Abstract
By reacting to local environmental stimuli, ant workers excavate a nest that offers suitable climatic conditions. Workers may face increasing CO2 levels while digging across the soil profile, and it is an open question whether these levels are used as cues during nest excavation. Here, we explored the influence of different underground CO2 concentrations on digging behavior in the leaf-cutting ant Acromyrmex lundii. We first quantified digging rates and transport of excavated material as a function of CO2 levels, ranging from atmospheric values to 11% CO2. The mass of both the excavated soil and the pellets transported out of the digging chamber were quantified. CO2 preferences for excavation were investigated in a second experiment, in which workers were presented with a choice of two digging sites that differed in their CO2 levels, ranging from atmospheric to 4% CO2, and the mass of excavated material was quantified. Digging rates were similar for all tested CO2 levels up to 7%, and only significantly lower for 11%. The transport of excavated soil increased with increasing CO2 levels up to 7%, and then decreased at 11%. Workers preferred digging at 1% CO2 and avoided levels of 4%. We suggest that the observed CO2 preferences are likely driven by the requirements of their symbiotic fungus, and could be one of the reasons why colonies of A. lundii excavate superficial nest chambers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Social insects build the most complex nests in the animal kingdom. While building is a costly process, it is advantageous for the inhabiting colony. Erecting a barrier between the colony members and the physical environment provides a better defense against predators, helps to organize workers’ tasks and dampens climatic fluctuations to some degree (Hansell 1984). Excavating underground nests, as colonies of many ant species do, further aids climatic control. Climatic conditions underground, while still influenced by precipitation and solar radiation, fluctuate less and are not characterized by extreme high and low temperature or humidity conditions as aboveground.
Despite the complex nature of social insect nests, they result from simple responses of building workers to local biotic or abiotic stimuli (Deneubourg and Franks 1995; Theraulaz et al. 1998; Bonabeau 1998), without workers having an overview of the complete process or a central control. For example, the presence of other nestmates as biotic cues triggers nest digging behavior in ants, and decreasing local worker density leads to cessation of excavation (Deneubourg and Franks 1995; Rasse and Deneubourg 2001). Other biotic stimuli help to intensify the excavation process. In the leaf-cutting ant Acromyrmex lundii, locally-deposited brood and symbiotic fungus increase worker density at these sites as they are attractive to workers (Römer and Roces 2014). Vibrational signals produced by digging workers attract more workers to the site, leading to stronger aggregation (Pielström and Roces 2012). Building can also be organized by stigmergic responses to the abiotic environment without a direct interaction among workers (Grassé 1959; Theraulaz and Bonabeau 1999). For example, the deposition of freshly excavated soil pellets can influence ant workers’ decision where to engage in excavation and it, therefore, spatially organizes building (Pielström and Roces 2013).
Microclimatic factors are important abiotic cues for nest building as nests should provide adequate temperature or humidity ranges for their inhabitants as well as for the performance of colony tasks, especially the raising of brood (Roces and Núñez 1989; Bollazzi and Roces 2002; Falibene et al. 2016), as it ensures colony growth. Excavating or enlarging a nest where proper conditions are encountered should provide the colony with an appropriate climate. Indeed, leaf-cutting ants show coinciding preferences for nest excavation (Bollazzi et al. 2008; Pielström and Roces 2014) and for brood and fungus rearing (Roces and Kleineidam 2000; Bollazzi and Roces 2002), i.e., between 20 and 30 °C and high soil moistures, well-suited for the development of the symbiotic fungus these ants grow (Quinlan and Cherrett 1978; Powell and Stradling 1986). As a general rule, climatic conditions underground fluctuate more strongly close to the soil surface, and temperature decreases and moisture increases with depth (Hillel 1998). To some degree, the microclimate of underground nests can also be adjusted by building responses, i.e., the opening and closing of nest openings to hinder airflows and to counteract humidity losses of the nest air (Bollazzi and Roces 2007).
Building an underground nest or controlling ventilatory airflows through building responses in an existing nest presents the colony with a problem. Depending on soil characteristics, the gas exchange may be hindered, leading to underground hypercapnic (high carbon dioxide) and hypoxic (low oxygen) conditions. Decaying organic material in the soil as well as the respiration of the inhabiting colony itself further increase the CO2 levels. Generally, the underground CO2 levels close to the soil surface are much higher than the atmospheric levels, currently around 0.04% CO2. Close to the surface of sandy soils, for instance, CO2 levels range from 0.2 to 0.4% CO2 (Tschinkel 2013), while levels of 1–2.7% CO2 can be detected in more organic soils (unpublished data). At deeper soil layers, levels of 5–7% can be reached (Kleineidam and Roces 2000; Bollazzi et al. 2012), as the underground CO2 concentration increases with depth (Schwartz and Bazzaz 1973; Hamada and Tanaka 2001).
Leaf-cutting ants are an ideal organism to study the potential influence of underground CO2 concentrations on nest excavation. They build the most complex nests among ants, with nests of some species having thousands of chambers and encompassing millions of individuals (Moreira et al. 2004a, b). In addition, they are the only insects known equipped with antennal sensilla that respond to changes and to absolute CO2 levels (Kleineidam and Tautz 1996; Kleineidam et al. 2000). High CO2 concentrations inside the nest may influence colony growth by negatively influencing the respiration rate of the fungal symbiont (Kleineidam and Roces 2000), the sole food source of the brood. In our recent study, we showed that workers of A. lundii avoided high CO2 concentrations to maintain their fungus, and rather preferred intermediate CO2 values as found at levels close to the soil surface (Römer et al. 2017).
As of yet, it is unclear whether long-term exposure to the CO2 levels encountered in ant nests might affect growth or development of their brood, as observed in some Lepidoptera (Coelho Junior and Parra 2013; Coelho Junior et al. 2017). Much higher CO2 values, as used for instance for pest control or anesthesia, can negatively affect not only brood development, but also some aspects of the adult physiology (Beckmann 1974; Nicolas and Sillans 1989; Guerenstein and Hildebrand 2008; Klaiber et al. 2013). Leaf-cutting ants show distinct CO2 preferences for the placement of their brood (unpublished data) and also show specific, regulatory building responses when constructing nest turrets in response to high CO2 levels inside nest tunnels (Halboth and Roces 2017).
Considering both the negative effect of CO2 on fungus growth and the observed CO2 preferences for the placement of fungus and brood inside the nest, leaf-cutting ants are expected to use CO2 as a cue when they excavate their nests. We, therefore, asked whether CO2 influences the digging behavior of workers of the leaf-cutting ant A. lundii, and whether workers select a digging site based on the prevailing CO2 levels. To answer the first question, we offered a group of workers single digging chambers with different CO2 concentrations, ranging from atmospheric values to 7% CO2. As leaf-cutting ants are tolerant to very low O2 concentrations (Hebling et al. 1992), we also asked whether they may still excavate at the extreme levels of 11% CO2, although such high levels are unlikely to occur in their nests. We quantified both the total amount of soil excavated at different CO2 levels and the removal of excavated soil pellets after 5 h. The selection of a digging site depending on the CO2 levels was explored by presenting workers with a binary choice setup offering two digging chambers with different CO2 concentrations. Here, atmospheric, 1% CO2 (as occurring at superficial soil layers) and 4% CO2 (as found at deeper soil layers) were offered, and the total amount of excavated soil was quantified.
Materials and methods
Study animals
All experiments were performed with worker groups of the leaf-cutting ant A. lundii. Founding colonies were collected near Montevideo, Uruguay, and raised in the Department of Behavioral Physiology and Sociobiology at the University of Würzburg, Germany. Colonies were kept under constant temperature (25 °C) and relative humidity (50%), under a 12:12 h LD cycle. At the time of the experiments, all colonies were mature and at least 4 years old. They consisted of eight nest boxes (19 × 19 × 9 cm) filled with symbiotic fungus, a feeding box and a box for waste disposal. Food (Rubus fruticosus leaves, diluted honey) and water were provided ad libitum.
Experimental setups and procedure
Effect of CO2 on digging rates
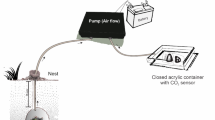
A digging chamber (9.5 × 9.5 × 3 cm) was connected to a second, open box (Fig. 1a; ant release box, 19 × 19 × 9 cm) by a piece of plastic tubing (diameter 1.5 cm). The back half of the digging chamber was filled with a clay–sand mixture (2:1, Claytec Baulehm gemahlen 0–0.5 mm, Viersen, Germany and Dorsilit Kristall-Quarzsand 0.1–0.5 mm, Hirschau, Germany; 16% water content) for excavation. To generate a constant CO2 concentration inside the digging box, a small rubber hose (diameter 0.3 cm) was inserted through the back-wall of the chamber and buried up to half-way into the clay. Extending from the rubber hose, a small tunnel was drilled into the clay through which the airstream was pumped (flow rate 50 ml min−1) into the empty part of the digging chamber. To prevent desiccation of the clay mixture by the inflowing air, the airstream was directed through a wash bottle filled with water before the hose was inserted into the arena. To maintain a stable CO2 concentration, the chamber air was pumped out (miniature vane pump, 135 FZ, Schwarzer Precision, Germany) at an equal flow rate of 50 ml min−1 through two small rubber hoses (diameter 0.3 cm) inserted in the entrance wall of the chamber. To quantify digging rates at atmospheric values (ca. 0.04% CO2), the standard laboratory compressed air was used as source. Elevated CO2 concentrations were generated by mixing compressed air with pure carbon dioxide using gas-mixing devices (Mass Flow Controller MFC-4 and Mass Flow Controller v1.0, Sable Systems International, USA).
Experimental setups a to quantify digging rates: digging chamber connected to an ant release box. Inflow of air with different CO2 concentrations through rubber hose inserted into the clay mixture through the back chamber wall. Outflow of air through hoses located in the front wall if the chamber, b choice of a digging site: two digging chambers connected by a Y-shaped tube to an ant release box. Inflow of air with different CO2 concentrations into the chambers through rubber hoses inserted at the bifurcation point of the Y-shaped tunnel leading to the nest chambers. The injected air left passively through the upper openings of the sliding wall
On the day of the experiment, 100 media-sized workers (mean body mass: 5.3 ± 1.2 mg SD, n = 80) were collected from 1 of 5 mother colonies. We started the assay only after the establishment of the chosen CO2 level inside the chamber (CO2 sensor: Gasmitter, Sensor Devices, Germany; range 0–10%, resolution: 0.01%), since a local CO2 stimulus could lead to ant aggregation at the spot and to subsequent digging activity because of the increased ant density, and not as a result of the offered CO2 concentration. Then, a group of 100 workers was placed into the ant release box from where ants could freely explore the whole setup and start to dig. After 5 h, all ants were removed and the total amount of excavated material was quantified by weighing the digging chamber, cleared of all excavated soil pellets, to the nearest 0.1 g (1 g of the clay mixture corresponds to an excavated volume of 0.41 cm3), and by subtracting the weight from the full arena weighed at the beginning of the assay. As leaf-cutting ants may not remove all excavated material from the digging site, but use it to subsequently reduce the created nest space (Römer and Roces 2015), we also separately quantified to the nearest 0.1 g the mass of excavated clay pellets carried out of the digging chamber.
Six series were performed. First, we quantified digging rates at atmospheric CO2 levels as a control baseline. Further, we assayed concentrations of 1 and 2%, to investigate the effects of levels measured close to the soil surface (unpublished data), where nest chambers of A. lundii are usually found (Zolessi and González 1978). Additionally, levels that occur at deeper soil layers, 4 and 7%, were tested. Finally, we assayed an extreme CO2 value of 11%, overreaching the highest levels measured around leaf-cutting ant nests, to test workers’ tolerance levels. We chose this level because workers of the mangrove-dwelling ant Camponotus anderseni switch to anaerobic respiration during nest flooding at this concentration (Nielsen et al. 2006). We had also used this concentration in previous unpublished studies on leaf-cutting ants and detected no signs of lethargy by workers during current tasks or deaths. It is important to indicate that the O2 concentrations of the CO2-enriched gas mixtures used in the present series only decreased by 3% over the assayed CO2 range, from 20.95% O2 at atmospheric CO2 levels, to 18.1% O2 at 11% CO2. Since leaf-cutting ant workers show respiratory regulation and are not affected by O2 levels down to 10% (Hebling et al. 1992), we are confident that any effect of the used gas mixtures should be attributed to the experimentally increased CO2 levels.
Choice of a digging site based on CO2 levels
Two digging chambers (9.5 × 9.5 × 3 cm) were connected to a third, open box (ant release box, 9.5 × 9.5 × 5.5 cm, Fig. 1b) by a Y-shaped plastic tube (diameter 1.7 cm). The back half of each chamber was filled with a clay–sand mixture (2:1) with a water content of 19%. A perforated, sliding wall was inserted in each lid of the digging chambers, effectively preventing initial digging but allowing the diffusion of volatiles from the clay mixture. For the establishment of the different CO2 concentrations in the digging chambers, specific CO2 mixtures were pumped into each of the two digging chambers through two small rubber hoses (diameter 0.3 cm; flow rate 50 ml min− 1), inserted into the Y-shaped tube at the bifurcation point and terminating in each chamber. Measurements of air humidity inside the digging chambers without ants revealed values between 70 and 90%, which prevented desiccation of the clay mixture.
Analogous to the previous experiment, 100 media-sized workers were collected from one of three mother colonies. When the chosen CO2 concentrations were established in both digging chambers, workers were placed into the ant release box. From there they had free access to both digging chambers, but were initially prevented from initiating excavation at once by the sliding walls in front of the clay mixture. After a familiarization period of 1 h, the sliding walls were lifted and ants could excavate for 3 h. Afterwards, the ants were removed and the total amount of excavated material was quantified to the nearest 0.1 g (1 g of the clay mixture corresponds to an excavated volume of 0.28 cm3). As the aim of this experiment was to determine worker preferences for the task of nest digging, pellet transport out of the digging arenas was not recorded.
We tested different combinations of three CO2 concentrations, namely atmospheric levels, 1% and 4%, as they occur at superficial and deep soil layers, respectively. Four different series were performed offering the following choices: atmospheric vs. atmospheric levels (control for side biases), atmospheric vs. 1% CO2, atmospheric vs. 4% CO2, and 1% CO2 vs. 4% CO2.
Data analysis
Normal distribution of data was tested using the Shapiro–Wilk test. Digging rates and pellet transport were compared using either ANOVA and Scheffé post hoc tests or the Kruskal–Wallis test and multiple Dunn comparisons with a Bonferroni–Holm correction as post hoc tests. Datasets of digging choices were compared using paired t tests. For the sake of homogeneity, data on digging rates and pellet transport are presented as boxplots in Fig. 2, although data were analyzed based on the outcome of the test of normal distribution. All graphs were created with SigmaPlot 10 (Systat Software, Inc., San Jose, California, USA) and later edited using Microsoft PowerPoint (Microsoft Corporation, Redmond, Washington, USA).
Digging behavior a mass of excavated material (in g) over 5 h, depending on the prevailing CO2 concentration: ANOVA and Scheffé post hoc tests, n = 20 per series, b excavated material removed from the chamber, depending on the CO2 concentration: Kruskal–Wallis test and multiple Dunn comparisons with Bonferroni–Holm correction as post hoc test, n = 20 per series, line: median, box: 25–75% percentiles, whiskers: min–max values, presentation without outliers, data groups sharing the same letters do not differ statistically, α ≤ 0.05
Results
Effect of CO2 on digging rates
Not all released ants were present in the digging arena during the experiment (usually between 25 and 40 workers), and not all ants present in the arena engaged in digging. Rather, workers moved between the release box and digging arena, so that a large proportion of ants was also present either in the connecting tube or in the release box at any given time. The total mass of material excavated over 5 h was similar in the range from atmospheric values to 7% CO2 (Fig. 2a; atmospheric: 21.17 ± 0.72 g mean ± SE, 1%: 23.6 ± 1.59 g, 2%: 23.03 ± 1.0 g, 4%: 23.09 ± 0.93 g, 7%: 21.76 ± 1.04 g; sample size of each group = 20). Only digging activity at the extremely high value of 11% CO2 (12.36 ± 0.84 g) was significantly lower (ANOVA: F = 16.19, p < 0.0001, Table 1).
The mass of excavated pellets removed from the digging site increased with increasing CO2 concentration. Only at 11%, at which a low digging rate was displayed as indicated above, did the amount of removed pellets significantly decrease (Fig. 2b; atmospheric: 1.6 g median, 1%: 5.95 g, 2%: 9 g, 4%: 12.45 g, 7%: 14.2 g, 11%: 9.15 g; sample size = 20 in each group). The statistical comparisons among levels indicated that pellet transport was comparable between atmospheric values and 1%, but increased significantly at 2% CO2. There were no differences between 1 and 2%, but pellet transport differed between 1 and 4% as well as 7%. Transport at 2% was significantly lower than at 7%, but comparable to 4%. In summary, most material was removed from the chambers at 4 and 7%, followed by 2 and 11% CO2 (Kruskal–Wallis test: H = 54.86, p < 0.00001; Table 1).
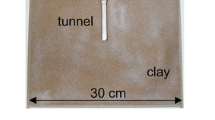
In relation to the total amount of material excavated (Fig. 2a), only 7% of the pellets were removed outside of the digging chamber at atmospheric values, while nearly 70% of them were removed at the highest levels of 7% CO2 and 11% CO2. Exemplary photos of digging chambers showing areas of excavation and pellets still left there at the end of the assay are presented in Fig. 3a-c.
Exemplary photos of digging chambers showing the excavated areas (upper chamber half) and the pellets remaining in the chamber (lower chamber half), a digging at atmospheric values, b digging at 4% CO2 concentration, c digging at 11% CO2 concentration, En = Entrance to chamber, gray arrow = direction of air inflow, black bar = 2 cm
Choice of a digging site based on CO2 levels
During this experiment, most workers were inside the digging chambers and only a small proportion was seen, at any given time, in the ant release box. Worker numbers in the chambers also fluctuated throughout the time of the assay. Similar to the first experiment, not all workers at the digging sites actually engaged in excavation. Workers showed no side preference when presented with the control choice situation offering atmospheric CO2 values at both digging chambers, i.e., digging activity was equally distributed (Fig. 4a; paired t test: t = − 2.04, n = 12, p = 0.07). More material was excavated under atmospheric conditions than in the chamber with elevated CO2 levels, whether these were 1 or 4%, yet the differences were not statistically significant (Fig. 4b, c; paired t test: atmosph. vs 1%. t = 1.32, n = 12, p = 0.21; atmosph. vs. 4%. t = 1.82, n = 12, p = 0.1). There was a significant preference when two different CO2 levels that occur underground were presented as choice, i.e., 1 vs. 4%. Workers excavated more in the chamber with 1% CO2, a level found at shallow soil strata, and avoided 4% CO2, a value expected to occur at deep soil layers (Fig. 4d; paired t test: t = 3.8, n = 12, p = 0.003).
Choice of a digging site based on CO2 levels, a atmospheric vs. atmospheric (control), b atmospheric vs. 1% CO2, c atmospheric vs. 4% CO2, d 1 vs. 4% CO2, expressed as excavated material per chamber after 3 h, n = 12 in each group, round symbols—means, whiskers—standard error, ns not significant, **p < 0.01
While no comparisons between the two experiments were planned because of their different aims, note that the measured digging rates at a given CO2 level largely differed between the experiments. For instance, an average of 5 g/h at atmospheric levels in Exp. 1—digging rates—(Fig. 2, left), and of 14 g/h (or a mean of 7 g/h in each of the two chambers at atmospheric levels, Fig. 4a) in Exp. 2—digging choices—were excavated. The higher digging rates in the choice experiment likely resulted from the higher water content of the offered clay mixture (19%, compared to 16% in the first experiment), which significantly increases both excavation speed and mass of the excavated pellets (Pielström and Roces 2014).
Discussion
Underground CO2 levels and digging rates
Digging rates of A. lundii were only negatively influenced by the very high CO2 concentration of 11%. Such high CO2 levels have never been measured in or around leaf-cutting ant nests, not even for deep nests of the genus Atta, which can reach 5–7 m (Moreira et al. 2004a) and levels of 6–7% CO2 (Kleineidam and Roces 2000; Bollazzi et al. 2012). It is unknown under which conditions leaf-cutting ants would face such high levels of 11%. It is also unclear whether such high levels lead to a reduction in individual digging performance, or in the number of ants that engage in digging because of individual differences in response thresholds. However, this shows that leaf-cutting ants are not only tolerant to decreasing levels of O2 down to 10% (Hebling et al. 1992), but also to high levels of CO2, and that they are still able to excavate to some degree and engage in transport under such high hypercapnic conditions.
Increased CO2 levels are known to act as a releaser of digging behavior in the ant Solenopsis geminata (Hangartner 1969). Our digging experiments did not reveal this effect, as digging rates at levels between 1 and 7% were similar to atmospheric levels. However, S. geminata workers were attracted to a local CO2 source and aggregated closely to such a source. The local increase of CO2 at the site could have led to a self-organized digging response triggered by the increased ant aggregation at the site, rather than a direct effect of CO2 on excavation activity. This is the reason why we started the assays only when the CO2 level had been established inside the digging chamber and, therefore, prevented workers to initially respond to the CO2 provided at the local outflowing site on the digging face.
Underground CO2 levels and the transport of excavated material
While there was no detectable effect of CO2 on digging rates at CO2 levels surrounding natural leaf-cutting ant nests, the same cannot be said about the effect on transport of the excavated soil pellets. Workers carried more of their excavated material outside of the digging chamber, the higher the CO2 concentration. Usually, not all excavated soil pellets are transported outside of the nest. A recent study found that when digging over 2 days, up to 30% of soil pellets is deposited inside the excavated nest space and reworked (Römer and Roces 2015), either to create narrow tunnels or reduce cavity space. Probably due to the short experimental time of 5 h, ants did not appear to use the material to build or form certain structures in the present experiments, and simply placed the excavated pellets on the ground a few centimeters behind the active digging zone.
As we did not observe individual workers, it is unclear whether all excavated pellets were deposited inside the digging chamber by the initial excavators and then picked-up and carried away by different ants, or if some, if not all excavators carried their pellets further away themselves. Pellet transport in leaf-cutting ants seems to be organized analogous to leaf-fragment transport into transport chains (Röschard and Roces 2003) where the original excavators only carry their loads a very short distance before dropping it, and short-distance and long-distance carriers pick the pellets up and transport them further away from the digging site (Pielström and Roces 2013).
Two scenarios may account for the increased removal of excavated material from the chamber with increasing CO2 concentration. CO2 could have, as mentioned above, a negative effect on digging activity, even at concentrations of 1–7%, and increasing numbers of ants could have stopped excavating, with increasing CO2 concentration. However, as workers were not individually observed, we cannot rule out that alternative workers gaining access to the digging face kept overall digging rates at high levels. Digging rates would only be noticeable reduced when not enough ants could replace the ones that gave up digging. This might have been the case at the CO2 level of 11%. Ants no longer engaging in excavation could then have switched to soil transport, so that an increasing number of workers transported soil pellets out of the digging chamber, with higher CO2 concentration. This would be an analogous behavioral switch known from foraging leaf-cutting ants, which change from leaf cutting to leaf transport when their mandibles are worn down and they are no longer physically able to cut leaves (Schofield et al. 2011). On the other hand, removing the pellets from the digging site could be a regulatory response at unsuitable, high CO2 concentrations aimed at generating more free space to increase local airflows and so to facilitate nest ventilation. As O2 values in our experiments only slightly decreased at high CO2 levels, as indicated above, the observed pellet transport is unlikely to be a response to changes in O2 values.
CO2 preferences for the selection of a digging site
Acromyrme lundii displayed CO2 preferences for the selection of a digging site, yet only when the offered concentrations in both chambers corresponded to values that occur underground. When one chamber offered atmospheric values as occurring aboveground, digging activity was equal in both chambers. This is not due to the inability of the ants to perceive the difference between the two CO2 concentrations, since leaf-cutting ants are able to detect very low absolute CO2 values (Kleineidam and Tautz 1996; Kleineidam et al. 2000). Also, a recent study showed that A. lundii does display CO2 preferences in experiments with atmospheric levels offered as an alternative to elevated values, and avoids atmospheric values to maintain the fungus (Römer et al. 2017). It remains unknown why ants showed no preferences for excavation between chambers with atmospheric and underground CO2 values in the present study. Interestingly, the displayed preference for digging at 1% is similar to the CO2 preference shown for the maintenance of the fungus (Römer et al. 2017), i.e., such digging preference may lead to the excavation of chambers at suitable levels for this crucial nest task.
The effects of carbon dioxide on nest excavation and determination of nest depth in leaf-cutting ants
Ants should excavate their nests at soil strata that offer suitable environmental conditions to successfully perform their inside-nest tasks. Indeed, leaf-cutting ants do also display preferences for other microclimatic variables when they excavate (Bollazzi et al. 2008; Pielström and Roces 2014). As climatic variables change with soil depth, ants could use gradients in the soil as cues to initiate nest excavation. Many ant nests have a very distinct stratification of their underground chambers, for example the Florida harvester ant Pogonomyrmex badius, with a distinct top-heavy chamber stratification (Tschinkel 2004). As CO2 increases with depth, ants could use this gradient as a template for nest excavation. However, experimentally reversing the CO2 gradient did not lead to the excavation of upside-down nests by P. badius (Tschinkel 2013). Lack of use of the soil CO2 gradient may be due to the better aerated soil type in which P. badius excavates their nests. This sandy soil does not have a steep CO2 gradient with depth, whereas in the more organic and clayish soil many leaf-cutting ant species dig their nests in, CO2 levels increase very steeply with depth (Bollazzi et al. 2012).
Our study revealed that leaf-cutting ants do use the carbon dioxide levels in the soil as a cue for self-organized nest excavation. Based on previous findings for temperature and moisture levels, the range of the reported microclimatic preferences for digging is broader (Bollazzi et al. 2008; Pielström and Roces 2014) than the range for the placement and maintenance of brood and fungus (Roces and Kleineidam 2000; Bollazzi and Roces 2002). The same seems to be the case for leaf-cutting ants’ CO2 preferences. Here, they not only show narrow, but also distinct preferred CO2 ranges for fungus and brood tending (between 1 and 3% for fungus: Römer et al. 2017; 3% for brood, unpublished results). Avoiding too high CO2 values, as they occur at deep soil layers, but accepting elevated ones as found closer to the soil surface (our present results), would provide workers with broader microclimatic ranges that enable short-term responses to changing environmental conditions, i.e., brood or fungus relocation across different nest depths to follow their preferred values.
Even though preferences for specific environmental variables may concentrate digging activity at certain sites across the soil profile, they may not be the direct cause for the emergence of a nest chamber, since brood or fungus need to be first placed at a selected site, as demonstrated in a recent study (Römer and Roces 2014). Environmental variables determine where brood and fungus are placed (Roces and Kleineidam 2000; Bollazzi and Roces 2002; Römer et al. 2017). The relocated items, which are attractive to workers, lead to their aggregation at the site, which in turn triggers digging behavior (Rasse and Deneubourg 2001). Other factors, such as vibrational communication signals produced by workers engaged in digging (Pielström and Roces 2012), stigmergic responses to excavated soil pellets (Pielström and Roces 2013), and microclimatic preferences for digging could further increase digging activity at the selected site.
The observed choices for a CO2 concentration found closely to the soil surface also help to understand A. lundii’s subterranean nesting biology, with nest chambers excavated in the uppermost soil layers. Since the respiration of the fungus is compromised at high CO2 levels as occurring deep underground (Kleineidam and Roces 2000), avoidance of 4% CO2 and preference for 1% during excavation could, therefore, be a behavioral adaptation of A. lundii to provide a long-term, suitable environment for fungus farming in nest chambers dug at superficial soil levels. Colonies of other Acromyrmex species and of most species of the genus Atta excavate much deeper nests with chambers found at 4 m (Verza et al. 2007) or even at 7 m (Moser 1963; Moreira et al. 2004a) below the surface. Deeper underground, some environmental variables such as humidity or temperature might be stable and more critical for brood and fungus survival than others. Depending on location and soil characteristics, ants are, therefore, expected to trade-off their competing microclimatic preferences when excavating new chambers at the best suitable sites. This aspect of self-organized nesting behavior remains to be explored in the future.
References
Beckmann HE (1974) The damaging effect of supercooling, narcosis and stress on the memory of the honeybee. J Comp Physiol 94:249–266
Bollazzi M, Forti LC, Roces F (2012) Ventilation of the giant nests of Atta leaf-cutting ants: does underground circulating air enter the fungus chambers? Insectes Soc 59:487–498. https://doi.org/10.1007/s00040-012-0243-9
Bollazzi M, Kronenbitter J, Roces F (2008) Soil temperature, digging behaviour, and the adaptive value of nest depth in South American species of Acromyrmex leaf-cutting ants. Oecologia 158:165–175. https://doi.org/10.1007/s00442-008-1113-z
Bollazzi M, Roces F (2002) Thermal preference for fungus culturing and brood location by workers of the thatching grass-cutting ant Acromyrmex heyeri. Insectes Soc 49:153–157. https://doi.org/10.1007/s00040-002-8295-x
Bollazzi M, Roces F (2007) To build or not to build: circulating dry air organizes collective building for climate control in the leaf-cutting ant Acromyrmex ambiguus. Anim Behav 74:1349–1355. https://doi.org/10.1016/j.anbehav.2007.02.021
Bonabeau E (1998) Fixed response thresholds and the regulation of division of labor in insect societies. Bull Math Biol 60:753–807. https://doi.org/10.1006/bulm.1998.0041
Coelho Junior A, Geremias LD, Alves GR et al (2017) The biology of Trichogramma pretiosum as atmospheric O2 becomes depleted and CO2 accumulates. Biol Control 105:1–5. https://doi.org/10.1016/j.biocontrol.2016.11.005
Coelho Junior A, Parra JRP (2013) Effect of carbon dioxide (CO2) on mortality and reproduction of Anagasta kuehniella (Zeller 1879), in mass rearing, aiming at the production of Trichogramma spp. An Acad Bras Cienc 85:823–831. https://doi.org/10.1590/S0001-37652013000200021
Deneubourg JL, Franks NR (1995) Collective control without explicit coding: the case of communal nest excavation. J Insect Behav 8:417–432. https://doi.org/10.1007/BF01995316
Falibene A, Roces F, Rössler W, Groh C (2016) Daily thermal fluctuations experienced by pupae via rhythmic nursing behavior increase numbers of mushroom body microglomeruli in the adult ant brain. Front Behav Neurosci 10:1–12. https://doi.org/10.3389/fnbeh.2016.00073
Grassé P-P (1959) La reconstruction du nid et les coordinations interindividuelles chez Bellicositermes natalensis et Cubitermes sp. La theorie de la stigmergie. Insectes Soc 6:41–83
Guerenstein PG, Hildebrand JG (2008) Roles and effects of environmental carbon dioxide in insect life. Annu Rev Entomol 53:161–178. https://doi.org/10.1146/annurev.ento.53.103106.093402
Halboth F, Roces F (2017) The construction of ventilation turrets in Atta vollenweideri leaf-cutting ants: carbon dioxide levels in the nest tunnels, but not airflow or air humidity, influence turret structure. PLoS One 12:e0188162. https://doi.org/10.1371/journal.pone.0188162
Hamada Y, Tanaka T (2001) Dynamics of carbon dioxide in soil profiles based on long-term field observation. Hydrol Process 15:1829–1845. https://doi.org/10.1002/hyp.242
Hangartner W (1969) Carbon dioxide, a releaser for digging behavior in Solenopsis geminata (Hymenoptera: Formicidae). Psyche (Stuttg) 76:58–67. https://doi.org/10.1155/1969/58428
Hansell MH (1984) Animal architecture and building behavior. Longman Inc., New York
Hebling MJA, Penteado CHS, Mendes EG (1992) Respiratory regulation in workers of the leaf cutting ant Atta sexdens rubropilosa Forel, 1908. Comp Biochem Physiol 101:319–322
Hillel D (1998) Environmental soil physics. Academic Press, London
Klaiber J, Najar-Rodriguez A, Dialer E, Dorn S (2013) Elevated carbon dioxide impairs the performance of a specialized parasitoid of an aphid host feeding on Brassica plants. Biol Control 66:49–55
Kleineidam C, Roces F (2000) Carbon dioxide concentrations and nest ventilation in nests of the leaf-cutting ant Atta vollenweideri. Insectes Soc 47:241–248. https://doi.org/10.1007/PL00001710
Kleineidam C, Romani R, Tautz J, Isidoro N (2000) Ultrastructure and physiology of the CO2 sensitive sensillum ampullaceum in the leaf-cutting ant Atta sexdens. Arthropod Struct Dev 29:43–55
Kleineidam C, Tautz J (1996) Perception of carbon dioxide and other “air-condition” parameters in the leaf cutting ant Atta cephalotes. Naturwissenschaften 83:566–568. https://doi.org/10.1007/BF01141981
Moreira AA, Forti LC, Andrade APP et al (2004a) Nest architecture of Atta laevigata (F. Smith 1958) (Hymenoptera: Formicidae). Stud Neotrop Fauna Environ 39:109–116. https://doi.org/10.1080/01650520412331333756
Moreira A, Forti L, Boaretto M et al (2004b) External and internal structure of Atta bisphaerica Forel (Hymenoptera: Formicidae) nests. J Appl Entomol 128:204–211. https://doi.org/10.1111/j.1439-0418.2004.00839.x
Moser JC (1963) Contents and structure of Atta texana nest in summer. Ann Entomol Soc Am 56:286–291
Nicolas G, Sillans D (1989) Immediate and latent effects of carbon dioxide on insects. Annu Rev Entomol 34:97–116. https://doi.org/10.1146/annurev.en.34.010189.000525
Nielsen MG, Christian K, Henriksen PG, Birkmose D (2006) Respiration by mangrove ants Camponotus anderseni during nest submersion associated with tidal inundation in Northern Australia. Physiol Entomol 31:120–126. https://doi.org/10.1111/j.1365-3032.2005.00492.x
Pielström S, Roces F (2012) Vibrational communication in the spatial organization of collective digging in the leaf-cutting ant Atta vollenweideri. Anim Behav 84:743–752. https://doi.org/10.1016/j.anbehav.2012.07.008
Pielström S, Roces F (2013) Sequential soil transport and its influence on the spatial organisation of collective digging in leaf-cutting ants. PLoS One 8:e57040. https://doi.org/10.1371/journal.pone.0057040
Pielström S, Roces F (2014) Soil moisture and excavation behaviour in the chaco leaf-cutting ant (Atta vollenweideri): digging performance and prevention of water inflow into the nest. PLoS One. https://doi.org/10.1371/journal.pone.0095658
Powell RJ, Stradling DJ (1986) Factors influencing the growth of Attamyces bromatificus, a symbiont of attine ants. Trans Br Mycol Soc 87:205–213. https://doi.org/10.1016/S0007-1536(86)80022-5
Quinlan RJ, Cherrett JM (1978) Aspects of the symbiosis of the leaf-cutting ant Acromyrmex octospinosus (Reich) and its food fungus. Ecol Entomol 3:221–230. https://doi.org/10.1111/j.1365-2311.1977.tb00877.x doi
Rasse P, Deneubourg JL (2001) Dynamics of nest excavation and nest size regulation of Lasius niger (Hymenoptera: Formicidae). J Insect Behav 14:433–449. https://doi.org/10.1023/A:1011163804217
Roces F, Kleineidam C (2000) Humidity preference for fungus culturing by workers of the leaf-cutting ant Atta sexdens rubropilosa. Insectes Soc 47:348–350. https://doi.org/10.1007/PL00001728
Roces F, Núñez J (1989) Brood translocation and circadian variation of temperature preference in the ant Camponotus mus. Oecologia 81:33–37. https://doi.org/10.1007/BF00377006
Römer D, Bollazzi M, Roces F (2017) Carbon dioxide sensing in an obligate insect fungus symbiosis: CO2 preferences of leaf-cutting ants to rear their mutualistic fungus. PLoS One 12:e0174597. https://doi.org/10.1371/journal.pone.0174597
Römer D, Roces F (2014) Nest enlargement in leaf-cutting ants: Relocated brood and fungus trigger the excavation of new chambers. PLoS One 9:e97872. https://doi.org/10.1371/journal.pone.0097872
Römer D, Roces F (2015) Available space, symbiotic fungus and colony brood influence excavation and lead to the adjustment of nest enlargement in leaf-cutting ants. Insectes Soc 62:401–413. https://doi.org/10.1007/s00040-015-0419-1
Röschard J, Roces F (2003) Cutters, carriers and transport chains: Distance-dependent foraging strategies in the grass-cutting ant Atta vollenweideri. Insectes Soc 50:237–244. https://doi.org/10.1007/s00040-003-0663-7
Schofield RMS, Emmett KD, Niedbala JC, Nesson MH (2011) Leaf-cutter ants with worn mandibles cut half as fast, spend twice the energy, and tend to carry instead of cut. Behav Ecol Sociobiol 65:969–982. https://doi.org/10.1007/s00265-010-1098-6
Schwartz DM, Bazzaz FA (1973) In situ measurements of carbon dioxide gradients in a soil-plant-atmosphere system. Oecologia 12:161–167. https://doi.org/10.1007/BF00345515
Theraulaz G, Bonabeau E (1999) A brief history of stigmergy. Artif Life 5:97–116
Theraulaz G, Bonabeau E, Deneubourg J-L (1998) The origin of nest complexity in social insects. Complexity 3:15–25
Tschinkel WR (2013) Florida harvester ant nest architecture, nest relocation and soil carbon dioxide gradients. PLoS One 8:e59911. https://doi.org/10.1371/journal.pone.0059911
Tschinkel WR (2004) The nest architecture of the Florida harvester ant, Pogonomyrmex badius. J Insect Sci 4:21. https://doi.org/10.1672/1536-2442(2004)004
Verza SS, Forti LC, Lopes JFS, Hughes WOH (2007) Nest architecture of the leaf-cutting ant Acromyrmex rugosus rugosus. Insectes Soc 54:303–309. https://doi.org/10.1007/s00040-007-0943-8
Zolessi LC, González LA (1978) Observaciones sobre el género Acromyrmex en el Uruguay. IV. A. (Acromyrmex) lundi (Guérin, 1938) (Hymenoptera: Formicidae). Rev la Fac Humanidades y Ciencias Ser Ciencias Biológicas 1:9–28
Acknowledgements
We would like to thank David Urbaniec, Lisa Weidner and Baris Düdükcü for their help during the experiments, and Griselda Roces for the ant drawing. We are grateful to two anonymous reviewers, whose comments greatly improved the manuscript. Daniela Römer was supported by a grant from the Postdoc Plus funding program of the Graduate School of Life Sciences (GSLS) of the University of Würzburg, Germany, and a Grant of the Agencia Nacional de Investigación e Innovación (ANII, Grant Number PD_NAC_2015_1_108641), Uruguay. Florian Halboth was supported by a Grant from the German Excellence Initiative to the Graduate School of Life Sciences, University of Würzburg.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Römer, D., Halboth, F., Bollazzi, M. et al. Underground nest building: the effect of CO2 on digging rates, soil transport and choice of a digging site in leaf-cutting ants. Insect. Soc. 65, 305–313 (2018). https://doi.org/10.1007/s00040-018-0615-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-018-0615-x