Abstract
The size of underground ant nests positively correlates with their worker number, suggesting that during colony growth, workers respond to increased space demands by enlarging the existing nest. In leaf-cutting ants, the presence of brood and a growing symbiotic fungus are expected to generate spatial demands workers should respond to. We investigated to what extent the presence of in-nest stores, such as brood and fungus, and actual space availability influence the digging behavior of leaf-cutting ants and lead to the adjustment of nest enlargement. In the laboratory, we offered worker groups of Acromyrmex lundi a nest site consisting of either a tunnel, representing reduced available space, or a tunnel and a small chamber, representing ample available space as a starting point for nest enlargement. Workers could move and store offered brood and fungus into the nest structure, and enlarge it as needed. The presence of brood and fungus influenced digging activity as well as the architecture of the nest. In the presence of relocated brood, digging activity was higher in reduced space than in ample space, suggesting that high worker density stimulated excavation. When workers relocated fungus and brood into ample space, digging activity was similar as in their absence, yet a larger chamber and fewer tunnels were excavated. During the process of nest enlargement, workers were observed to initially excavate space in excess, which was refilled with part of the removed soil pellets, thus leading to a reduction of available space. Pellet deposition seemed to be opportunistic, with workers refilling unused space. Results indicate that the size of a leaf-cutting ant nest does not simply depend on the number of inhabiting workers. Rather, workers adjust the enlargement of their nest space depending on the current space available and the presence of in-nest stores.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Social insects build complex nests that protect their colonies and dampen environmental fluctuations (Hansell 1984). Unlike the majority of eusocial bee, wasp and termite species, which usually construct their nests out of wax, carton and other building materials above ground, most ant species excavate and inhabit subterranean nests (Hölldobler and Wilson 1990). By removing soil, workers create the basic architectural structures of a nest, i.e., chambers and tunnels. Chambers are usually horizontally oriented, flat and have a round base (Antonialli and Giannotti 2001; Tschinkel 2003). Here workers store the brood, food, and in some species, accumulate waste material (Jonkman 1980a, b; Mikheyev and Tschinkel 2004; Tschinkel 2004). In the case of fungus-growing ants, the chambers are spherical and dome shaped (Jacoby 1936; Stahel and Geijskes 1941; Bollazzi et al. 2012), because they are used to grow a voluminous, sponge-like fungus (Weber 1966). Nest chambers are connected to each other and to the soil surface through oblong and narrow tunnels (Jacoby 1937). They accommodate the colonies’ traffic as well as contribute to nest ventilation (Bollazzi et al. 2012).
It is often argued that one of the main mechanisms underlying the emergence of the architecture of ant nests is self-organization, a process in which cooperating individuals have only access to local information (Deneubourg and Franks 1995; Camazine et al. 2001). While these arguments are well established in the literature, few studies have identified the specific environmental stimuli and decision-making processes involved in complex excavation behaviors. Ants may react to local stimuli originating from their environment, other nearby workers, and the by-products of the building process itself (Franks and Deneubourg 1997; Rasse and Deneubourg 2001; Bollazzi et al. 2008; Pielström and Roces 2013). The enlargement of a nest from its founding state, which in most ant species is comprised of a short, downward leading tunnel with a small chamber, appears to be a regulated process. Nest size increases with time and correlates with colony population (Tschinkel 1987; Deneubourg and Franks 1995; Rasse and Deneubourg 2001; Tschinkel 2004; Mikheyev and Tschinkel 2004; Buhl et al. 2005), and these observations have led to the conclusion that worker number is the primary factor responsible for nest size determination.
For example, in the ant Lasius niger, when ant groups of different sizes could excavate in an experimental arena, larger groups excavated larger nests (Rasse and Deneubourg 2001). When more ants were added to simulate colony growth, ants responded with enhanced excavation or restarting halted excavation, which led to the enlargement of the nest. A density-dependent stimulation of digging activity is thought to be one of the possible underlying mechanisms of this response, where increased worker density at the beginning of nest enlargement initiates excavation. Through positive feedback, excavation activity could be maintained or increased, and later be counteracted via negative feedback, by which excavation would diminish or cease. Positive feedback may also occur through a stimulation of digging by perceived CO2 (Hangartner 1969), stigmergic responses to excavated soil pellets (Pielström and Roces 2013) or through stridulating excavators (Pielström and Roces 2012), which draws a workforce to a site where they excavate. When space has been created, the reduced worker density, or the possibility for workers to move across an extended space, could act as a negative feedback, without requiring any explicit measure of nest and/or population size by workers. Nest excavation appears, therefore, to be self-regulated via positive feedback, leading to nest enlargement, and negative feedbacks provided directly by the generated space itself, or indirectly via a reduction of worker density (Rasse and Deneubourg 2001; Halley et al. 2005; Fröhle 2009; Pielström 2013).
A further hypothetical mechanism involved in the adjustment of nest enlargement is workers obtaining spatial knowledge about the size of the excavated structure itself, using idiothetic information, as founding queens of leaf-cutting ants do for the determination of nest depth (Fröhle and Roces 2012). Additionally, digging activity may act as a negative feedback, because workers may simply excavate less or stop digging as time progresses because of exhausted energy stores or changes in their response thresholds, as known for leaf-cutting ant queens (Camargo et al. 2011; Fröhle and Roces 2012). This would lead to a final nest size without explicit information about the space already created or the presence of nestmates.
In recent years, a few studies offered further insight into the mechanisms involved in building a nest by investigating the emergence of fungus chambers in leaf-cutting ant nests. Acromyrmex lundi leaf-cutting ants are known to enlarge existing chambers only if they contain fungus; otherwise they excavate tunnels (Fröhle and Roces 2009). They appear to use their voluminous fungus as a template to adjust the size and shape of the nest chambers (Fröhle and Roces 2009).
In addition, it was recently demonstrated that the presence of brood and fungus at a given site triggers the excavation of new nest chambers (Römer and Roces 2014). The brood of A. lundi was shown to be attractive to workers, which aggregate around these items and thereby increase local ant density (Römer and Roces 2014). This locally increased worker density was thought to be responsible for increased excavation activity at the site of brood placement, as compared to alternative sites without brood. The occurrence of brood at a site also led to a change in the shape of the excavated structure, which was round and chamber-like, as compared to a tunnel-like structure excavated at a site without brood (Römer and Roces 2014). A study on Atta sexdens, which excavates deeper nests in contrast to the more superficially nesting A. lundi, also reported the same effect of brood and fungus presence on chamber excavation (Camargo and Forti 2015). This suggests that the presence of in-nest stores can influence the internal architecture of a nest through a self-organized, likely worker aggregation-based adjustment of digging activity.
An additional behavior likely involved in the adjustment of nest space was observed in Atta vollenweideri (Pielström and Roces 2013) as well as in small worker groups in A. lundi (Fröhle 2009), although its significance remained unexplored: workers were observed to deposit part of their excavated soil pellets within the excavated structure itself, thus reducing the free nest space.
In this work, we investigated how the available nest space and the presence of relocated brood and fungus as stored items influence the magnitude of both digging behavior and pellet deposition, thus leading to the adjustment of nest enlargement in the leaf-cutting ant Acromyrmex lundi. Two series of laboratory experiments were performed. The first series was aimed at investigating the effect of relocated brood and available space on nest enlargement. For that, we presented groups of A. lundi workers with a nest site consisting of either a single tunnel, representing a reduced available nest space, or of a tunnel and a small chamber, representing ample nest space. Workers could move and store brood into the offered structure, and enlarge it as needed. The second experimental series was aimed at evaluating the effects of relocated fungus, with and without embedded brood, on nest enlargement, since the voluminous fungus is expected to generate large space demands because of both its own volume and its influence on worker aggregation around its structure.
Materials and methods
All experiments were performed in the laboratory between June 2010 and June 2011 with 3 colonies of the leaf-cutting ant A. lundi, founded in 2006. They were reared in a climate chamber at the University of Würzburg, Germany, in the Department of Behavioral Physiology and Sociobiology, at 25 °C, 50 % humidity and a 12:12 h light:dark cycle on an ad libitum diet of blackberry (Rubus fruticosus) leaves, honey water and water. Each colony was kept in a system of plastic boxes (19 × 19 × 9 cm) that served as artificial fungus chambers, a waste disposal box and a feeding arena, all interconnected by transparent plastic tubing.
Each assay was performed with a subcolony consisting of 150 medium-sized workers (mean body mass calculated from a ubiquitous sample of 80 medium workers = 5.3 ± 1.2 mg SD), 75 of which were collected out of a randomly chosen fungus box and 75 out of the feeding arena. Depending on the experimental series, as described below, brood or fungus alone, or a combination of both were provided to the workers. Such items were collected from the same fungus box as the workers. If only brood was provided, all visible traces of fungus mycelium were carefully removed from the brood cuticle. If only fungus was provided, eggs, brood and all small gardening workers were removed. Workers, brood and fungus were not reintroduced into their colonies after the assays.
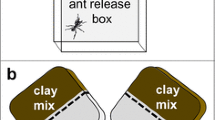
The rationale of the experimental design was to offer single subcolonies one nest site for excavation, into which workers could relocate the brood and/or fungus, and enlarge the pre-given space if needed. In independent experiments, two plastic boxes, representing the ‘outside’ of a nest (Fig. 1a) were connected to a nest site. This site was either of reduced space, consisting of a tunnel (7 × 1 × 1 cm = 7 cm3, Fig. 1b), or ample space, consisting of a similar tunnel leading to a circular cavity (diameter 5 cm = 19.6 cm3, Fig. 1c).
Experimental setup. a on the left: the pellet deposition box (box 1) with connection to digging arena; on the right: the feeding box (box 2) with water (w) and honey water (f), green arrow the site for the introduction of ants, brood and fungus, depending on the experiments; b setup reduced space with a tunnel (7 × 7 × 1 cm) as offered nest space; c setup ample space with a tunnel and cavity (diameter 5 cm, height 1 cm) as offered nest space. Black arrows the direction of the ants coming from the feeding and pellet-deposition box
The duration of each assay was 48 h. While workers had not ceased nest excavation after that time, this was considered as a sufficient timeframe to evaluate the adjustment of nest enlargement, as Solenopsis invicta as well as A. lundi workers were observed to display their highest digging rates within the first 24 h, which then decreased considerably over time (Chen 2009; Fröhle and Roces 2009). In addition, our preliminary experiments showed that chamber excavation takes place within the first 24 h after fungus and brood relocation into a nest site, and that only slight tunneling activity continues thereafter.
Series
Adjustment of nest enlargement: influence of brood and available space
In the first series, the influence of brood and available space on nest enlargement was investigated. Four different experiments were performed, which combined presence and absence of brood, as well as reduced and ample space as follows. Experiment 1a: brood relocated into reduced nest space (n = 15); Experiment 1b: brood relocated into ample space (n = 36); Experiment 1c: reduced space without brood as control (n = 9) and Experiment 1d: ample space without brood as control (n = 12). Experiments 1a and 1b were performed using different quantities of brood to account for a possible effect of brood number on digging activity (for details see supplementary material S1 and S2). However, data analysis showed no effect, so that data for the different brood quantities were pooled and presented as two groups, ‘reduced space with brood’ and ‘ample space with brood’. The average number of brood used was 40.
Adjustment of nest enlargement: influence of fungus with and without embedded brood
In the second series, the influence of relocated fungus on nest enlargement was investigated by offering an empty tunnel and a cavity (setup “ample space”) to accommodate the voluminous fungus mass during the relocation process. Two different experiments were performed, as follows. Experiment 2a: relocation of only fungus (n = 12) and Experiment 2b: relocation of fungus and brood, to investigate potential synergistic effects of brood on excavation (n = 28). For Experiment 2b, brood was also given in differing quantities, averaging 55 brood items. As for Series 1, there was no relationship between brood quantity and workers’ digging activity (for details on brood number given and statistics see supplementary material S3). All assays with brood embedded in the fungus were, therefore, pooled and presented as one group ‘ample space with fungus and brood’.
Experimental procedure
The nest site was offered within a horizontal square arena (30 × 30 × 1 cm) made out of clear polycarbonate with an entrance hole (diameter 1 cm) in the bottom and a removable lid (Fig. 1b, c). The arena was filled with moist clay (Claytec Baulehm gemahlen 0–0.5 mm, Viersen, Germany) with a water content of 21 % to facilitate excavation (Pielström and Roces 2014). Some of the clay was then removed to create the free space offered to the ants. The arena containing the nest site was connected to an empty box for the deposition of the removed soil pellets (Fig. 1a, box 1 on the left; 19 × 19 × 9 cm), which led to a feeding box (Fig. 1a, box 2 on the right; 19 × 19 × 9 cm) via a wooden bridge. By separating the soil deposit from the food supply, we ensured that the offered honey water and water remained unspoiled by dropped-in soil pellets, which could compromise worker survival.
Each assay began with the placement of workers and items to be relocated into the feeding box, and ended after 48 h. Digging and relocation of brood and fungus into the humid nest site usually began within the first 1 or 2 h after workers had explored the setup and discovered the nest site.
After the end of the assay, the excavated nest was photographed with a piece of millimeter paper as size reference. The relocated fungus inside the excavated nest structures was collected and weighed to the nearest 0.1 mg, and the brood items were counted. As described by Fröhle (2009) for small A. lundi worker groups, it was observed that part of the excavated soil pellets, which are formed following a grabbing and raking sequence (Sudd 1969), were not removed to the outside box, but deposited inside the excavated structure, usually adhered to the sides of the chamber or tunnel walls. Therefore, pellets deposited in tunnels or inside the chamber were carefully removed and weighed separately to the nearest 0.1 g. The outline of the excavated chamber could be easily distinguished from that of tunnels, as in the former the ants excavated from the top to the bottom of the arena (1 cm). Conversely, tunnels were more narrow and shallow, and usually excavated in the upper half of the soil layer. After pellet removal, a plaster cast was made of the emerged or enlarged cavity.
Measurements and calculations
Three different variables were compared across the experiments to investigate the mechanisms underlying the adjustment of nest space. (a) The total digging activity, which corresponds to the total volume excavated as chamber and tunnels. Total digging activity was quantified in cm3 by converting the mass of all excavated pellets into volume (1 g clay = 1.8 cm3). A part of the total digging activity represented the volume of an excavated chamber, which was measured to the nearest 0.5 cm3 using the method of water displacement of the corresponding plaster cast, minus the volume of the offered nest space at the beginning. The digging activity corresponding to the excavated tunnels was calculated by subtracting the volume of the excavated or enlarged chamber from the volume corresponding to the total digging activity. (b) The free nest volume at the end of the assay, calculated as the total digging activity plus the initial empty space of the offered structures minus the volume occupied by the loosely packed soil pellets deposited inside. (c) The volume occupied by the internal pellet deposit, calculated by converting the total mass of the deposited pellets using the following empirically determined factor: 0.79 g pellets = 1 cm3, and presented in addition as proportion of the total excavated pellets.
We used box plots in all figures of the results for conformity reasons. However, some data sets were normally distributed. Appropriate parametric or non-parametric statistical tests were performed according to the distribution of the data sets.
Results
General observations
After the discovery of the empty nest site inside the clay-filled arena, workers started to remove pellets to the deposition box within the first hour. The offered items in the different experiments, i.e., brood, fungus or both combined, were readily transported inside the nest site, where excavation simultaneously took place. After relocation of items inside the nest site, some workers could be observed sitting on top of fungus and brood, seemingly inactive. Most of the digging activity took place straight ahead from the entrance hole, indicating that ants maintained their heading when coming from the outside box into the arena to excavate. Once an excavated tunnel hit the opposite arena wall, workers excavated along it, forming U-shaped tunnels located at the edge of the digging arena. As already mentioned, part of the excavated soil pellets were not transported outside, but deposited inside the excavated space and initially offered structures. An example of the excavation observed in Experiment 1a with relocated brood into reduced space is presented in Fig. 2, in which the pellets deposited inside visibly narrow and smooth the wider and more irregular tunnels initially excavated.
a Example of an excavated nest site with 5 pieces of brood after 48 h; b same picture; black line boundary of the excavated nest; white area free nest space; yellow dashed line outline of offered tunnel and entrance hole. Arrows the direction of the ants coming from the pellet-deposition box. Scale of black bar = 5 cm
Adjustment of nest enlargement: influence of brood and available space
The ants’ digging activity, i.e., the space excavated by ants, differed among the four experiments of Series 1 (Fig. 3a, One-way ANOVA, p < 0.001, n = 15; see table S1 in supplementary material for detailed statistics). Ants relocating brood inside reduced space (Experiment 1a) excavated the largest volume. When ants relocated brood inside ample space (Experiment 1b), the digging activity was significantly reduced. In the absence of brood to be relocated, but having only reduced space available (Experiment 1c), the digging activity was also increased and statistically similar to that of Experiment 1a, but also statistically comparable with both ample space experiments (Experiment 1b and 1d). Without brood but under conditions of ample space (Experiment 1d), digging activity was lower and similar to that with the same available space but with relocated brood (Experiment 1b). Taken together, the experiments indicate that reduced space increases digging activity only in the presence of brood.
a Digging activity, i.e., excavated volume of all 4 experiments of Series 1. from left to right: reduced space with brood (Experiment 1a), ample space with brood (Experiment 1b), reduced space without brood (Experiment 1c) and ample space without brood (Experiment 1d); b free nest size of Experiments 1a–1d of Series 1. Dashed line the volume of offered space at the beginning of the excavation; line median, box 25–75 % percentiles, whiskers min–max values. One-way ANOVA, post hoc test Scheffé, groups with the same letter do not differ statistically; p < 0.05
The largest free nest volume at the end of Series 1 was observed when workers relocated brood into a reduced space (Fig. 3b, Experiment 1a); otherwise the free volumes were comparable (One-way ANOVA, p < 0.001; for detailed statistics see supplementary material, Table S2).
While excavation took place, there was a continuous deposition of pellets inside the excavated space. The volume occupied by the pellets deposited inside the nest was high, covering more than one half of the total nest site, yet independent of the presence of brood or initially available space (Fig. 4a; One-way ANOVA, p = 0.41). However, the proportion of the total excavated pellets deposited inside the nest varied among the experiments (One-way ANOVA, p < 0.001, for detailed statistics see supplementary material, Table S3). Deposition inside the nest was lower when ants relocated brood inside and excavated in a reduced space, i.e., a large proportion of pellets was carried to the pellet-deposition box (Fig. 4b). When ample space was available, inside pellet deposition was higher, independent of the presence of brood. Deposition in reduced space without brood was also high but not statistically different from that with brood.
Ants excavated a chamber in almost all experiments offering reduced space, i.e., those that offered only a pre-given tunnel. When brood was available and could be relocated, a chamber emerged in all 15 assays. When no brood was present, a chamber emerged in 8 out of 9 assays. The volume of the excavated chamber, however, was larger when brood could be relocated inside the excavated structure (Fig. 5, gray box plots; statistics see figure caption). In both experiments, the excavated volume was significantly reduced by the deposition of soil pellets adhered to the chamber wall. However, the free chamber volume at the end of the experiments was larger when brood was present in the chamber (Fig. 5, white box plots). Without brood, all initially excavated cavities were almost completely filled with pellets, so that only a tunnel structure remained at the end (examples of chambers with and without brood are presented in Fig. 6).
Excavated chamber or cavity size (cm3) depending on brood presence. Left Experiment 1a with brood; right Experiment 1c without brood. Excavated volume (gray box plots), and free volume (white box plots) after pellet deposition. (Unpaired t test, comparison excavated volume Experiment 1a vs. Experiment 1c: t = 2.89, df = 21, p < 0.01, n 1a = 15, n 1c = 8; comparison free volume Experiment 1a vs. Experiment 1c: t = 4.44, df = 21, p < 0.001, n 1a = 15, n 1c = 8; analysis reduction of excavated volume through pellet deposition in each experiment: paired t test: Experiment 1a: t = 4.47, df = 14, p < 0.001, n = 15; Experiment 1c: t = 6.39, df = 7, p < 0.001, n = 8); line median, box 25–75 % percentiles, whiskers min–max values; ** = p<0.01; *** = p<0.001
Examples of digging sites, 48 h after start of the experiment; close-up of self-excavated chambers or cavities in both reduced space series (Experiment 1a and 1c). a Brood present: a large chamber has been excavated, which was then slightly reduced by pellet deposition. b Same image, with excavated chamber size (black line) and free chamber size (white line) after pellet deposition is outlined. c No brood present: a smaller cavity was excavated and significantly reduced to a tunnel through pellet deposition. d Same image, with cavity size (black line) and free structure (white line) after pellet deposition are outlined. Yellow dashed line part of the initially offered tunnel. Scale of black bar = 2 cm
Adjustment of nest enlargement: influence of fungus with and without embedded brood
Workers displayed the same digging activity, whether they relocated fungus inside a pre-given cavity, or whether only brood or no items at all were relocated, as depicted in Fig. 7a (Kruskal–Wallis test; p = 0.29). To allow comparisons with the previous series without fungus, results from the two experiments using ample space (with or without brood) were plotted again on the right side of the figure. The free nest space after pellet deposition, however, differed among the experiments, being significantly larger when fungus was relocated inside the nest (Fig. 7b; Kruskal–Wallis test; p < 0.001 for detailed statistics see supplementary material, Table S4), independent of embedded brood. When no fungus was available, the remaining free nest volume was smaller because of the greater deposition of pellets inside the excavated structures, and was independent of the presence of brood (Fig. 7b, on the right).
a Digging activity of Experiment 2a (with fungus) and Experiment 2b (with fungus and brood, left side), and of the previous series without fungus for comparisons (Experiment 1b with brood and Experiment 1d without brood. b Free nest volume of the four experiments. Dashed line the volume of the free space offered at the beginning of excavation; line median, box 25–75 % percentiles, whiskers min–max values. Kruskal–Wallis test, post hoc test Dunn; groups with the same letter do not differ statistically; p < 0.05
Workers not only enlarged the offered nest cavity, but also excavated tunnels. Figure 8 presents the digging activity from the previous figure, separated into chamber and tunnel volumes. There was a significantly greater enlargement of the offered cavity when relocated fungus was present, with and without embedded brood (Fig. 8a, gray box plots without pattern; Kruskal–Wallis test, p < 0.001; for detailed statistical analysis see Table S5 in the supplementary material). When only brood was relocated or no items were offered, the cavity was less enlarged (Fig. 8a, on the right). After the deposition of pellets, the free chamber volumes also differed among the experiments (Fig. 8b, white box plots without pattern; Kruskal–Wallis test, p < 0.001; for detailed statistical analysis see Table S6 in the supplementary material), with a significantly larger free chamber volume when fungus with or without embedded brood was relocated into the nest site (Fig. 8b, on the left). After relocation of only brood or without relocation of items, the deposition of excavated pellets strongly reduced the initially offered cavity (Fig. 8b, on the right). Figures 9 and 10 present two examples of the excavated structures, one with relocated fungus and brood, and the other without item relocation.
Digging activity and free volume of Fig. 7, now divided into chamber (box plots without pattern) or tunnel volume (box plots with pattern). Depicted are Experiment 2a (with fungus) and 2b (with fungus and brood) on the left side, and Experiment 1b (with brood) and Experiment 1d (without items) from Series 1 for comparison. a Digging activity for chamber and tunnels; b free chamber and tunnel volume; dashed line the volume of offered chamber or tunnel space at the beginning of excavation; line median, box 25–75 % percentiles, whiskers min–max values. Kruskal–Wallis test, post hoc test Dunn; groups with the same letter do not differ statistically; p < 0.05
Example of enlargement of the offered cavity and tunnel excavation with fungus and brood in ample space (Experiment 2b). The offered cavity was majorly enlarged and only a small tunnel volume was excavated. Tunnels were also reduced by the deposition of soil pellets. a Digging arena; b close-up of the enlarged cavity; c same picture as (a), with the offered structures marked with a dashed, yellow line; d same picture as (b), with the cavity enlargement marked with a black line, and the margin of deposited soil pellets marked with a white line. Note the tunnel initially excavated and later completely filled with soil pellets (at the bottom right corner). Scale of black bar = 2 cm
Example of enlargement of the offered cavity and tunnel excavation without stored items in ample space (Experiment 1d). The offered cavity was marginally enlarged and a large tunnel volume was excavated. Enlarged cavity and tunnels were reduced by the deposition of soil pellets. a Digging arena; b close-up of the offered cavity; c same picture as (a), with the offered structures marked with a dashed, yellow line; d same picture as (b), with the cavity enlargement marked with a black line, and the margin of deposited soil pellets marked with a white line. Scale of black bar = 2 cm
The magnitude of tunnel excavation was also affected by the presence of stored items (Fig. 8a, gray box plots with pattern; Kruskal–Wallis test, p < 0.001; for detailed statistical analysis see Table S7 in the supplementary material). When fungus was present, significantly fewer tunnels were excavated, independent of the presence of embedded brood (Fig. 8a, on the left). When only brood but no fungus was offered, or no items at all were present, larger tunnel volumes were excavated (Fig. 8a, on the right). In all conditions, the excavated tunnels were also reduced in size through pellet deposition. The resulting free tunnel volumes were similar, except for a statistical difference between the experiment with brood embedded in the fungus and only brood relocated inside the nest (Fig. 8b, white box plots with pattern; Kruskal–Wallis test, p < 0.01; for detailed statistical analysis see Table S8 in the supplementary material).
Discussion
In our experiments, a group of workers separated from their colony excavated nest structures of varying size, consisting of tunnels and rounder, more chamber-like shapes, depending on variables other than worker number. The presence of stored items, brood and fungus, as well as the existing nest space at the initiation of digging, affected nest excavation and nest architecture. Not all of the excavated and initially offered space was used for storing items or facilitating the traffic flow. Rather, unused space, which may have arisen because of an initially high density of workers that triggered digging activity (Römer and Roces 2014), was opportunistically refilled by the deposition of excavated soil pellets in a dynamic process. The spatial demands of stored items, particularly the symbiotic fungus, led to a flexible adjustment of nest space, with the resulting free nest volume not simply determined by the number of inhabiting workers, but adjusted to the current conditions in the nest.
Feedback mechanisms during nest excavation
Studies with Lasius niger and Messor sancta (Rasse and Deneubourg 2001; Buhl et al. 2005, Toffin et al. 2009, 2010) emphasize the existence of feedback mechanisms that stimulate ants to dig, and also to cease digging after some time has passed. High ant density, i.e., the local aggregation of workers, is considered one of the main triggers for the initiation of digging (Rasse and Deneubourg 2001; Toffin et al. 2009; 2010). Workers may respond to their rate of encounter with nestmates, which likely increases with the local ant density, and start to excavate. As the space available inside a nest is expected to influence the aggregation of its inhabitants, it should also impact the probability that an ant initiates digging. In fact, it has been reported that the creation of nest space acts as an inhibitor for further nest enlargement (Rasse and Deneubourg 2001; Buhl et al. 2005, Fröhle 2009; Pielström 2013). When local worker density decreases due to the larger available nest space, less ants should start or continue excavating, as the encounter rates are expected to decrease, and signals or cues that may attract workers to the digging sites would be more dispersed and hence harder to find (Buhl et al. 2005; Pielström and Roces 2012). Römer and Roces (2014) hypothesized that this mechanism of aggregation-dependent initiation and inhibition of digging was responsible for the difference in digging activity of A. lundi workers in two simultaneously offered sites, one with brood and the other empty. Brood had a significant positive effect on the worker density at the site, rather than having a stimulating effect on digging activity itself. Series 1 of the present study seems to confirm this conclusion, because workers relocating brood inside reduced space excavated the largest volumes, as compared to the situation with ample space, under which workers could disperse more and, therefore, relax the local ant density.
The presence of fungus did not increase workers digging activity. As previous experiments with A. lundi but with a larger fungus mass did show an increase of digging activity (Fröhle, 2009), it seems likely that the amount of fungus offered in the present study was just too low to have this effect. It is suggested that in our experiments, the offered ample nest volume at the beginning of the digging process precluded the occurrence of a high local ant density and conditioned the intensity of the workers’ digging response by acting as a strong negative feedback. We, therefore, conclude that the mechanism by which the ants adjust their nest enlargement is based on positive and negative feedback loops triggering and inhibiting digging based on changes in local ant density. Any factor affecting local ant density should, therefore, affect the digging behavior of ants present at this site and with it the enlargement of the nest. These could either be the number of inhabiting ants itself (Rasse and Deneubourg 2001), the existing space inside the nest or the presence of worker-attracting stimuli.
An indirect assessment of ant density is also hypothesized to operate in quorum sensing during collective decision-making in house hunting ants (Pratt, 2005), although its precise mechanisms remain to be elucidated. Encounter rates with nestmates may be the cue that indirectly informs workers about the local ant density (Gordon et al. 1993). It is also possible that ants can perceive the space directly (Pratt and Pierce 2001), but the means by which they may judge the size of the available space remains unclear. Scouts of the rock-dwelling ant Lepthothorax albipennis appear to assess the area size of a potential new nest using the intersection frequency of their own, pheromone marked path (Mallon and Franks 2000). Leaf-cutting ant queens use idiothetic information to judge the length of the tunnel connecting the founding chamber with the surface (Fröhle and Roces 2012). By walking around, or back and forth, leaf-cutting ant workers could also hypothetically gain spatial knowledge about existing structures.
The formation of nest chambers and tunnels
Investigations on the process of nest enlargement as a whole can lead to a better understanding of the mechanisms underlying digging behavior in ants. However, since nests not only grow but differentiate into architectural features such as chambers and tunnels, the analysis of their emergence may provide insights into the colony demands that drive their construction. Nest architecture is expected to be functional, i.e., to offer the inhabitants structures for the necessary in-nest tasks. In general terms, chambers are excavated to rear brood or store food, and tunnels connect different chambers and allow food and information to be distributed. Very little is known about the mechanisms underlying the excavation of tunnels and chambers in ant nests. Studies with Lasius niger provided new insights into the mechanisms involved in the transition between chamber and tunnel excavation (Toffin et al. 2009, 2010). In these studies, workers that had access to one digging site without any pre-given nest space first excavated in a centrifugal way, leading to the initial emergence of a round cavity. As the digging process continued, workers switched to tunnel excavation, so that the cavity became ramified into several tunnels. It can be assumed that local ant density at the beginning of excavation was high due to the lack of alternative digging sites, thus leading to the excavation in a concentrated manner. Because of the excavation of free space, the number of digging workers was no longer sufficient to excavate along the entire digging face, with the consequence that not all available digging sites were occupied. Likely due to the positive feedbacks mentioned above, ants concentrated at discrete sites along the digging face, thus leading to the emergence of ramifications and later tunnels. It appears that for a chamber to emerge, the ant density at a spot should be high while more dispersed ants dig less as well as differently, leading to the emergence of tunnels.
Our study supports and extends these results, as A. lundi workers also excavated first a chamber and then tunnels when offered only a reduced nest space (Series 1), an effect that was even stronger when brood was relocated inside the structure. This effect was likely based on the aggregation of a large number of workers around the brood, as recently shown (Römer and Roces 2014). In addition, the CO2 produced by the brood could have also enhanced digging intensity at the site, because high CO2 levels trigger digging in ants (Hangartner 1969).
The presence of fungus with or without embedded brood also led to the emergence of larger chambers and fewer tunnels (Series 2), with the inverse pattern when no items were offered. The excavation of tunnels when a large space was available and no brood was present was likely due to the reduced local ant density, which precluded workers to excavate in a centrifugal manner along the available digging face. It appears that those factors that positively affect ant density delay the transition from concentrated digging (cavity excavation) to more dispersed excavation (tunnel excavation). Finally, it remains to be investigated whether the intensity of tunnel excavation in our horizontal arenas is comparable to the pattern observed in natural nests, in which workers usually excavate using gravity for orientation (Sudd 1972). An additional open question is whether the digging activity observed in our laboratory settings may be larger than that occurring in natural nests, because workers were suddenly confronted with insufficient nest space in the laboratory, while field colonies may grow regularly and gradually expand the nest accordingly.
Adjustment of nest space by pellet deposition
As shown in the present study, the adjustment of nest enlargement is not only brought about by changes in digging activity. We demonstrated that leaf-cutting ants refill parts of the excavated nest with soil pellets in a dynamic process during excavation, effectively reducing the free space within the nest. Pellets were usually deposited alongside the walls of cavities or tunnels, where the ants manipulated them into position with their mandibles and front legs.
A considerable pellet deposition was observed under conditions of ample space without relocated fungus, and also when cavities were excavated in absence of stored items because of an initial reduced space. After pellet deposition, the former cavities were narrowed to tunnels. Direct observations suggest that soil carriers simply deposit their loads at unused places, where the movements of nestmates are not obstructed or items are not stored. Refilling appears, therefore, to be opportunistic, rather than being an active means to reduce the available nest space. We argue that the unused space is created due to the self-organizing nature of the digging process, where digging diminishes slowly and not abruptly. Because in our experiments ants were suddenly confronted with a nest site that needed to be enlarged to house brood or fungus, it is likely that digging activity, and also the refilling of the excavated space with pellets, were higher as compared to natural conditions. However, there have also been reports about pellet deposition inside natural fungus-growing ant nests, where cavities were refilled with soil (Autuori 1942; Solomon et al. 2011; Moser, 1963; Moreira et al. 2004a; b). In other ant species, pellets are also often deposited in formerly excavated tunnels (Sudd and Franks 1987).
From an energetic perspective, refilling unused space with pellets may be the most economical way to dispose of the soil, without the need to carry the pellets outside of the nest. Indirectly, the reduction of chamber and tunnel space through pellet deposition might help to promote a more stable microclimate, as it could lead to a reduction of airflow through the nest preventing humidity losses. The deposition of moist clay pellets close to brood and fungus could also increase local air humidity.
The mechanism of internal pellet deposition could be based on stigmergy, the interaction of workers through the product of their work (Grassé 1959). The placement of some pellets in unused space, where traffic flow was not disrupted or items were not stored, could have stimulated other ants carrying a pellet, to deposit theirs nearby, resulting in the accumulation of more pellets at this site. The unloading of particles for wall-building of Leptothorax ants is also thought to be based on such a stigmergic mechanism (Franks et al. 1992). When comparing the deposition in reduced space and ample space, internal deposition was higher when crowding was lower, i.e., under conditions of ample space. It can be hypothesized that a low rate of encounters with nestmates, because of the low local ant density, stimulates loaded workers to deposit their pellets in the excavated structures. When workers excavated mainly tunnels, they also deposited more pellets inside. This could be due to a negative relationship between carrying distance and probability of unloading, as found in the wall-building behavior of Leptothorax tuberointerruptus (Franks and Deneubourg 1997) and in soil deposition behavior of Atta vollenweideri (Pielström and Roces 2013). A distance-dependent unloading behavior would lead to a higher deposition inside nests with a higher tunnel volume, i.e., when the offered cavity was less enhanced, as ants would have to walk a greater distance towards the nest entrance. After pellet deposition, interestingly, the free tunnel volumes were similar in all experiments, indicating that the volume of the tunnel system was adjusted to the worker number, invariable across experiments.
Taken together, our results indicate that the adjustment of both nest size and internal architecture does not simply depend on the number of workers that inhabit a colony. The mechanisms underlying the determination of nest size are flexible and are affected by the available nest space and the presence of in-nest stores. They involve positive and negative feedback loops, such as worker aggregation around stored items and inhibition via the generated space, thus leading to a self-regulated onset and lessening of excavation. The extent of local worker density also influences the internal nest architecture, as ants create cavities when excavating in a concentrated manner, and tunnels when they are more dispersed. Another important mechanism by which ants dynamically adjust the size of their nests is the opportunistic deposition of excavated soil pellets at unused spaces, effectively downsizing their nest. The presence of in-nest stores reduces unused space inside the nest. While the deposition of pellets clearly indicates that workers recognize nest space that is not currently in use, its adaptive value remains elusive.
References
Antonialli WF, Giannotti E (2001) Nest architecture and population dynamics of the ponerine ant Ectatomma edentatum. Sociobiology 38:475–486
Autuori M (1942) Contribuição para o conhecimento da saúva (Atta spp. Hymenoptera–Formicidae). III. Excavação de um saúveiro (Atta sexdens rubropilosa Forel, 1908). Arq Inst Biol 13:137–148
Bollazzi M, Kronenbitter J, Roces F (2008) Soil temperature, digging behaviour, and the adaptive value of nest depth in South American species of Acromyrmex leaf-cutting ants. Oecologia 158:165–175. doi:10.1007/s00442-008-1113-z
Bollazzi M, Forti LC, Roces F (2012) Ventilation of the giant nests of Atta leaf-cutting ants: does underground circulating air enter the fungus chambers? Insect Soc 59:487–498. doi:10.1007/s00040-012-0243-9
Buhl J, Deneubourg JL, Grimal A, Theraulaz G (2005) Self-organized digging activity in ant colonies. Behav Ecol Sociobiol 58:9–17. doi:10.1007/s00265-004-0906-2
Camargo RS, Forti LC (2015) What is the stimulus for excavation of fungus chamber in leaf-cutting ants? Acta Ethol 18:31–35. doi:10.1007/s10211-014-0181-9
Camargo RS, Forti LC, Fujihara RT, Roces F (2011) Digging effort in leaf-cutting ant queens (Atta sexdens rubropilosa) and its effects on colony survival in the claustral phase. Insect Soc 58:17–22. doi:10.1007/s00040-010-0110-5
Camazine S, Deneubourg JL, Franks NR, Sneyd J, Theraulaz G, Bonabeau E (2001) Self-organization in biological systems. Princeton studies in complexity. Princeton University Press, Princeton
Chen J (2009) Automatic system for continuously monitoring digging volume of Red imported fire ants (Hymenoptera: Formicidae) in the laboratory. J Econ Entomol 102:1393–1395
Deneubourg JL, Franks NR (1995) Collective control without explicit coding: the case of communal nest excavation. J Insect Behav 8:417–432. doi:10.1007/BF01995316
Franks NR, Deneubourg JL (1997) Self-organizing nest construction in ants: individual worker behavior and the nest’s dynamics. Anim Behav 54:779–796. doi:10.1006/anbe.1996.0496
Franks NR, Wilby A, Silverman BW, Tofts C (1992) Self-organizing nest construction in ants: sophisticated building by blind bulldozing. Anim Behav 44:357–375
Fröhle K (2009) Mechanismen zur Regulierung der Nestgröße während des Koloniewachstums bei Blattschneiderameisen. PhD dissertation, Julius-Maximilians Universität Würzburg, Germany
Fröhle K, Roces F (2009) Underground agriculture: the control of nest size in fungus-growing ants. In: Theraulaz G, Solé R, Kuntz P (eds) From insect nests to human architecture - Workshop on engineering principles of innovation in swarm-made architecture. European Centre for Living Technology, Venice, pp 95–104
Fröhle K, Roces F (2012) The determination of nest depth in founding queens of leaf-cutting ants (Atta vollenweideri): idiothetic and temporal control. J Exp Biol 215:1642–1650. doi:10.1242/jeb.066217
Gordon DM, Paul RE, Thorpe K (1993) What is the function of encounter patterns in ant colonies? Anim Behav 45:1083–1100
Grassé PP (1959) La reconstruction du nid et les coordinations inter-individuelles chez Bellicositermes natalensis et Cubitermes sp. La Théorie de la stigmergie: Essai d’interprétation du comportement des termites constructeurs. Insect Soc 6:41–81
Halley JD, Burd M, Wells P (2005) Excavation and architecture of Argentine ant nests. Insect Soc 52:350–356. doi:10.1007/s00040-005-0818-9
Hangartner W (1969) Carbon dioxide, a releaser for digging behavior in Solenopsis geminata (Hymenoptera: Formicidae). Psyche 76:58–67
Hansell MH (1984) Animal architecture and building behavior. Longman Inc., New York
Hölldobler B, Wilson EO (1990) The ants. Harvard University Press, Berlin
Jacoby M (1936) Über das Wachsen des Atta-Nestes im ersten Jahre nach der Gründung (Hym. Formicidae). Rev Entomol 6:120–126
Jacoby M (1937) Das räumliche Wachsen des Atta-Nestes vom 50. bis zum 90. Tage (Hym. Formicidae). Rev Entomol 7:416–425
Jonkman JCM (1980a) The external and internal structure and growth of nests of the leaf-cutting ant Atta vollenweideri Forel, 1983 (Hym.:Formicidae) Part I. Z angew Entomol 89:158–173
Jonkman JCM (1980b) The external and internal structure and growth of nests of the leaf-cutting ant Atta vollenweideri Forel, 1983 (Hym.:Formicidae) Part II. Z angew Entomol 89:217–246
Mallon EB, Franks NR (2000) Ants estimate area using Buffon’s needle. Proc R Soc Lond B 267:765–770
Mikheyev AS, Tschinkel WR (2004) Nest architecture of the ant Formica pallidefulva: structure, costs and rules of excavation. Insect Soc 51:30–36. doi:10.1007/s00040-003-0703-3
Moreira AA, Forti LC, Andrade APP, Boaretto MAC, Lopes JFS (2004a) Nest architecture of Atta laevigata (F. Smith, 1958) (Hymenoptera: Formicidae). Stud Neotrop Fauna En 39:109–116. doi:10.1080/01650520412331333756
Moreira AA, Forti LC, Boaretto MAC, Andrade APP, Lopes JFS, Ramos VM (2004b) External and internal structure of Atta bisphaerica Forel (Hymenoptera: Formicidae) nests. J Appl Entomol 128:204–211
Moser JC (1963) Contents and structure of Atta texana nest in summer. Ann Entomol Soc Am 56:286–291
Pielström S (2013) On the role of local information in the spatial organization of collective nest digging in the leaf-cutting ant Atta vollenweideri (Forel, 1893). PhD dissertation, Julius-Maximilians Universität Würzburg, Germany
Pielström S, Roces F (2012) Vibrational communication in the spatial organization of collective digging in the leaf-cutting ant Atta vollenweideri. Anim Behav 84:743–752. doi:10.1016/j.anbehav.2012.07.008
Pielström S, Roces F (2013) Sequential soil transport and its influence on the spatial organization of collective digging in leaf-cutting ants. PLoS One 8:e57040. doi:10.1371/journal.pone.0057040
Pielström S, Roces F (2014) Soil moisture and excavation behaviour in the Chaco leaf-cutting ant (Atta vollenweideri): digging performance and prevention of water inflow into the nest. PLoS One 9:e95658. doi:10.1371/journal.pone.0095658
Pratt SC (2005) Quorum sensing by encounter rates in the ant Temnothorax albipennis. Behav Ecol 16:488–496. doi:10.1093/beheco/ari020
Pratt SC, Pierce NE (2001) The cavity-dwelling ant Leptothorax curvispinosus uses nest geometry to discriminate between potential homes. Anim Behav 62:281–287. doi:10.1006/anbe.2001.1777
Rasse P, Deneubourg JL (2001) Dynamics of nest excavation and nest size regulation of Lasius niger (Hymenoptera: formicidae). J Insect Behav 14:433–449. doi:10.1023/A:1011163804217
Römer D, Roces F (2014) Nest enlargement in leaf-cutting ants: relocated brood and fungus trigger the excavation of new chambers. PLoS One 9:e97872. doi:10.1371/journal.pone.0097872
Solomon SE, Lopes CT, Mueller UG, Rodrigues A, Sosa-Calvo J, Schultz TR, Vasconcelos HL (2011) Nesting biology and fungiculture of the fungus-growing ant, Mycetagroicus cerradensis: New light on the origin of higher attine agriculture. J Ins Sci 11:12. http://www.insectscience.org/11.12
Stahel G, Geijskes DC (1941) Weitere Untersuchungen über Nestbau und Gartenpilz von Atta cephalotes L. und Atta sexdens L. (Hym. Formicidae). Rev Entomol 12:243–268
Sudd JH (1969) The excavation of soil by ants. Z Tierpsychol 26:257–276
Sudd JH (1972) The response of digging ants to gravity. Insect Soc 19:243–250
Sudd JH, Franks NR (1987) The behavioral ecology of ants. Chapman and Hall, New York, pp 55–64
Toffin E, Di Paolo D, Campo A, Detrain C, Deneubourg JL (2009) Shape transition during nest digging in ants. P Natl Acad Sci USA 106:18616–18620. doi:10.1073/pnas.0902685106
Toffin E, Kindekens J, Denoubourg JL (2010) Excavated substrate modulates growth instability during nest building in ants. P Roy Soc Lond B Bio 277:2617–2625. doi:10.1098/rspb.2010.0176
Tschinkel WR (1987) Seasonal life history and nest architecture of a winter-active ant, Prenolepsis imparis. Insect Soc 34:143–164
Tschinkel WR (2003) Subterranean ant nests: trace fossils past and future? Palaeogeogr Palaeocl Palaeoecol 192:321–333
Tschinkel WR (2004) The nest architecture of the Florida harvester ant. Pogonomyrmex badius. J Ins Sci 4:21
Weber NA (1966) Fungus-growing ants. Science 153:587–604. doi:10.1126/science.153.3736.587
Acknowledgments
We would like to thank Kerstin Fröhle for sharing her experience with the laboratory methods to investigate ant digging behavior, and Steffen Pielström, James Waters, and two anonymous reviewers for their helpful comments and suggestions on the manuscript. Annette Laudahn and Adrienne Gerber-Kurz assisted with experimental preparation and were responsible for ant rearing. This study was partially supported by funds from the German Research Foundation (DFG, Grant SFB 554/TP E1).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Römer, D., Roces, F. Available space, symbiotic fungus and colony brood influence excavation and lead to the adjustment of nest enlargement in leaf-cutting ants. Insect. Soc. 62, 401–413 (2015). https://doi.org/10.1007/s00040-015-0419-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-015-0419-1