting the mean EAG response of the two nearest hexane controls (Raguso and Light 1998). To compensate for the decrease in the antennal responsiveness during the experiment, the resulting EAG amplitude was corrected according to the reduction in the EAG response to the standard stimulus (Den Otter et al. 1991). Each extract or test solution of a pure compound was tested on five antennae of different males and females.
Within each sex, one-way analysis of variance (ANOVA) followed by Tukey’s-HSD test was used to rank the mean EAG responses to different extracts. In dose–response curves, the activation threshold was considered to be the lowest dose at which the lower limit of the standard error of the mean response was greater than the upper limit of the standard error for the lowest dilution tested (Sant’ana and Dickens 1998). Saturation level was taken as the lowest dose at which the mean response was equal to or less than the previous dose (Germinara et al. 2009). In each sex, mean EAG responses to the same dose of olean and muscalure were compared using Student’s t test.
Gas chromatography–electroantennographic detection (GC/EAD)
To evaluate the antennal capability of B. oleae females to detect the presence of (Z)-9-tricosene in the male rectal glands, GC/EAD analyses were carried out using antennae from 7 days old virgin females and a rectal gland extract from 7 days old virgin males. The gland extract was prepared as above and concentrated to a 10 μl volume. One μl of extract (1 gland equivalent) was injected in a Fisons (Milan, Italy) 9000 series chromatograph equipped with a splitless injection system and a SPB-5 capillary column (30 m × 0.32 mm i.d., 0.25 μm film thickness) (Supelco Inc., Bellefonte, PA, USA). Conditions were carrier gas, helium at 20 psi; oven program, 60 °C for 2 min, 10 °C/min to 270 °C; injector and detector temperature, 260 °C. To prevent condensation during cooling, a universal ‘X’ Press Tight® connector (Restek Corporation, Bellefonte, PA, USA) was used to dilute the column effluent with 15 ml/min helium, supplied through a make-up inlet of the GC, and to split it 1:1 between the flame ionization detector and EAG antennal preparation (see above). Electroantennographic detection responses were amplified (50×) with an AC/DC UN-6 amplifier in DC mode (Syntech Laboratories, Hilversum, The Netherlands).
Gaschromatography (GC) and gaschromatography–electron impact mass spectrometry (GC/EI-MS)
To study the presence of muscalure and olean in rectal and urotergal glands of B. oleae males as a function of age, extracts from rectal to urotergal glands of virgin males at 1, 3, 6, 9, 11, 13, 16, and 20 days after emergence (starting from early February 2011) were analysed by GC and GC/EI-MS. For each gland type and age, three extracts (each from 10 virgin males) were examined.
GC analyses were performed using a Dani GC 1000 instrument with PTV injectors, equipped with a Dani DDS 1000 data station and two bonded FSOT column (Dani DN-5 and Dani DN-20, both 30 m × 0.25 mm i.d.). GC/MS analyses were performed with a mass selective detector 5973 Network interfaced with an Agilent Technologies 6890N Network GC system, equipped with an Agilent HP-5MS bonded FSOT column (30 m × 0.25 mm i.d.). Both GC and GC/MS analyses of extracts were carried out under splitless conditions, injecting 3 μl of hexane extract and using helium as carrier gas (1 ml/min); usually, the oven temperature was programmed as follows: 1 min at 40 °C, to 250 °C at 10 °C/min, 5 min at 250 °C, to 280 °C at 20 °C/min, 30 min at 280 °C. To compare retention times, a slower temperature program was also used (1 min at 40 °C, to 200 at 10 °C/min, to 280 at 6 °C/min, 30 min at 280 °C).
Compounds were identified comparing their mass spectra and retention times to those of commercial or synthesized standards. Olean was purchased from Sigma-Aldrich®, muscalure was synthesized in our laboratory following exactly the procedure reported by Fisher and Tyman (1998): it was 99 % chemically and stereoisomerically pure. The quantifications of olean and (Z)-9-tricosene were performed by GC, using absolute calibration curves obtained with pure olean or (Z)-9-tricosene, respectively (Carpita et al. 2012).
Behavioural assays
The attractiveness of male rectal and urotergal glands was evaluated in a two-choice bioassay by using a Plexiglas unit (15 × 15 × 1.5 cm) as a still-air arena. In the centre of this unit, there was a circular chamber (i.e. the specimen release chamber, diameter 4 cm) connected to two other identical chambers by means of two linear paths (2 cm in length, 1 cm in width), forming a 90° angle. The top of the arena was covered with of a removable panel of glass. At the beginning of the tests, an individual was gently transferred to the release chamber using a glass vial and released on the floor of the chamber. The choice for a given cue was recorded if the fly moved to the cue within 3 min after being released and if it engaged in searching behaviours on the chosen cue for at least 30 s.
The attractiveness of rectal glands from virgin, 5 or 15 days old males, to virgin, 15 days old females and males, was evaluated by crushing 2 or 5 rectal glands on a filter-paper dish (diameter: 1.5 cm) then placed in a side chamber of the arena. An equal clean filter paper dish was placed in the other side chamber, and a single individual of B. oleae was exposed in the release chamber. The same method was followed for testing the attractiveness of 5 and 15 days old male urotergal glands. In this case 5 or 10 glands were extracted as described above and crushed on filter paper. In all bioassays, with each new specimen, the arena was rotated clockwise 90° to avoid positional effects. Moreover, the relative position of the sources was randomised, to avoid that one source was always on the right and the other on the left (Benelli et al. 2013a). Between each behavioural treatment, the odour-cleaning procedure was as follows: the Plexiglas® arena (and the glass lid) was first washed for about 30 s with hexane, then with warm water at 35–40 °C, thus cleaned in a water bath with mild soap for about 5 min, rinsed with hot water for about 30 s, and finally rinsed with distilled water at room temperature (Carpita et al. 2012; Benelli and Canale 2013a). After 10 replicates, the glands were renewed. Individuals that did not make any choice (i.e., the fly remains steadfast in the release chamber and does not show any particular behaviour) were excluded.
For each bioassay, 60 replicates were performed, a χ 2 test (with Yates’s correction) was used to evaluate behavioural data from two-choice bioassays, and a probability of 0.05 was considered (Sokal and Rohlf 1981). For each replicate, each fly was replaced by a new one of the same age. The assays were carried out over several days to account for any daily variability. All experiments were performed between 15.30 and 19.30 and conducted in a room uniformly lit with daylight fluorescent tubes (Philips 30 W/33). Light intensity, measured inside the Plexiglas cage, was approximately 1,000 lux (estimated over the 300–1,100 nm waveband using a LI-1800 spectroradiometer LI-COR Inc., Lincoln, NE, USA equipped with a remote cosine receptor). The temperature was set at 23 °C ± 1, whereas the relative humidity was kept at 45 % ± 5.
Video recordings
B. oleae courtship behaviour was recorded with the HotShot® 512 SC high-speed video camera (NAC Image Technology Inc, Simi Valley, CA, USA). A young (5 days old) or old (15 days old) B. oleae virgin male was gently transferred in a testing arena containing ten virgin females (15 days old). Sequential images from each detail were recorded at a rate of 8,000 fps with an exposure time of 0.125 ms (Benelli et al. 2013b, c; Bonsignori et al. 2013). The HotShot® 512 SC video camera stored images with a resolution of 512 × 256 pixels directly to its internal memory. Then, these images were downloaded into a dedicated computer for data analysis. The area in which insects were expected to perform mating behaviour was lightened with four LED illuminators (RODER SRL, Oglianico, TO, Italy) that emit light (420 lm each) at λ = 628 nm. The red light has been chosen both because it matches the maximum absorption frequency of the camera and because it does not damage the visual apparatus of the insects, not possessing receptors for that wavelength (Briscoe and Chittka 2001). Both for young and adult males, twenty male courtships were filmed and analysed through frame-by-frame analysis, to describe the peculiar male grooming reaction from rectal to urotergal glands.
Results
EAG responses to crude extracts
Rectal and urotergal gland extracts of B. oleae males and females elicited measurable EAG responses in both sexes of the same species. Significant differences were found among EAG responses of males (F = 9.92; df = 3; P < 0.001) and females (F = 29.30; df = 3; P < 0.001) to different stimuli (Fig. 1). Male EAG responses to male and female rectal gland extracts were similar and significantly higher than those to the corresponding urotergal gland extracts. The highest female EAG response was elicited by the female rectal gland extracts, followed by the male rectal gland extracts. These responses were significantly higher than those to the urotergal gland extracts of the corresponding sex.
Chemical and electrophysiological analyses
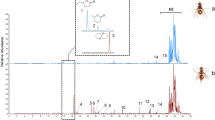
In GC/EAD analysis, female antennae consistently detected two active peaks in rectal gland extracts from 7 days old males at the retention times of (A) 8.22 min and (B) 22.90 min, respectively (Fig. 2). Comparative GC/MS analyses showed that retention times and fragmentation patterns of compounds A and B precisely matched those of synthetic olean and muscalure, respectively. Both chemicals were present also in the urotergal glands of coeval B. oleae males.
It was almost evident that in urotergal and rectal glands the amounts of olean and muscalure varied according to the fly’s age. Under laboratory conditions, olive fruit fly males begin to produce detectable olean in rectal glands from the 1st day after emergence (Fig. 3a), then the amount of olean reached a maximum when gonad maturation was complete (5–8 days old), thereafter decreasing to 0 by the 11th day of life. Muscalure production in urotergal and rectal glands of males was low until the 7th day of life, thereafter increasing until the 21st day (Fig. 3b). Moreover, both the muscalure and olean production in rectal glands were relatively higher than in urotergal glands, suggesting that the rectal ampulla may be the site where these compounds are produced.
Bactrocera oleae a quantification of 1,7-dioxaspiro[5.5]undecane (olean) in glands associated with the rectal ampulla (olean R) and in urotergal glands (olean T) of virgin males; b quantification of (Z)-9-tricosene (muscalure) in glands associated with the rectal ampulla (muscalure R) and in urotergal glands (muscalure T) of virgin males. Values are mean ± SD from three replicates
EAG dose–response curves to synthetic compounds
The antennal sensitivity of B. oleae females and males towards increasing concentrations of muscalure and olean are reported in Fig. 4. Both compounds elicited EAG dose-dependent responses. In females, the activation threshold in response to muscalure (0.01 μM) was lower that to olean (0.1 μM). In males, the activation threshold was 0.1 μM for both compounds. In females, the EAG response to muscalure was significantly higher than to olean from 0.01 to 1 μM (t = 2.41 to 4.25; df = 14; P < 0.05) and significantly lower at 10 μM (t = 3.77; df = 14; P < 0.05). In males, the EAG response to muscalure was significantly higher when compared with olean from 0.01 to 10 μM (t = 2.26 to 3.30; df = 14; P < 0.05).
Behavioural assays
As shown in Fig. 5, two male rectal glands (15 days old) were more attractive to B. oleae females than the control (χ 2 = 8.817, df = 1, P = 0.003), but a higher number of adult male rectal glands was not more attractive than the control. When concerning urotergal glands from adult males, it was observed that only ten glands were able to attract more females than the control (χ 2 = 4.817, df = 1, P = 0.028). Two and five rectal glands from young males attracted males (χ 2 = 3.750, df = 1, P = 0.052; χ 2 = 7.350, df = 1, P = 0.007, respectively) but not females. None of the tested glands from young males attracted females.
Attractiveness to 15 day-old Bactrocera oleae virgin a females or b males of 5 (young) and 15 (adult) day-old male rectal and urotergal glands crushed on a little dish of filter paper (diameter: 1.5 cm). Control clean filter paper; **significantly different at 0.01 probability level, *significantly different at 0.05 probability level, n.s. not significantly different (χ 2 test, Yates’s correction), 60 flies were tested for each treatment
Video recordings
The analysis of video recordings revealed a peculiar grooming reaction performed by B. oleae adult males during courtship (Fig. 6). A courting male vibrates its wings fast in a dorso-ventral fashion, and rolls them on their longitudinal axes. During wing vibration, it periodically rubs a hind tarsus on the distal tip of the abdomen, in close proximity of the anus, to imbue the tarsal brush of the 1st tarsomere with the rectal secretion. Subsequently, it rubs the tarsus on the urotergal area (see Electronic Supplementary Material “Video 1” for details). This behaviour was not observed in B. oleae young males.
The peculiar grooming reaction performed by Bactrocera oleae males during courtship: a courting males vibrate their wings fast in a dorso-ventral fashion, and rolling them on their longitudinal axes. b During wing vibration, it periodically rubs a hind tarsus on the distal tip of the abdomen, in close proximity of the anus, to imbue the tarsal brush of the 1st tarsomere with the rectal secretion. Subsequently c it rubs the tarsus on the urotergal area (see Electronic Supplementary Material “Video 1” for details)
Discussion
EAG experiments with crude extracts indicated the presence of EAG-active compounds in both rectal and urotergal glands of B. oleae males and females, the EAG activity of rectal gland extracts being generally higher than that of urotergal ones. GC/EAD results highlighted that the main chemicals responsible of the electrophysiological activity elicited by the male rectal gland secretion were olean and muscalure. Moreover, EAG dose–response experiments clearly indicated that the peripheral olfactory systems of both sexes are able to perceive olean and muscalure in a wide range of doses, which was almost similar for both compounds. The electrophysiological activity of olean, as well as other three compounds, α-pinene, nonanal and ethyl dodecanoate, has been proved also by Van Der Pers et al. (1984) on B. oleae males and females. Even if the presence of α-pinene and nonanal was never confirmed in B. oleae females, ethyl dodecanoate was recently found as a minor component in the rectal glands of virgin females (Carpita et al. 2011). Concerning (Z)-9-tricosene, our results reported for the first time its electrophysiological activity towards both sexes of B. oleae and chemical analysis confirmed that its production in rectal glands was related to the male’s age (Carpita et al. 2012).
From a behavioural point of view, the bioactivity of male rectal glands in vivo was demonstrated. It was observed that two rectal glands from sexually mature males (15 days old) were able to exert attraction towards females, because they contain muscalure. A larger number of glands were not more attractive than the control, probably because the high concentration of muscalure inside the arena saturates the female’s antennal receptors. Furthermore, rectal glands from young males (5 days old) attracted other males, since they contain olean, but they did not show any attraction towards virgin females. Indeed, B. oleae males were able to produce olean in their rectal glands from the 1st day after emergence, and this production was a function of age. According to Canale et al. (2012), it was noted that the olean content in the rectal ampulla of young males reached a maximum in coincidence with the sexual maturity and decreased to 0 by the 11th day of life. The production of female-typical chemical compounds in young males is not uncommon among Diptera (Curcillo and Tompkins 1987; Vaias et al. 1993). The role of olean secreted by B. oleae males is still unclear. Mazomenos and Pomonis (1983) reported that the high synthesis of olean by wild males during June and July may suggest that olean produced by males acts as a male aggregation signal, but this hypothesis was never verified. Recently, Benelli et al. (2013c) have ruled out that the production of olean in young males may be a case of female chemical mimicry, because young males do not have a mating advantage over older ones, as already noted for other insect species (Ruther and Steiner 2008; Benelli and Canale 2012; 2013b). It seems that young males are really perceived as females by older males, and it cannot be excluded that the B. oleae males could benefit indirectly from the olean production before being sexually mature by distracting competitors away from females. After having reached sexual maturity, B. oleae males do not need to produce the female scent and stop its biosynthesis (Benelli et al. 2013c).
First evidences about the involvement of male urotergal glands in the chemical sexual communication of B. oleae were provided. It was demonstrated that the presence of muscalure on urotergal glands and the trend of its amount per individual was strictly related to its production in the rectal ampulla. The urotergal glands from adult males attracted females, as a result of their muscalure content. This attractiveness appears to be dose-dependent, since only ten urotergal glands from sexually mature males were able to induce attraction towards virgin females. The presence of (Z)-9-tricosene could be explained as a consequence of the transfer of the rectal content to urotergal glands through the peculiar leg rubbing showed by frame-by-frame analysis of courtship video recordings. The transfer of muscalure on the urotergal areas could be functional to enhance its release. In fact, the wing vibrations performed contemporarily to the above-mentioned leg-rubbing may allow the spreading of muscalure towards conspecifics. Our hypothesis is substantiated by several findings on other tephritid species (Shelly and Kaneshiro 1991). In Anastrepha suspensa (Loew), it has been observed that wing vibrations are performed in connection with pheromone emission to attract females (Webb et al. 1976). Similarly, in the Mediterranean fruit fly, Ceratitis capitata (Wiedemann), the male produces a droplet of scent substances from the anus and then starts wing vibration behaviour (Briceño et al. 1996). On the other hand, urotergal glands from young B. oleae males (5 days old) did not attract other males. Indeed, they contained very small amounts of olean, and it was consistently found in their rectal glands. The lack of olean on the urotergal glands of young males could be due to the absence of the peculiar leg-rubbing behaviour among these males as well as to the extreme volatility of this compound.
Overall, we believe that our findings can contribute to a more detailed understanding of the mating system of the olive fruit fly. Further research is needed to evaluate the attractiveness of (Z)-9-tricosene in field and semi-field trials, as well as to provide a detailed chemical characterization of the other compounds that are present in B. oleae urotergal and rectal glandular secretions (Carpita et al. 2011).
References
Baker R, Herbert RH, Howse PE, Jones OT, Francke W, Reith W (1980) Identification and synthesis of the major sex pheromone of the olive fly, Dacus oleae. J Chem Soc 1:52–54
Benelli G, Canale A (2012) Do Psyttalia concolor (Hymenoptera: Braconidae) males gain in mating competitiveness from being courted by other males while still young? Entomol Sci 15:257–260
Benelli G, Canale A (2013a) Do tephritid-induced fruit volatiles attract males of the fruit flies parasitoid Psyttalia concolor (Szépligeti) (Hymenoptera: Braconidae)? Chemoecology. doi:10.1007/s00049-013-0127-0
Benelli G, Canale A (2013b) Male-male sexual behaviour in the parasitic wasp Psyttalia concolor. J Ins Sci 13:25. Available at http://www.insectscience.org/13.25
Benelli G, Canale A, Bonsignori G, Ragni G, Stefanini C, Raspi A (2012) Male wing vibration in the mating behavior of the olive fruit fly Bactrocera oleae (Rossi) (Diptera: Tephritidae). J Ins Behav 25:590–603
Benelli G, Pacini N, Conti B, Canale A (2013a) Following a scented beetle: larval faeces as a key olfactory cue in host location of Stegobium paniceum (Coleoptera: Anobiidae) by Lariophagus distinguendus (Hymenoptera: Pteromalidae). Chemoecology. doi:10.1007/s00049-012-0125-7
Benelli G, Bonsignori G, Stefanini C, Dario P, Canale A (2013b) Male wing fanning performance during successful and unsuccessful mating in the parasitic wasp Lariophagus distinguendus Förster (Hymenoptera: Pteromalidae). J Ins Behav 26:228–237
Benelli G, Bonsignori G, Stefanini C, Raspi A, Canale A (2013c) The production of female sex pheromone in Bactrocera oleae (Rossi) young males does not influence their mating chances. Entomol Sci 16:47–53
Bonsignori G, Stefanini C, Scarfogliero U, Mintchev S, Benelli G, Dario P (2013) The Green Leafhopper, Cicadella viridis (Hemiptera, Auchenorrhyncha, Cicadellidae), jumps with near-constant acceleration. J Exp Biol 216:1270–1279
Briceño D, Ramos D, Eberhard W (1996) Courtship behaviour of male Ceratitis capitata (Diptera: Tephritidae) in captivity. Fla Entomol 79:130–143
Briscoe AD, Chittka L (2001) The evolution of color vision in insects. Annu Rev Entomol 46:471–510
Canale A, Benelli G (2012) Impact of mass-rearing on the host-seeking behaviour and parasitism by the fruit fly parasitoid Psyttalia concolor (Szépligeti) (Hymenoptera: Braconidae). J Pest Sci 85:65–74
Canale A, Carpita A, Conti B, Canovai R, Raspi A (2012) Effect of age on 1,7-dioxaspiro-[5.5]-undecane production in both sexes of olive fruit fly, Bactrocera oleae (Rossi) (Diptera Tephritidae). IOBC Bull 72:219–225
Carpita A, Canale A, Benelli G, Conti B, Raspi A (2011) Componenti secondari presenti nelle ghiandole associate all’ampolla rettale delle femmine di Bactrocera oleae (Rossi). XXIII Congresso Nazionale Italiano di Entomologia. Genova, Italy, 13–16 Giugno 2011, Atti p 169
Carpita A, Canale A, Raffaelli A, Saba A, Benelli G, Raspi A (2012) (Z)-9-tricosene identified in rectal gland extracts of Bactrocera oleae males: first evidence of a male-produced female attractant in olive fruit fly. Naturwissenschaften 99:77–81
Cavalloro R, Delrio G (1970) Rilievi sul comportamento sessuale di Dacus oleae (Gmelin) (Diptera, Tripetidae) in laboratorio. Redia LII:201–230
Curcillo PG, Tompkins L (1987) The ontogeny of sex appeal in Drosophila melanogaster males. Behav Genet 17:81–86
Daane KM, Johnson MW (2010) Olive fruit fly: managing an ancient pest in modern times. Annu Rev Entomol 55:151–169
De Marzo L, Nuzzaci L, Solinas M (1978) Studio anatomico, istologico, ultrastrutturale e fisiologico del retto ed osservazioni etologiche in relazione alla possibile produzione di feromoni sessuali nel maschio di Dacus oleae (Gmelin). Entomologica, XIV:203–266
Den Otter CJ, Tchicaya T, Schutte AM (1991) Effects of age, sex and hunger on the antennal olfactory sensitivity of tsetse flies. Phys Entomol 16:173–182
Economopoulos AP, Gianakakis A, Tzanakakis ME, Voyatzoglou A (1971) Reproductive behaviour and physiology of the olive fruit fly. 1. Anatomy of the adult rectum and odours emitted by adults. Ann Entomol Soc Am 64:1112–1116
Fisher IG, Tyman JHP (1998) The synthesis of long chain dialkylalkynes, dialkyldiynes and their hydrogenation to monoenes and dienes. Synth Comm 28:1323–1338
Fletcher MT, Kitching W (1995) Chemistry of Fruit Flies. Chem Rev 95:789–928
Germinara GS, De Cristofaro A, Rotundo G (2009) Antennal olfactory responses to individual cereal volatiles in Theocolax elegans (Westwood) (Hymenoptera: Pteromalidae). J Stored Prod Res 45:195–200
Gucci R, Caruso G, Canale A, Loni A, Raspi A, Urbani S, Taticchi A, Esposto S, Servili M (2012) Changes in free acidity, peroxide value and phenolic compounds of olive oils obtained from fruits (cv. Frantoio) damaged by Bactrocera oleae (Rossi). HortScience 47:301–306
Kitching W, Lewis JA, Perkins MV, Drew R, Moore CJ, Schurig V, König WA, Francke W (1989) Chemistry of fruit flies. Composition of the rectal gland secretion of (male) Dacus cucumis (Cucumber fly) and Dacus halfordiae. Characterization of (Z, Z)-2,8-Dimethyl-1,7-dioxaspiro 5.5]undecane. J Org Chem 54:3893–3902
Levi-Zada A, Nestel D, Fefer D, Nemni-Lavy E, Deloya-Kahane I, David M (2012) Analyzing diurnal and age-related pheromone emission of the olive fruit fly, Bactrocera oleae by sequential SPME-GCMS analysis. J Chem Ecol 38:1036–1041
Mavraganis VG, Papadopoulos NT, Kouloussis NA (2010) Extract of olive fruit fly males (Diptera: Tephritidae) attract virgin females. Entomol Hell 19:14–20
Mazomenos BE (1989) Dacus oleae. In: Helle W Word Crop Pests (ed) Vol 3A: Fruit Flies: their biology, natural enemies and control. p 169
Mazomenos BE, Haniotakis GE (1981) A multicomponent female sex pheromone of Dacus oleae Gmelin: isolation and bioassay. J Chem Ecol 7:1561–1573
Mazomenos BE, Haniotakis GE (1985) Male olive fruit fly attraction to synthetic sex pheromone components in laboratory and field tests. J Chem Ecol 11:397–405
Mazomenos BE, Pomonis JG (1983) Male olive fruit fly pheromone: isolation, identification and lab-bioassays. In: Cavalloro R Proceedings of the CEC/IOBC International Symposium “Fruit Flies of Economic Importance” (ed), Athens, Greece, 16–19 November 1982. Balkema, Rotterdam, pp 96–103
Raguso RA, Light DM (1998) Electroantennogram responses of male Sphinx perelegans hawkmoths to floral and ‘green leaf volatiles’. Entomol Exp Appl 86:287–293
Raspi A, Canale A, Lucchi A (1997) Preliminary observations on integumentary urotergal glands in Bactrocera oleae (Gmelin) (Diptera Tephritidae). Frustula Entomol XX:193–202
Rice RE, Phillips PA, Stewart-Leslie J, Sibbett GS (2003) Olive fruit fly populations measured in central and southern California. Calif Agric 57:122–127
Rotundo G, Germinara GS, De Cristofaro A, Rama F (2001) Identificazione di composti volatili in estratti da diverse cultivar di Olea europaea L. biologicamente attivi su Bactrocera oleae (Gmelin) (Diptera: Tephritidae). Boll Lab Entomol Agr Filippo Silvestri 57:25–34
Ruther J, Steiner S (2008) Costs of female odour in males of the parasitic wasp Lariophagus distinguendus (Hymenoptera: Pteromalidae). Naturwissenschaften 95:547–552
Sant’ana J, Dickens JC (1998) Comparative electrophysiological studies of olfaction in predaceous bugs, Podisus maculiventris and P. nigrispinus. J Chem Ecol 24:965–984
Schultz CA, Boush GM (1971) Suspected sex pheromone glands in three economically important species of Dacus. J Econ Entomol 64:347–349
Shelly TE, Kaneshiro KY (1991) Lek behavior of the Oriental fruit fly, Dacus dorsalis, in Hawaii (Diptera: Tephritidae). J Ins Behav 4:235–241
Sokal RK, Rohlf FJ (1981) Biometry. Freeman and Company, New York
Tzanakakis ME (2006) Insects and mites feeding on olive: distribution, importance, habits, seasonal development and dormancy. Brill Acad Publ Leiden
Vaias LJ, Napolitano LM, Tompkins L (1993) Identification of stimuli that mediate experience-dependent modification of homosexual courtship in Drosophila melanogaster. Behav Genet 23:91–97
Van der Pers JNC, Haniotakis GE, King BM (1984) Electroantennogram responses from olfactory receptors in Dacus oleae. Entomol Ellen 2:47–53
Webb JC, Sharp L, Chambers DL, McDow JJ, Benner JC (1976) Analysis and identification of sounds produced by the male Caribbean fruit fly, Anastrepha suspensa. Ann Entomol Soc Am 69:415–420
Wicker-Thomas C (2007) Pheromonal communication involved in courtship behaviour in Diptera. J Ins Physiol 53:1089–1100
Zervas GA (1982) Reproductive physiology of Dacus oleae (Gmel.) (Diptera: Trypetidae). Comparison of a wild and artificially reared flies. Geoponica 282:10–14
Zouros E, Krimbas CB (1970) Frequency of male bigamy in natural population of the olive fruit fly Dacus oleae as found by using enzyme polymorphism. Entomol Exp Appl 13:1–9
Acknowledgments
We thank two anonymous reviewers for their insightful comments on an earlier version of the manuscript, Helen Romito and Marco Colucci (Sant’Anna School of Advanced Studies, Pisa) for proofreading the English, Francesco Lanzo (University of Pisa) for the creation of artworks, Giacomo Ragni (The BioRobotics Institute, Sant’Anna School of Advances Studies, Pisa) for technical support during high-speed video recordings, Giulia Fiore and Giulia Giunti (University of Pisa) for their kind assistance during manuscript preparation. This research was financially supported by M.I.U.R. (Ministry of Education, University and Research, Italy)—P.R.I.N. 2007.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MPG 7380 kb)
Rights and permissions
About this article
Cite this article
Canale, A., Germinara, S.G., Carpita, A. et al. Behavioural and electrophysiological responses of the olive fruit fly, Bactrocera oleae (Rossi) (Diptera: Tephritidae), to male- and female-borne sex attractants. Chemoecology 23, 155–164 (2013). https://doi.org/10.1007/s00049-013-0131-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-013-0131-4