Abstract
In leaf-cutting ants, workers are expected to excavate the nest at a soil depth that provides suitable temperatures, since the symbiotic fungus cultivated inside nest chambers is highly dependent on temperature for proper growth. We hypothesize that the different nesting habits observed in Acromyrmex leaf-cutting ants in the South American continent, i.e. superficial and subterranean nests, depend on the occurrence, across the soil profile, of the temperature range preferred by workers for digging. To test this hypothesis, we first explored whether the nesting habits in the genus Acromyrmex are correlated with the prevailing soil temperature regimes at the reported nest locations. Second, we experimentally investigated whether Acromyrmex workers engaged in digging use soil temperature as a cue to decide where to excavate the nest. A bibliographic survey of nesting habits of 21 South American Acromyrmex species indicated that nesting habits are correlated with the soil temperature regimes: the warmer the soil at the nesting site, the higher the number of species inhabiting subterranean nests, as compared to superficial nests. For those species showing nesting plasticity, subterranean nests occurred in hot soils, and superficial nests in cold ones. Experimental results indicated that Acromyrmex lundi workers use soil temperature as an orientation cue to decide where to start digging, and respond to rising and falling soil temperatures by moving to alternative digging places, or by stopping digging, respectively. The soil temperature range preferred for digging, between 20°C and maximally 30.6°C, matched the range at which colony growth would be maximized. It is suggested that temperature-sensitive digging guides digging workers towards their preferred range of soil temperature. Workers’ thermopreferences lead to a concentration of digging activity at the soil layers where the preferred range occurs, and therefore, to the construction of superficial nests in cold soils, and subterranean ones in hot soils. The adaptive value of the temperature-related nesting habits, and the temperature-sensitive digging, is further discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For ants inhabiting nests excavated in soil, nest depth largely affects microclimatic conditions faced by colonies (Seeley and Heinrich 1981; Sudd 1982), since temperature, humidity, and air composition strongly vary with soil depth (Hillel 1998). Therefore, ants are expected to dig their nests at those soil layers providing a proper microclimate for colony growth.
The selection of an adequate soil layer for nest location should be particularly relevant for leaf-cutting ants. Leaf-cutting ants of the genera Atta and Acromyrmex cut leaves as a substrate for a symbiotic fungus they cultivate inside the nest chambers. This fungus has strict demands of high humidity and temperatures between 25 and 30°C for proper growth (Powell and Stradling 1986; Quinlan and Cherrett 1978). It represents the only food source for the developing brood, and to a lesser extent, for the adults (Bass and Cherrett 1995; Quinlan and Cherrett 1979; Weber 1972). Workers are therefore expected to provide it with an adequate nest microclimate so as to ensure its growth.
In the leaf-cutting ant genus Acromyrmex, nests show interspecific differences regarding their depth (Fowler and Claver 1991). Some species construct mound-shaped superficial nests, with the fungus garden located at the soil surface level and covered by a thatch mound composed of soil and plant fragments. In contrast, other species inhabit nests with multiple chambers excavated up to a depth of 3 m (Bonetto 1959; Fowler 1985; Gonçalves 1961; Lapointe et al. 1998). Acromyrmex species inhabiting mound-shaped superficial nests are widespread in the southernmost colder regions of South America, thus suggesting that thatched nests have the same properties as the mound nests of wood ants (genus Formica) in the northern hemisphere (Seeley and Heinrich 1981), i.e. the thatch helps the colony to achieve higher and more stable temperatures than those of the surrounding environment (Farji-Brener 2000). The predominant occurrence of superficial nests with increasing latitude in Acromyrmex and other ants (Seeley and Heinrich 1981) correlates with the concomitant decrease in average soil temperatures with latitude (Hillel 1998; Rosenberg et al. 1983). We propose the hypothesis that the two nesting habits observed in Acromyrmex, i.e. superficial and subterranean nests, are brought about by the temperature preferences of workers when excavating the fungus chambers. Given that soil temperature is negatively correlated with soil depth (Campbell 1977; Hillel 1998; Rosenberg et al. 1983), the preferred temperature range for digging would occur in hot soils at deeper layers than in cold soils. The prevailing soil temperature regime is therefore expected to influence the determination of nest depth in Acromyrmex leaf-cutting ants.
Even though other variables like soil moisture or air composition also depend on soil depth, we argue that soil temperature is the most relevant selective force that has influenced the determination of nest depth in Acromyrmex species over evolutionary time. Without disregarding the influence of soil moisture on nest depth, as reported for a number of ant species (Seeley and Heinrich 1981; Sudd and Franks 1987), temperature is known to largely determine growth, brood production and survival in ant colonies (Brian and Brian 1951; Callcott et al. 2000; Korzukhin et al. 2001; Markin et al. 1974; Porter 1988; Southerland 1988). For leaf-cutting ants in particular, temperature is a powerful variable predicting the occurrence and density of Paraguayan and Argentinean species (Farji-Brener 1994; Fowler 1983), and workers show temperature-sensitive behavioural responses in different contexts, such as brood or fungus relocation (Bollazzi and Roces 2002), and food search (Kleineidam et al. 2007).
We first hypothesize that Acromyrmex species present a subterranean nesting habit in hot soils, and a superficial one in cold soils. To evaluate this hypothesis, a bibliographic survey on 21 South American Acromyrmex species was carried out, and the relationship between nesting habit and soil temperature at the nesting site was established. We further hypothesize that the observed nesting habits are brought about by the temperature preferences of workers while digging. Therefore, we have performed a series of laboratory experiments with the species Acromyrmex lundi aimed at assessing: (1) the influence of soil temperature on workers’ digging performance; (2) whether workers show thermopreferences and use soil temperature as a cue to decide where to start digging; (3) whether digging workers respond to changes in soil temperature by giving up their activity and by changing digging places, thus allowing them to seek for their preferred soil temperatures for further digging.
Materials and methods
Soil temperature and observed nest depths in South American species of the genus Acromyrmex
The bibliographic survey on South American Acromyrmex species was carried out so as to: (1) classify the species as having either subterranean or superficial nests, and (2) determine the soil temperature regime (see below) of the locations where species have been reported to occur.
First, nests were assigned as either subterranean or superficial depending on the location of the fungus chambers in the soil profile. In superficial nests, an imaginary line representing the soil surface would cut through a fungus chamber at a given point. In such cases, the fungus gardens are usually covered by a thatch of plant fragments and soil, as in Acromyrmex heyeri (Fig. 1). In contrast, in subterranean nests one or multiple fungus chambers are entirely located under the soil surface level, as in Acromyrmex striatus (Fig. 1).
Second, the locations where the surveyed Acromyrmex species have been recorded were assigned as having one of two possible soil temperature regimes, i.e. the mean annual soil temperature at a depth of 50 cm. The data of Van Wambeke (1981) and the Global Soil Temperature Regimes map (USDA 2005) were used. Both the thermic and isothermic soils belong to the first category, called thermic soils (USDA 1975), which present a mean annual soil temperature between 15 and 22°C. Both the hyperthermic and isohyperthermic soils belong to the second category, called hyperthermic soils (USDA 1975), which present a mean annual soil temperature higher than 22°C. Since shading alters soil temperature regimes by locally diminishing soil temperature (Alvalá et al. 2002; Rosenberg et al. 1983; Weber 1959), the nesting sites were scored as exposed or shaded. Such division has commonly been employed in several surveys of the Acromyrmex genus throughout South America (Fowler 1985; Fowler and Claver 1991; Gonçalves 1961; Kusnezov 1978). It considers the vegetation cover at the nesting site: nests in exposed grasslands versus nests under the shade of trees or inside woods. This classification results in four categories of nesting sites, ranging from the colder to the warmer ones as follows: shaded thermic soils, exposed thermic soils, shaded hyperthermic soils, and exposed hyperthermic soils. The bibliographic survey was limited to Argentina, Brazil, Colombia, Paraguay, Venezuela and Uruguay, since the most complete investigations regarding nesting habits of Acromyrmex species have only been made in these countries.
Effect of soil temperature on workers’ digging performance
Laboratory experiments were performed with 1-one-year old colony of Acromyrmex lundi collected in Sarandi del Yi, Uruguay (33 20′25″S, 55 37′53″W), and transported to the Department of Behavioural Physiology and Sociobiology at the University of Würzburg, Germany. A. lundi was chosen because colonies show plasticity in their nesting habits. Colonies dig subterranean nests (Fowler 1985) in the hot soils of Paraguay (Van Wambeke 1981), but inhabit in addition a thatch mound of leaf fragments and debris between aerial tree roots (Bonetto 1959) in the milder soils of west Argentina (Van Wambeke 1981). The soil used during the laboratory experiments was a mixture of sand and clay (2:1) with a mass water content of 12% (range 10–15%).
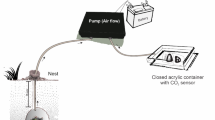
In order to assess how soil temperature affects workers’ digging performance, groups of five workers (mean body mass = 2.46 mg) were presented with soils at either 10, 15, 20, 25, 30, 35 or 40°C, and the total amount of soil excavated over 4 h was recorded. A plastic tube of 10 cm length and 1 cm diameter filled with soil was used as a digging tube (Fig. 2a). It was fitted within an aluminium plate fixed to a thermostatic plate, which maintained the soil at the desired temperature by means of a thermoregulated water bath. The ants were introduced in a small box attached to the digging tube, and allowed to dig for 4 h. Twenty-four replicates were done for each soil temperature.
Ant box (A), aluminium plate (a), digging tube (D), thermostatic plate (Th) attached to a thermal bath (Tb) to regulate its temperature, digging boxes with different temperatures (D1, D2), initial digging box (Di) and optional digging box (Do), computer-controlled Peltier element (Pe). Grey areas represent the soil. Scale bar 2 cm (only for the digging boxes)
Soil temperature selected by workers for commencement of digging
In order to know whether workers decide where to start digging depending on the sensed soil temperature, groups of three workers were simultaneously confronted with two soils at different temperatures, and their preferences were recorded. For this, a simultaneous binary choice between a soil at 25°C and a soil at either 15, 20, 30 or 35°C was used. The experimental set up consisted of three interconnected boxes (Fig. 2b). The two lateral ones, called the digging boxes, were filled with soil and placed on separated plates independently thermoregulated by two thermal baths attached to them. When the soil inside the digging boxes reached the two temperatures to be tested, three workers were introduced in the middle box. The temperature selected for the commencement of digging was defined as that of the digging box where workers started and continued digging over 30 min. Having once selected a box, workers were not observed changing to the other side. In control assays both soils were maintained at 25°C, and preferences for a particular side were recorded in the same way. Forty replicates were carried out for each pair of tested temperatures.
Workers’ responses to increasing and decreasing soil temperatures while digging
In natural soils, temperature varies continuously over time, so that workers are expected to be exposed to changing temperatures while engaged in digging. Therefore, workers were confronted with either decreasing or increasing soil temperatures while digging, and their performance was recorded. It was quantified as the rate of soil pellets removed from a soil undergoing a continuous temperature change, compared with a soil at constant temperature. A group of five workers was placed inside an ant box (Fig. 2c) located between two digging boxes filled with soil initially maintained at room temperature, 24°C. Workers could start digging only inside the so-called initial digging box, because the access to the opposite one, called the optional digging box, was blocked by a sliding door. Thirty minutes later, this door was opened, so that workers could choose between either continuing digging inside the initial digging box or moving to the optional digging box. Digging activity was monitored over 100 min (see below). Through this procedure, it was assessed whether workers show site fidelity to the initial digging place when soil temperature remained unchanged. This series was considered the control one. In two other independent experimental series, the sliding door was opened 30 min after the assays began, and the soil temperature at the initial digging place was either increased or decreased at a rate of 0.1°C/min for 100 min. Thus, the soil temperature changed either from 24 to ca. 34°C, or from 24 to ca. 14°C. Inside the optional digging box the soil was maintained at the same temperature workers experienced in the initial box at the beginning (ca. 24°C). By measuring workers’ digging activity in the initial and optional digging boxes, the temperature at which workers stopped digging in the first box and eventually moved into the optional box was assessed. As a measure of digging activity, the number of soil pellets removed by workers from each digging box over time was recorded by four video cameras placed at each side of both digging boxes. From the video recording, the number of pellets removed every 5 min (with constant, increasing or decreasing temperatures) was counted. A very accurate change of soil temperature inside the initial box was achieved using a computer-controlled Peltier element located inside an aluminium plate, above which the initial digging box was placed (Fig. 2c). Soil and air temperature were recorded inside the initial and optional digging boxes using a temperature data logger equipped with four wire sensors (Voltcraft® K204). Twelve replicates of the series with increasing and decreasing temperatures, as well as the control ones, were performed.
Finally, in order to know whether the threshold temperature at which workers stop digging may compromise worker survival, mortality rates of A. lundi workers as a function of temperature were evaluated. For this, independent groups of 50 workers (mean body mass = 2.43 mg) were placed in Petri dishes inside an incubator at 98% relative humidity, and exposed to constant air temperatures ranging from 25 to 42°C, in 1°C steps. After 6 h, the number of dead workers in each group was counted.
Results
Soil temperature and nesting habits in Acromyrmex
For the 21 South American Acromyrmex species, the warmer the soil at the nesting site, as a consequence of soil temperature regime and nesting-site exposition, the higher the number of species inhabiting subterranean nests (G-test = 13.65, df = 3, P = 0.003) (Fig. 3a, Table 1). Whereas Acromymrex species exclusively inhabit superficial nests in colder soils, mostly subterranean nests occur in the hottest soils. In the milder soils, Acromyrmex inhabit both nest types. Figure 3b presents the distribution of thermic and hyperthermic soil temperature regimes in South America, as well as examples of the soil temperature variation over 48 h at locations subjected to either a hyperthermic or a thermic regime.
a Nest types recorded for 21 Acromyrmex species depending on the soil temperature regimes (thermic or hyperthermic soil) and nesting site exposition (shaded or exposed) at the site of occurrence. bLeft Distribution of thermic and hyperthermic soils in South America, partially based on the Global Soil Temperature Regimes map (USDA 2005). Right Soil temperature at 10 cm (continuous line) and 40 cm depth (dashed line) over 48 h in a hyperthermic (10°56′S, 45°69′W) and a thermic (29°17′S, 53°69′W) soil in spring 2005. Data obtained from the PCD Program (CPTEC 2006)
Table 1 presents the data of nesting habits of the surveyed species depending on the soil temperature regime. Two Acromyrmex subgenera are recognized based on workers’ morphology and the substrate used to cultivate the fungus (Gonçalves 1961; Weber 1972). First, the subgenus Acromyrmex that comprises species cutting dicotyledoneous leaves, i.e. leaf-cutting ants (Table 1). For this subgenus, the warmer the soil at the nesting site, the higher the number of species inhabiting subterranean nests (G-test = 12.2, df = 3, P = 0.006). Five out of the 16 species in the Acromyrmex subgenus, i.e. ambiguus, aspersus, crassispinus, lundi and rugosus, showed intraspecific nesting plasticity (Table 1), i.e. the nesting habit depended on soil temperature, being superficial in colder soils and subterranean in the hottest ones (G-Test = 9.64, df = 3, P = 0.02). Second, the subgenus Moellerius, which comprises five species of grass-cutting ants (Table 1). Within this subgenus, there was no correlation between nesting habit and soil temperature at the nesting site (G-test for exposed hyperthermic vs. exposed thermic = 0.17, df = 1, P = 0.67). With the exception of A. heyeri that inhabits a superficial nest, the other four Moellerius species occurred in subterranean nests irrespective of soil temperature.
Soil temperature and digging behaviour
Soil temperature strongly influenced digging performance of A. lundi workers. Workers dug more in soils at 25°C than in soils at lower or higher temperatures (Fig. 4).
When presented with a choice between two constant temperatures, i.e. 25°C and one alternative, workers preferred to start digging in soils at 25°C than in soils at 15 and 35°C, and there were no differences in preference in the range of 20–30°C (Fig. 5).
Workers responded to changes in soil temperature experienced while digging, but qualitatively differently to increasing and decreasing temperatures. During the control experiments with no temperature change, workers mostly continued digging at the initial digging place after the sliding door was opened (Fig. 6a). In contrast, workers exposed to increasing temperature at the initial digging place moved to the optional digging place after a while (Fig. 6b), and their digging activity was almost completely concentrated there. In the experiments with decreasing soil temperature (Fig. 6c), digging activity in the initial box decreased as temperature changed, but no concomitant increase in activity in the optional box was observed. The total digging activity was therefore lower than that observed in the two previous experiments (Fig. 6; one-way ANOVA, F (34,2) = 17.26, P < 0.001; post hoc Scheffe test at P < 0.001). There was no difference in the total digging activity between the control series and the series with increasing temperatures (one-way ANOVA, F (34,2) = 17.26, P < 0.001; post hoc Scheffe test at P < 0.001).
Number of soil pellets removed every 5 min by workers either from the initial digging box (grey portion) or the optional digging box (white portion). a Control experiments with constant soil temperature (24°C) in both digging boxes. b Experiments with increasing soil temperature in the initial digging box, and constant in the optional one. c Experiments with decreasing soil temperature in the initial digging box, and constant in the optional one. Bars far right Total digging activity in the initial and optional digging boxes
For the series with increasing temperatures, the average threshold temperature at which workers decided to give up and to change the digging place was evaluated by plotting digging activity in the initial digging box (as a percentage of the total activity in the two boxes) as a function of the actual temperature (Fig. 7a). The sigmoidal fit shows that the soil temperature at which digging activity decreased to 50% at the initial box was 30.3°C. Figure 7b presents the relationship between worker mortality and temperature established in the laboratory assays. The sigmoidal fit shows that the lethal air temperature (50% mortality) was 39.7°C.
a Digging activity in the initial box, as a percentage of the total number of pellets removed from both digging boxes, as a function of the average temperature of the 5-min interval (data from the grey/white bars, see Fig. 6b). Continuous line Digging activity (%) = 98.13 {1 + exp[−(T − 30.31)/−1.67]} (T in °C), R2 = 0.59, P < 0.001. b Worker mortality as a function of air temperature in 1°C steps (n = 7, ±SE). Dashed line Mortality (%) = 101.63 {1 + exp[-(T − 39.72)/−0.36]} (T in °C), R2 = 0.98, P < 0.001. Dotted lines Soil temperature at which digging activity fell to 50% (a), and air temperature at which worker mortality reached 50% (b)
Discussion
Nest depth as an adaptation based on thermoregulatory needs
Our bibliographic survey suggests that the nest depths in Acromyrmex leaf-cutting ants are adaptive responses based on the colony’s thermoregulatory needs. The warmer the soil, i.e. from shaded thermic soils to exposed hyperthermic soils, the higher the number of species inhabiting subterranean nests (Fig. 3a). We argue that inhabiting subterranean nests in warm soils is advantageous because of the avoidance of higher temperatures at the superficial soil layers (Fig. 3b). Conversely, living in superficial nests in cold soils benefits from the milder temperatures at the superficial soil layers (Fig. 3b). The observed trend, however, should be interpreted with caution. First, although each species contributed only once to each soil temperature category, most of them contributed more than one observation to the whole data set. Second, species are not necessarily independent sampling units, because closely related species often inherit traits from common ancestors. These considerations make it difficult to perform a comparative interspecific analysis of what we consider an adaptive trait (Harvey and Pagel 1991). Therefore, it is worthwhile to consider the conditions under which the Acromyrmex genus originated in South America, as well as the phylogenetic relationship among species.
The Attini ant-fungus mutualism originated in South America during the early Cenozoic, 45–65 million years ago (Mueller et al. 2001; Schultz and Brady 2008), when the South American climate was warm, wet and non-seasonal (Ortiz-Jaureguizar and Cladera 2006). The leaf-cutting ant genera Atta and Acromyrmex originated in southern South America later during the Miocene, around 8–12 million years ago (Schultz and Brady 2008). The South American climate started to become dryer then, and colder and seasonal in regions south of 15°S, and the biomes were dominated by park and grassland savannas (Ortiz-Jaureguizar and Cladera 2006). Given that Acromyrmex species originated under climatic conditions that could be regarded as unsuitable for fungal growth (Griffin 1994), colonies are therefore expected to have developed adaptations to maintain their fungus gardens under proper microclimatic conditions inside nests.
A closer examination of Table 1 suggests that the adaptation to soil temperature through the different nesting habits mostly occurred in the subgenus Acromyrmex, because members of the subgenus Moellerius with the exception of A. heyeri, present a subterranean nesting habit independently of soil temperature. Since species belonging to the subgenus Acromyrmex probably originated from Moellerius ancestors (Fowler 1982), it can be argued that the superficial nesting habit is a derived trait. This hypothetical scenario is supported, first, by the fact that the fungus-growing ant genus Trachymyrmex, which represents the transition between the lower attines and the Acromyrmex (Brandão and Mayhé-Nunes 2007; Mayhé-Nunes and Jaffé 1998; Schultz and Brady 2008), inhabit subterranean nests (Weber 1972) that show marked morphological similarities to the subterranean nests of four out of the five Moellerius species. Both in the genus Trachymyrmex and the Moellerius group, nests comprise multiple regularly-spaced fungus chambers interconnected by tunnels (Weber 1972). Second, from the time of the postulated origin of Acromyrmex, 8–12 million years ago (Schultz and Brady 2008), temperatures have continuously decreased in South American latitudes south of 15°S (Ortiz-Jaureguizar and Cladera 2006). Therefore, adaptations to colder environments are expected to have evolved, such as the acquisition of a superficial nesting habit and the construction of thatched nests with thermoregulatory benefits. As mentioned above, the likely basal Moellerius species inhabit subterranean nests, yet as grass-cutting ants they are restricted to grasslands and exposed nesting sites, and are more abundant in hot hyperthermic soils (Table 1). Inhabiting subterranean nests under these conditions would have the advantage of avoidance of higher soil temperatures near the soil surface. In this line of argument, it is noteworthy that the only Moellerius species that build superficial thatched nests, A. heyeri, is one of the southernmost distributed species of Acromyrmex. It reinforces the idea that the construction of superficial thatched nests is an adaptive response to soil temperature.
In addition to the observed temperature-related pattern of nesting habits across species, intraspecific plasticity in nesting habits depending on soil temperature has also been reported for members of the Acromyrmex subgenus (Table 1). To what extent this plasticity can be accounted for by the experimentally explored temperature-sensitive digging behaviour is discussed in the next sections.
Temperature-sensitive digging and nest depth
Our laboratory investigations showed that A. lundi workers use soil temperature as an orientation cue to decide where to start digging. In addition, workers respond to rising and falling soil temperatures by either moving to alternative digging places, or by stopping digging, respectively. These results indicate that A. lundi workers show a marked thermopreference while digging, and prefer to dig in soils with temperatures between 20°C and maximal ca. 30°C. This is the first experimental demonstration that temperature is used as a orientation cue in the context of collective digging in ants. We suggest that during nest growth, digging workers orient towards their preferred range of soil temperature, which leads to a concentration of digging activity at the layers where it occurs. For instance, the higher the average soil temperature, the deeper the nest location, since the preferred temperature range will occur at deeper layers. Such a mechanism may account for the construction of superficial nests in cold soils, and subterranean ones in warm soils.
As in most ants, nest excavation in Acromyrmex begins as a founding chamber dug by the mated queen at 5–20 cm depth (Bruch 1923; Camargo et al. 2004; Montenegro 1973). In exposed habitats of tropical areas, where temperatures at the soil surface may exceed 30°C during daily oscillations (Passerat de Silans et al. 2006) (Fig. 3b), workers are expected to dig downwards to avoid them. Our results support this view, because workers avoided a soil at 35°C to start digging, yet started in a soil at 25°C (Fig. 5). In addition, workers digging in a soil with increasing temperature stopped digging around 30–32°C, but continued digging at the same rate at a neighbouring location at 25°C (Fig. 6b). In both shaded hyperthermic soils and in thermic soils, however, the need to avoid the superficial soil layers during nest enlargement would not necessarily be so marked. There, the location of the superficial founding chamber may already offer a suitable temperature range for colony growth (Fig. 3b). Therefore, workers may enlarge the nest without the need to go deeper into the soil profile, where soil temperatures are likely lower than near the surface. This view is also supported by our results. Workers were reluctant to start digging in soils at 15°C (Fig. 5), and when confronted with decreasing soil temperatures, diminished digging activity and did not show a tendency to change digging places (Fig. 6c). Acromyrmex superficial nests would therefore be expected to comprise one single large chamber. This is indeed the fact for nine species inhabiting superficial nests, with the only exception being Acromyrmex lobicornis, which excavates multiple small chambers inside a solid mound constructed with soil and debris (Bonetto 1959; Gonçalves 1961; Zolessi and González 1974).
As mentioned in the Introduction, other variables such as soil moisture, or colony size, are also expected to influence the determination of nest depth. Nest depth in Acromyrmex landolti, as an example, has been shown to be negatively correlated with soil moisture (Lapointe et al. 1998), and colonies are known to relocate fungus gardens across the soil profile as a response to soil moisture. Humidity control is highly relevant for fungal growth (Roces and Kleineidam 2000), but it appears unlikely that humidity requirements alone may account for the reported patterns of nesting habits in the genus Acromyrmex. Soil temperature seems to be a more powerful predictor of nesting habits. For instance, Acromyrmex crassispinus colonies build subterranean nests in the hot soils of Paraguay, yet superficial ones in the colder thermic soils of central Argentina and south Brazil (Bonetto 1959; Fowler 1985; Gonçalves 1961; Guerra de Gusmão 1998; Link et al. 2001c), although both regions present the same soil moisture regime (USDA 1975).
Regarding the effect of colony size on nest depth, it is known for ants that the excavated nest volume depends on the number of colony workers (Buhl et al. 2005; Deneubourg and Franks 1995; Halley et al. 2005; Rasse and Deneubourg 2001). Therefore, colonies would simply go deeper as they grow because of the increasing excavated volume, and differences in nest depth among colonies could merely be regarded as a by-product of differences in the sizes of mature colonies. In addition, ant colonies in temperate areas tend to be larger than those of tropical areas (Kaspari and Vargo 1995). Assuming this trend for the genus Acromyrmex, and considering that nest depth would depend on colony size as indicated above, Acromyrmex nests should occur deeper in temperate areas than in tropical ones. However, our results do not support this hypothesis, since the subterranean nesting habit mainly occurs in the tropical areas, where hyperthermic soils are predominant, and the superficial nesting habit in the temperate areas with thermic soils. Regarding colony size, while Acromyrmex rugosus colonies excavate subterranean nests in hyperthermic soils in north Brazil (Fernandes Soares et al. 2006), A. heyeri colonies build superficial nests on thermic soils of southern Brazil, even though the latter have a 10 times larger worker population (Diehl-Fleig and Droste 1992; Guerra de Gusmão 1998).
The adaptive value of temperature-sensitive digging
The adaptive value of temperature-sensitive digging is emphasized by the threshold temperature at which A. lundi stops digging when temperature increases, i.e. 30°C as determined in our study (Fig 7a). This temperature is not lethal at all for workers (Fig 7b), but matches the temperature at which brood development in ants starts being negatively affected, as reported for two common South American ants from subtropical and temperate areas, Camponotus mus and Solenopsis invicta (Porter 1988; Porter and Tschinkel 1993; Roces and Núñez 1989). Temperatures above 30°C were in addition found to be lethal for the symbiotic fungus Attamyces bromatificus isolated from colonies of Acromyrmex octospinosus, Atta cephalotes and Trachymyrmex urichi (Powell and Stradling 1986). Thus, A. lundi workers seem to avoid, while digging, the soil temperatures that are unsuitable for both fungus and brood development, and prefer those temperatures that are known to maximize fungal growth (Bollazzi and Roces 2002; Powell and Stradling 1986).
We suggest that plasticity in nesting habits based on temperature-sensitive digging has promoted the colonization of different habitats. It is noteworthy that the five Acromyrmex species with changing nesting habits occur in more habitats, from exposed hyperthermic soils to shaded thermic ones (Table 1), than those species lacking nesting plasticity, such as the Moellerius species confined to exposed soils (Table 1). This emphasizes the fact that for nest-building animals, an advance in their abilities to control the nest microclimate could lead to an extension of the habitats for the taxon (Hansell 2005). Although not necessarily linked to speciation, innovations in building behaviour that give rise to the invasion of new habitats might subsequently facilitate adaptive radiation (Hansell 2005). Interestingly, the subgenus Acromyrmex, with members that show plasticity in nesting habits depending on soil temperature, presents more species than the subgenus Moellerius, with members that inhabit invariant nests.
As for other ants, differences in nest depth in Acromyrmex have been regarded as adaptations aimed at achieving a suitable nest microclimate (Farji-Brener 2000; Lapointe et al. 1998; Navarro and Jaffé 1985; Seeley and Heinrich 1981). However, the question about what variable within the soil environment may account for the observed nest depths in ants remained unexplored. Our analysis suggests that soil temperature is a strong predictor of the observed nest depths in the genus Acromymrex. Further, our study provides, to our knowledge, the first experimental evidence for the use of soil temperature by digging workers as an orientation cue, suggesting its major role for the determination of nest depth across the soil profile. Temperature-sensitive digging behaviour in Acromyrmex would therefore help colonies to achieve a proper nest climate for fungus and brood development, and so maximize colony growth rates.
References
Alvalá RCS, Gielow R, da Rocha HR, Freitas HC, Lopes JM, Manzi AO, Diaz MAFS, Cabral OMR (2002) Intradiurnal and seasonal variability of soil temperature, heat flux, soil moisture content, and thermal properties under forest and pasture in Rondônia. J Geophys Res 107:8043
Bass M, Cherrett JM (1995) Fungal hyphae as a source of nutrients for the leaf-cutting ant Atta sexdens. Physiol Entomol 20:1–6
Bollazzi M, Roces F (2002) Thermal preference for fungus culturing and brood location by workers of the thatching grass-cutting ant Acromyrmex heyeri. Insectes Soc 49:153–157
Bonetto AA (1959) Las hormigas “cortadoras” de la provincia de Santa Fé (Géneros: Atta y Acromyrmex). Ministerio de Agricultura y Ganaderia, Provincia de Santa Fe, Argentina
Brandão CRF, Mayhé-Nunes AJ (2007) A phyologenetic hypothesis for the Trachymyrmex species groups, and the transition from fungus-growing to leaf-cutting in the Attini. In: Snelling RR, Fisher BL, Ward PS (eds) Advances in ant systematics (Hymenoptera:Formicidae): homage to E.O. Wilson: 50 years of contributions. Memoirs of the American Entomological Institute, vol 80. American Entomological Institute, Gainesville
Brian MV, Brian AD (1951) Insolation and ant populations in the west of Scotland. Trans R Entomol Soc Lond 102:303–330
Bruch C (1923) Estudios mirmecológicos con la descripción de nuevas especies de dípteros (Phoridae) por los Rr. Pp. H. Schmitz y Th. Borgmeier y de una araña (Gonyleptidae) por el Doctor Mello-Leitão. Rev Mus La Plata 27:172–220
Buhl J, Deneubourg J, Grimall AGT (2005) Self-organized digging activtiy in ant colonies. Behav Ecol Sociobiol 58:9–17
Callcott AMA, Oi DH, Collins HL, Williams DF, Lockley TC (2000) Seasonal studies of an isolated red imported fire ant (Hymenoptera: Formicidae) population in eastern Tennessee. Environ Entomol 29:788–794
Camargo R, Forti LC, Lopes JF, De Andrade APP (2004) Characterization of Acromyrmex subterraneus bruneus (Hymenoptera:Formicidae) young nests in a fragment of the neotropical forest. Rev Árvore 28:309–312
Campbell GS (1977) An introduction to environmental biophysics. Springer, New York
Carbonell CS (1943) Las hormigas cortadoras del Uruguay. Rev Asoc Ing Agron Montev 15:30–39
CPTEC (2006) Plataforma de Coleta de Dados. Centro de Previsao de Tempo e Estudos Climaticos. Ministerio de Ciencia e Tecnologia. Brasil. http://satelite.cptec.inpe.br/PCD/
De Andrade ML (1991) Bionomia e Distribução do Gênero Acromyrmex Mayr, 1865 (Hymenoptera: Formicidae) no estado de São Paulo, Brasil., M.S. thesis Universidade Estadual Paulista, Botucatu, São Paulo, Brasil
Deneubourg JL, Franks NR (1995) Collective control without explicit coding: the case of communal nest excavation. J Insect Behav 8:417–432
Diehl-Fleig E, Droste A (1992) Localizaçâo, morfologia externa e flutuações populacionais ao longo do ano de colônias de Acromyrmex heyeri (Hymenoptera: Formicidae). An Soc Entomol Bras 21:21–27
Espina ER, Timaure A (1977) Características de los nidos de Acromyrmex landolti (Forel), en el oeste de Venezuela. Rev Fac Agron Univ Zulia 4:53–62
Farji-Brener AG (1994) Leaf-cutting ants (Atta and Acromyrmex) inhabiting Argentina: patterns in species richness and geographical range sizes. J Biogeogr 21:391–399
Farji-Brener AG (2000) Leaf-cutting ant nests in temperate environments: mounds, mound damages and nest mortality in Acromyrmex lobicornis. Stud Neotrop Fauna Environ 35:131–138
Fernandes Soares IM, Della Lucia TMC, dos Santos AA, Cardoso Nascimento I, Delabie JHC (2006) Caracterizaçâo de ninhos e tamanho da colônia de Acromyrmex rugosus (F.Smith) (Hymenoptera, Formicidae, Attini) em restingas de Ilhéus, BA, Brasil. Rev Bras Entomol 50:128–130
Fowler HG (1982) Evolution of the foraging behaviour of leaf-cutting ants (Atta and Acromyrmex). In: Breed MD, Michener CD, Evans HE (eds)The biology of social insects. Proceedings of the 9th congress of the international union for the study of social insects. West View Press, Boulder
Fowler HG (1983) Distribution patterns of Paraguayan leaf-cutting ants (Atta and Acromyrmex) (Formicidae: Attini). Stud Neotrop Fauna Environ 18:121–138
Fowler HG (1985) Leaf-cuttings ants of the genera Atta and Acromyrmex of Paraguay. Dtsch Entomol Z 32:19–34
Fowler HG, Claver S (1991) Leaf-cutter ant assemblies: effects of latitude, vegetation, and behaviour. In: Huxley CR, Cutler DF (eds) Ant–plant interactions. Oxford University Press, Oxford, pp 51–59
Gonçalves CR (1961) O gênero Acromyrmex no Brasil (Hym. Formicidae). Stud Entomol 4:113–180
Griffin DH (1994) Fungal physiology. Wiley-Liss, New York
Guerra de Gusmão L (1998) Distribução geográfica de formigas cortadeiras do gênero Acromyrmex (Hymenoptera: Formicidae) na zona sul do estado do Rio Grande do Sul, Brasil., M.S. thesis Federal University of Pelotas. Pelotas, Rio Grande do Sul, Brazil
Halley JD, Burd M, Wells P (2005) Excavation and architechture of Argentine ant nests. Insectes Soc 52:350–356
Hansell M (2005) Animal architecture. Oxford University Press, Oxford
Harvey P, Pagel M (1991) The comparative method in evolutionary biology. Oxford Univ Press, London
Hillel D (1998) Environmental soil physics. Academic Press, London
Kaspari M, Vargo EL (1995) Colony size as a buffer against seasonality: Bergmann’s rule in social insects. Am Nat 145:610–632
Kleineidam C, Rutchy M, Casero-Montes ZA, Roces F (2007) Thermal radiation as a learned orientation cue in leaf-cutting ants (Atta volenweideri). J Insect Physiol 53:478–487
Korzukhin MD, Porter SD, Thompson LC, Wiley S (2001) Modeling temperature-dependent range limits for the fire ant Solenopsis invicta (Hymenoptera: Formicidae) in the United States. Environ Entomol 30:645–655
Kusnezov N (1956) Claves para la identificación de las hormigas de la fauna argentina. Idia August–September:1–58
Kusnezov N (1978) Hormigas argentinas–Clave para su identificación. Miscelánea 61, Ministerio de Cultura y Educación. Fundación Miguel Lillo, Tucumán
Lapointe SL, Serrano MS, Jones PG (1998) Microgeographic and vertical distribution of Acromyrmex landolti (Hymenoptera: Formicidae) nests in a neotropical savanna. Environ Entomol 27:636–641
Link D, Link FM, Grigoletto PM, Goulart L (2001a) Aspectos morfológicos externos do ninho de Acromyrmex ambiguus em bosques de Eucalipto. In: Anais do XV Encontro de Miremecologia. Londrina, Parana, Brazil
Link FM, Link D, Grigoletto PM, Goulart L (2001b) Aspectos morfológicos externos do ninho de Acromyrmex aspersus em bosques de Eucalipto. In: Anais do XV Encontro de Miremecologia. Londrina, Parana, Brazil
Link FM, Link D, Grigoletto PM, Goulart L (2001c) Aspectos morfológicos externos do ninho de Acromyrmex crassispinus em bosques de Eucalipto. In: Anais do XV Encontro de Miremecologia. Londrina, Parana, Brazil
Markin GP, O’Neal J, Dillier JH, Collins HL (1974) Regional variation in the seasonal activity of the imported fire ant, Solenopsis saevissima richteri. Environ Entomol 3:446–452
Mayhé-Nunes AJ, Jaffé K (1998) On the biogeography of Attini (Hymenoptera:Formicidae). Ecotropicos 11:45–54
Montenegro RA (1973) La fundación del hormiguero en Acromyrmex striatus (Rog.) (Hymenoptera, Formicidae). Stud Entomol 16:343–352
Mueller UG, Schultz TR, Currie CR, Adams RMM, Malloch D (2001) The origin of the attine ant-fungus mutualism. Q Rev Biol 76:169–197
Navarro JG, Jaffé K (1985) On the adaptive value of nest features in the grass-cutting ant Acromyrmex landolti. Biotropica 17:347–348
Ortiz-Jaureguizar E, Cladera GA (2006) Paleoenvironmental evolution of southern South America during the Cenozoic. J Arid Environ 66:498–532
Passerat de Silans A, Moreira da Silva F, de Assis dos Reis Barbosa F (2006) Determinação in loco da difusividade térmica num solo da região de Caatinga (PB). R Bras Sci Solo 30:41–48
Porter SD (1988) Impact of temperature on colony growth and developmental rates of the ant, Solenopsis invicta. J Insect Physiol 34:1127–1133
Porter SD, Tschinkel WR (1993) Fire ant thermal preferences: behavioral control of growth and metabolism. Behav Ecol Sociobiol 32:321–329
Powell RJ, Stradling DJ (1986) Factors influencing the growth of Attamyces bromatificus, a symbiont of attine ants. Trans Br Mycol Soc 87:205–213
Quinlan RJ, Cherrett JM (1978) Aspects of the symbiosis of the leaf-cutting ant Acromyrmex octospinosus (Reich) and its food fungus. Ecol Entomol 3:221–230
Quinlan RJ, Cherrett JM (1979) The role of fungus in the diet of the leaf-cutting ant Atta cephalotes (L.). Ecol Entomol 4:151–160
Rasse P, Deneubourg JL (2001) Dynamics of nest excavation and nest size regulation of Lasius niger (Hymenoptera: Formicidae). J Insect Behav 14:433–449
Roces F, Kleineidam C (2000) Humidity preference for fungus culturing by workers of the leaf-cutting ant Atta sexdens rubropilosa. Insectes Soc 47:348–350
Roces F, Núñez JA (1989) Brood translocation and circadian variation of temperature preference in the ant Camponotus mus. Oecologia 81:33–37
Rosenberg NJ, Blad BL, Verma SB (1983) Microclimate—the biological environment. Wiley, New York
Schultz TR, Brady SG (2008) Major evolutionary transitions in ant agriculture. PNAS 105:5435–5440
Seeley TD, Heinrich B (1981) Regulation of temperature in the nest of social insects. In: Heinrich B (ed) Insect thermoregulation. Wiley, New York, pp 160–234
Southerland MT (1988) The effects of temperature and food on the growth of laboratory colonies of Aphaenogaster rudis Emery (Hymenoptera: Formicidae). Insect Soc 35:304–309
Sudd JH (1982) Ants: foraging, nesting, brood behavior, and polyethism. In: Hermann HR (ed) Social insects, vol IV. Academic Press, New York, pp 107–155
Sudd JH, Franks NR (1987) The behavioural ecology of ants. Blackie, Glasgow
USDA (1975) Soil taxonomy: a basic system of soil classification for making and interpreting soil surveys. USDA-SCS. US Department of Agriculture handbook no. 436. US Government Printing Office
USDA (2005) Global soil temperature regimes map. World soil resources soil survey division, NRCS-USDA, Washington, DC. http://soils.usda.gov/use/worldsoils/mapindex/str.html
Van Wambeke A (1981) Calculated soil moisture and temperature regimes of South America. Techical monograph no. 2. USDA-SCS-SMSS, Washington DC
Weber NA (1959) Isothermal conditions in tropical soil. Ecology 40:153–154
Weber NA (1972) Gardening ants—the Attines. The American Philosophical Society, Philadelphia
Zolessi LC, Abenante YP (1973) Nidificación y mesoetología de Acromyrmex en el Uruguay. III. Acromyrmex (A.) hispidus Santschi, 1925 (Hymenoptera: Formicidae). Rev Biol Urug 1:151–165
Zolessi LC, González LA (1974) Nidificación y mesoetología de Acromyrmex en el Uruguay. II. Acromyrmex (Acromyrmex) lobicornis (Emery 1887). (Hymenoptera: Formicidae). Rev Biol Urug 1:37–57
Zolessi LC, González LA (1978) Observaciones sobre el género Acromyrmex en el Uruguay. IV. A. (Acromyrmex) lundi (Guérin, 1838) (Hymenoptera: Formicidae). Rev Fac Human Cienc (Cienc Biol) Montev 1:9–28
Zolessi LC, Philippi ME (1998) Las hormigas cortadoras del Uruguay del género Acromyrmex (Hymenoptera: Formicidae). In: Berti Fillo E, Mariconi FAM, Fontes LR (eds) Formigas cortadeiras dos países do mercosul. FEALQ, Piracicaba, pp 93–98
Acknowledgements
Thanks to Angel Vidal for developing the computer-controlled thermostatic system, and to Annette Laudahn, Steffanie Henkel and Silvia Cardozo for valuable help during the experiments. Thanks to Oliver Geissler for collecting the A.lundi colony. This research was supported by funds from the German Research Council (DFG, grant SFB 554/TP E1) and the German Academic Exchange Service (DAAD, PhD fellowship granted to M. B.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jay Rosenheim.
Rights and permissions
About this article
Cite this article
Bollazzi, M., Kronenbitter, J. & Roces, F. Soil temperature, digging behaviour, and the adaptive value of nest depth in South American species of Acromyrmex leaf-cutting ants. Oecologia 158, 165–175 (2008). https://doi.org/10.1007/s00442-008-1113-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-008-1113-z