Abstract
Ants build underground nests to protect their colonies and to improve conditions for their offspring. The excavation of nests, by ants, modifies the soil structure, facilitating gas exchange and circulation, which needs further studies. Do initial nests modify the initial chamber soil matrix and gas exchange? The objective was to study the soil micromorphology of the wall of the chamber of initial nests of the leaf-cutting ant A. sexdens and how the CO2 diffuses into the soil matrix. The CO2 concentration in initial four-month-old A. sexdens nests was measured for 24 h using a respirometric system with a gas meter and closed nest holes (obliterated). After this period, they were opened and the CO2 concentration measured again. In addition, 15 cm deep holes were drilled into the ground 15 and 60 cm away from the ant nest hole. The CO2 was measured in these orifices and then they were sealed for 24 h and new ones made after this period. The contents of the nest chambers were removed, after the CO2 measures and the soil micromorphology of the walls of the initial chamber analyzed. The CO2 concentration in the nest chamber was greater than that in the soil at 15 and 60 cm distant from it. The CO2 accumulation did not increase with the obliteration of the nest entrance for 24 h. Coarse material, mainly quartz and charcoal fragments, besides fine material of clay, organic matter and iron oxides composed the soil of the wall of initial nests. The soil porosity in the chamber walls of the initial nests was lower than that of the matrix of the adjacent soil and differed between those of the nests. Initial nests of A. sexdens modifies the soil matrix of their walls, allowing to CO2 exchange between its chamber and adjacent soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nests of the leaf-cutting ants Atta, Acromyrmex and Amoimyrmex, are elaborated during their construction and, normally, formed by chambers connected by galleries or channels opening on the soil surface (Cristiano et al. 2020). These chambers house the symbiotic fungus, the main food source for their larvae and adults. The excavation of digging the soil protects and allow to maintain favorable environmental conditions for the development of ant brood (Silva et al. 2003; Halley et al. 2005; Mota et al. 2021).
The nest initially excavated by a recently fertilized queen, which builds the first chamber (Camargo et al. 2011; Fujihara et al. 2012). This queen performs activities inside the nest until the first workers emerge and start foraging (Camargo et al. 2011), around four months after its foundation and then they made digging of channels and fungus and refuse chambers (Autuori 1942). The ants, when digging the nests, build chambers and tunnels, modifying the soil to facilitate gas exchange inside them. The internal architectural system created in the construction of these nests can also facilitate gas exchange and circulation (Bollazzi et al. 2012; Hasin et al. 2014; Halboth and Roces 2017). Soil micromorphology is suitable to study modifications by ants, identifying porosity, permeability and visualizing structural changes caused by soil compaction and densification (Cosarinsky and Roces 2012; Halboth and Roces 2017). However, the micromorphology gradually changed during the nest growth (Cosarinsky et al. 2021).
The nest architecture of Atta vollenweideri Forel (Hymenoptera: Formicidae), with ventilation towers over the central openings improving the wind-induced circulation, differs from that of other Atta species (Cosarinsky and Roces 2012; Halboth and Roces 2017). These openings are used as entrances connecting the foraging trails and, at the top of the nest by conspicuous towers facilitating gas exchange between the interior of the nest and the environment (Kleineidam and Roces 2000; Kleineidam et al. 2001; Halboth and Roces 2017).
Modifications in the soil structure by leaf-cutting ants, when digging their nests, increase gas exchange and gas circulation within them (Bollazzi et al. 2012), which needs further studies. Do initial nests modify the soil matrix of the initial chamber and do these modifications improve gas exchange in them? The soil micromorphology of the wall of the initial chamber of the leaf-cutting ant A. sexdens and the CO2 rate emitted by its initial nests were studied to answer these questions.
Methods and materials
Initial nests of atta sexdens
Initial nests of A. sexdens, four months old, were marked at Fazenda Santana, Botucatu, São Paulo, Brazil in 2018 (22°50’29.9"S 48°25’22.8"W).
Measuring the CO2 level in the nests
A respirometric system (Fig. 1) with a Bacharach brand gas meter using a fixed probe (http://www.bacharach-32inc.com) was used to measure the CO2 concentration in the initial A. sexdens nests. Ten consecutive readings of the CO2 concentration in the respirometric container were taken to obtain an average. These measurements were performed by introducing a heat exchanger into the inlet orifice and the air removed by a peristaltic pump (Camargo et al. 2016).
The holes in the nests were sealed (obliterated) for 24 h, after measuring the CO2 in them, and again opened when a new measurement was made. 5 cm deep holes in the ground, 15 and 60 cm away from the nest hole, were drilled. The CO2 concentration was measured in these holes, which were also sealed for 24 h, after which new measurements were taken. We followed the methodology of our previous study (Sousa et al. 2021).
Soil micromorphology of the wall of initial chamber nests
Three undisturbed soil samples were collected, air-dried for 15 days and subsequently in an oven at 40 °C for 24 h. Then, they were impregnated with polyester resin diluted in styrene monomer and fluorescent pigment (Tynopal OB) to observation and to define the pore space using ultraviolet light (UV) (Murphy 1986). Soil thin Sect. (5 × 9 cm) were obtained from the impregnated and dried blocks and described (Bullock et al. 1985; Castro and Cooper 2019). Small areas (0.8 × 0.6 cm) in these thin sections were selected, photographed and processed in the Noesis Visilog program, determining the total porosity (sum of the pore type areas/sum of the total analyzed area), pore morphology (rounded, irregular, elongated) and pore size classes 39–3900; 3900–39,000; >390,000 μm (Cooper and Vidal-Torrado 2005). Pedological features of the longitudinal section of the walls of the leaf-cutting ant fungus chambers were obtained by micromorphological description (Table 1).
Statistical analysis
The best fit of the CO2 concentration data to the model was obtained by the maximum likelihood estimation method of β and ϕ and its estimated coefficients related to the linear predictor in the transformed scale:
For example, the predicted expected value when X = 1 is:
The plyr, tidyverse, betareg, lmtest, emmeans, plotly, htmlwidgets and extrafont packages from the R free software environment for statistical computing and graphics version 4.0.4 were used.
Results
The soil fabric is defined by the total organization between the coarse, fine material and the pores (Bullock et al. 1985). The coarse material is composed of quartz and charcoal fragments varying in percentage of occurrence among the three studied nests, being 99, 97 and 98% for quartz in nests 1, 2 and 3, respectively; and 1, 3 and 2% for charcoal fragments in nests 1, 2 and 3. The fine material was characterized by the occurrence of clay minerals, organic matter and iron oxides inferred by matrix coloration. The relative distribution in the three nests is characterized as porphyric, with moderately developed subangular blocks microstructure and speckled b-fabric. Pedofeatures such as incomplete infillings and roots were found in nests 1 and 2, and complete and incomplete infillings, roots and excrements observed in nest 3 (Table 1).
The pores observed in the soil matrix and in the wall of the fungus chamber of A. sexdens nests were chambers, cavities, channels and fissures, with higher values in nest chamber 1 than in 2 and 3, these with predominance of elongated pores and irregular and presence of roots (Fig. 2d). The total soil porosity of the chambers walls of the initial ant nests was lower than the adjacent soil matrix (Table 2; Fig. 2a,b,c).
Photomicrographs of the soils of the walls of the chambers of initial nests of Atta sexdens (Hymenoptera: Formicidae). (a) Boundary between the soil matrix and the nest chamber 1 (FC– fungus chamber) (XPL (cross polarized light) + UV (UV light) (b) do 2 (FC) (XPL + UV); (c) and 3 (FC) (XPL + UV); d, e, f). Detail of the dominant pore type (P; chambers and cavities) in the soil matrix of the chamber of nests 1, 2 and 3 (PPL (plane polarized light)); Q = quartz; P = pore; FC = fungus chamber; R = root; C = charcoal fragment. Figure 2a, b and c, blue colored pores, black/brown soil matrix and quartz grains as bright/yellow spots
The total area occupied and the distribution of pores of the nest chambers, showed elongated and irregular pores in the soil matrix of the chambers of nests 2 and 3 and those of the nest 1 elongated and irregular (Fig. 3).
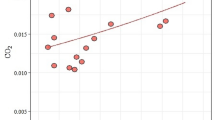
The significance of the negative coefficient estimate (β2= -0.036743, pval < 2e-16) indicated a decrease in CO2 values with increasing distance from the nest chamber of the leaf-cutting ant A. sexdens nests (Fig. 4; Table 3) without effect of the 24-hour obliteration on this gas emission (β1= -0.057043, pval = 0.737) (Table 3).
Discussion
The soil fabric with coarse material composed of quartz and charcoal, and fine material composed of clay, organic matter and iron oxides, related to the relative distribution of the porphyric type with microstructure in subangular blocks in nests 1, 2 and 3 was similar to the one reported those of Solenopsis invicta Buren (Hymenoptera: Formicidae) and A. vollenweideri (Green et al. 1999; Cosarinsky 2006). The absence of coatings and the presence of serrated and irregular edges of the walls of the fungus chambers of nests 1, 2 and 3, together with their lower porosity, indicate an incipient compaction of the walls of the nest chambers when excavating the first fungus chambers, leading to a more compact structure, which stabilizes the chamber walls of the three nests of A. sexdens (Humphreys 1994; Castro and Cooper 2019). However, the soil micromorphology of the underground structure of the initial nest chamber of A. sexdens differs from that of adult nests of A. vollenweideri with cemented microporous and massive intergrain structures alternating with pellicular and intergrain microaggregate structures (Table 1) (Cosarinsky and Roces 2007). In A. vollenweideri, based on morphological data, their surrounding soil profile is a Typic Haplustalf (Alfisol), while in A. sexdens is oxisol. This difference in structure is correlated with the maturity of the nests, the older and more mature the nest the greater reworking of soil particles by the ants generating complex structures such as those observed by (Cosarinsky and Roces 2007). As the original ferralsol is medium texture (sandy loamy) quartz is the mineral with the highest percentage in nests of A. sexdens and A. vollenweideri, but charcoal fragments were reported for those of A. sexdens and hematite grains (nodules) and some feldspar in those of A. vollenweideri (Cosarinsky and Roces 2007) formed over a less wheathered and poorly drained Planosol. The abundance of quartz grains provides larger stacking porosity, facilitating the diffusion of CO2 (Hillel 1998). Furthermore, clay particles and iron oxides (grains, coatings), were found in nests of these two species besides organic matter in the initial chamber wall of those of A. sexdens (Cosarinsky and Roces 2007). Micromorphological differences between A. sexdens and A. vollenweideri nests must be related to the nesting environments of these species, because, unlike A. sexdens, A. vollenweideri nests are often found in areas with flooded soil. Furthermore, as mentioned above, nest maturity can interfere with these micromorphological differences due to greater reworking of soil particles (Cosarinsky et al. 2021).
The greater porosity of the wall of the initial chamber of nest 1, of A. sexdens, than those of 2 and 3, is due to the differences during the nest excavation process by the workers, increasing them and the behavior to isolate the chamber with the matrix of adjacent soil (Cosarinsky and Roces 2007). The porosity of chambers in A. sexdens nests with very wide variation in pore area, from 39 µm2 to 390,000 µm2 differs from the microstructure of fungus chambers, garbage and tunnels in A. vollenweideri nests, very porous with equal or smaller voids than 100 μm (Cosarinsky and Roces 2007). This high variation in chamber wall porosity of A. sexdens nests may be mainly related to the fact that this ant initial nests are more superficial, approximately 15 cm deep and more exposed to roots (Fernandez-Bou et al. 2019). For this reason, differences in porosity between the wall of the nest chamber and the adjacent soil may be related to gas exchange in the nest, as they store CO2 from the soil and because they are not totally isolated, this gas diffuses from the outside to the inside of the chamber and vice versa (Fernandez‐Bou et al. 2019). The larger spaces and porosity of the nest chamber than the adjacent soil facilitates the CO2 entry from the soil as reported for A. vollemweideri, with mounds of soil excavated during colony increase and reinforcement to stabilize structures with tower as ventilation structures (Halboth and Roces 2017) to underground chambers by passive mechanism driven by the wind (Kleineidam and Roces 2000; Kleineidam et al. 2001). Gas exchange in initial nests of A. sexdens should be by passive diffusion, similar to that of most terrestrial substrates (Evans 1966), as structures such as towers were not reported in their soil mounds.
The rounded and elongated pores of nests 2 and 3 and those elongated and irregular in nest 1 of A. sexdens are due to variations during the excavation process by the ant, increasing its initial chamber and also to isolate the chamber from the matrix of adjacent soil (Cosarinsky and Roces 2007). These underground structures, resulting from the excavation to form chambers (for fungus and residues) and the tunnels in A. vollenweideri nests, are simple excavations. Furthermore, the micromorphology of these structures does not differ from the adjacent soil, with a maximum frequency of coarse components in the soil pellets of 30% and 50% in other regions such as the Bt Horizon (20–200 cm excavation limit). This difference may result from later incorporation of clay into the soil removed or from the way the pellets were formed, as reported for the digging behavior of ants in a natural environment with original microstructure and unknown fine and coarse components (Cosarinsky and Roces 2007).
The significance of the negative coefficient estimate, indicating a decrease in CO2 values with increasing distance from the nest chamber of the leaf-cutting ant A. sexdens, is due to the accumulation of this gas reducing its concentrations in the adjacent soil (Fernandez-Bou et al. 2019). Therefore, the lower concentration of this gas in more distant soils, even with similar depth, facilitates its penetration, by diffusion, into the initial nest chamber, reducing its concentration in the adjacent soil. The higher concentration of CO2 in the initial chamber makes wind-induced ventilation mechanisms necessary (Kleineidam et al. 2001) as reported for gas exchange in adult A. vollenweideri nests. Three mathematical models of gas diffusion have been studied to explain it in terrestrial substrates, probably by passive diffusion (Evans 1966). These models used empirical data and the physical and behavioral attributes of fossorial animals in simple cylindrical underwater and underground burrows (a) and spherical nest chamber and cavities (b) in impermeable medium, besides spherical nest chamber and cylindrical cavities in permeable medium (c). Ectothermic animals, in impermeable burrows, cannot depend on diffusion to meet their metabolic CO2 excretion needs, unless they if small in size (Withers 1978) and underground ectotherms may depend on gaseous diffusion, except when in large numbers (Withers 1978). Early nests of A. sexdens are much smaller with only one chamber, which possibly aids in gas exchange diffusion compared to adult ones with thousands of chambers.
Modifications in the soil matrix of the wall chamber of initial A. sexdens nests can help in the gas exchange between them and the adjacent soil by diffusion.
References
Autuori M (1942) Contribuição para o conhecimento da saúva (Atta spp. Hymenoptera Formicidae). II. O sauveiro inicial (Atta sexdens rubropilosa, Forel, 1908). Arq Inst Biol (Sao Paulo) 13:67–86
Bollazzi M, Forti LC, Roces F (2012) Ventilation of the giant nests of Atta leaf-cutting ants: does underground circulating air enter the fungus chambers? Insectes Soc 59:487–498
Bullock P, Fedoroff N, Jongerius A et al (1985) Handbook for soil thin section description. Waine Research
Camargo RS, Forti LC, Fujihara RT, Roces F (2011) Digging effort in leaf-cutting ant queens (Atta sexdens rubropilosa) and its effects on survival and colony growth during the claustral phase. Insectes Soc 58:17–22
Camargo RS, Silva EJ, Forti LC, Matos CAO (2016) Initial development and production of CO2 in colonies of the leaf-cutting ant Atta sexdens during the claustral foundation. Sociobiology 63:720–723
Castro SS, Cooper M (2019) Fundamentos de micromorfologia de Solos. Sociedade Brasileira de Ciência do Solo-SBCS, Viçosa
Cooper M, Vidal-Torrado P (2005) Caracterização morfológica, micromorfológica e físico-hídrica de solos com horizonte B nítico. Rev Bras Ciência do Solo 29:581–595
Cosarinsky MI (2006) Nest micromorphology of the neotropical mound building ants Camponotus punctulatus and Solenopsis sp. Sociobiology 47:329–344
Cosarinsky MI (2021) A review of micromorphological studies of ant and termite’s epigean nests located in neotropical soils of Argentina. J S Am Earth Sci 110:103380
Cosarinsky MI, Roces F (2007) Neighbor leaf-cutting ants and mound-building termites: comparative nest micromorphology. Geoderma 141:224–234
Cosarinsky MI, Roces F (2012) The construction of turrets for nest ventilation in the grass-cutting ant Atta vollenweideri: import and assembly of building materials. J Insect Behav 25:222–241
Cristiano MP, Cardoso DC, Sandoval-Gómez VE, Simões‐Gomes FC (2020) Amoimyrmex Cristiano, Cardoso & Sandoval, gen. nov. (Hymenoptera: Formicidae): a new genus of leaf‐cutting ants revealed by multilocus molecular phylogenetic and morphological analyses. Austral Entomol 59:643–676
Evans DD (1966) Gas movement. In: Black CA (ed) Methods of soil analysis. American Society of Agronomy, Madison, Wis, pp 318–330
Fernandez-Bou AS, Dierick D, Swanson AC et al (2019) The role of the ecosystem engineer, the leaf‐cutter ant Atta cephalotes, on soil CO2 dynamics in a wet tropical rainforest. J Geophys Res Biogeosciences 124:260–273
Fujihara RT, Camargo R, da Forti S LC (2012) Lipid and energy contents in the bodies of queens of Atta sexdens rubropilosa Forel (Hymenoptera, Formicidae): pre-and post-nuptial flight. Rev Bras Entomol 56:73–75
Green WP, Pettry DE, Switzer RE (1999) Structure and hydrology of mounds of the imported fire ants in the southeastern United States. Geoderma 93:1–17
Halboth F, Roces F (2017) The construction of ventilation turrets in Atta vollenweideri leaf-cutting ants: Carbon dioxide levels in the nest tunnels, but not airflow or air humidity, influence turret structure. PLoS ONE 12:e0188162
Halley JD, Burd M, Wells P (2005) Excavation and architecture of Argentine ant nests. Insectes Soc 52:350–356
Hasin S, Ohashi M, Yamada A et al (2014) CO2 efflux from subterranean nests of ant communities in a seasonal tropical forest, T hailand. Ecol Evol 4:3929–3939
Hillel D (1998) Environmental soil physics. Academic Press, San Diego
Humphreys GS (1994) Bioturbation, biofabrics and the biomantle: an example from the Sydney Basin. In: Ringrose-Voase A, Humphreys GS (eds) Micromorphology: studies in management and genesis. Elsevier, Amsterdam, pp 421–436
Kleineidam C, Roces F (2000) Carbon dioxide concentrations and nest ventilation in nests of the leaf-cutting ant Atta vollenweideri. Insectes Soc 47:241–248
Kleineidam C, Ernst R, Roces F (2001) Wind-induced ventilation of the giant nests of the leaf-cutting ant Atta vollenweideri. Naturwissenschaften 88:301–305
Mota TMM, Stefanelli LEP, Camargo RS et al (2021) Biological control in leaf-cutting ants. Atta sexdens. Formicidae, Hymenoptera. using pathogenic fungi.
Murphy CP (1986) Thin section preparation of soils and sediments. AB Academic Pub., Berkhamsted, UK
Silva A, Bacci M Jr, de Siqueira CG et al (2003) Survival of Atta sexdens workers on different food sources. J Insect Physiol 49:307–313
Sousa KKA, Camargo RS, Caldato N et al (2021) Carbon dioxide levels in initial nests of the leaf-cutting ant Atta sexdens (Hymenoptera: Formicidae). Sci Rep 11:20562. https://doi.org/10.1038/s41598-021-00099-8
Withers PC (1978) Models of diffusion-mediated gas exchange in animal burrows. Am Nat 112:1101–1112
Acknowledgements
To the Brazilian institutions “Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)”, “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES- Finance Code 001), “Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG)” and “Programa Cooperativo sobre Proteção Florestal (PROTEF) do Instituto de Pesquisas e Estudos Florestais (IPEF)” for financial support. We are also grateful to Dr. Pablo Vidal-Torrado and Mariane Chiapini from the Department of Soil Science, “Luiz de Queiroz” School of Agriculture, University of São Paulo (ESALQ/USP), who analyzed the soil samples and provided the photos of the soil micromorphology of the present study. We thank Dr. Julian Sabatini for reading and correcting the manuscript.
Author information
Authors and Affiliations
Contributions
R.S.C. and L.C.F. conceived the experiment(s), K.K.A.S., R.S.C., N.C., J.A.S. and A.P.F. conducted the experiment(s). All authors analysed the results and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sousa, K.K.A., Camargo, R.d., Caldato, N. et al. Soil micromorphology and CO2 exchange in initial Atta sexdens (Hymenoptera: Formicidae) nests. Int J Trop Insect Sci 43, 971–977 (2023). https://doi.org/10.1007/s42690-023-01009-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-023-01009-3