Abstract
Background
Climate change induces perturbation in the global water cycle, profoundly impacting water availability for agriculture and therefore global food security. Water stress encompasses both drought (i.e. water scarcity) that causes the drying of soil and subsequent plant desiccation, and flooding, which results in excess soil water and hypoxia for plant roots. Terrestrial plants have evolved diverse mechanisms to cope with soil water stress, with the root system serving as the first line of defense. The responses of roots to water stress can involve both structural and physiological changes, and their plasticity is a vital feature of these adaptations. Genetic methodologies have been extensively employed to identify numerous genetic loci linked to water stress-responsive root traits. This knowledge is immensely important for developing crops with optimal root systems that enhance yield and guarantee food security under water stress conditions.
Results
This review focused on the latest insights into modifications in the root system architecture and anatomical features of legume roots in response to drought and flooding stresses. Special attention was given to recent breakthroughs in understanding the genetic underpinnings of legume root development under water stress. The review also described various root phenotyping techniques and examples of their applications in different legume species. Finally, the prevailing challenges and prospective research avenues in this dynamic field as well as the potential for using root system architecture as a breeding target are discussed.
Conclusions
This review integrated the latest knowledge of the genetic components governing the adaptability of legume roots to water stress, providing a reference for using root traits as the new crop breeding targets.
Similar content being viewed by others
Introduction
The importance of legumes
Legume crops, such as soybean, common bean, pea, chickpea, pigeon pea, lentil, mungbean, black gram, and cowpea, are major sources of nutrition for both humans and livestock [1]. They offer abundant proteins, complex carbohydrates, dietary fiber, and oils, contributing to a balanced diet [2, 3]. These crops also offer great advantages in the form of sustainable agriculture. Through symbiosis with rhizobia to form root nodules, legume crops possess the ability to fix atmospheric nitrogen, thus enhancing soil fertility. This nitrogen fixation capacity plays a pivotal role in curbing greenhouse gas emissions and air pollution by reducing the necessity for synthetic nitrogen fertilizers derived from fossil fuels. Moreover, the incorporation of legumes into intercropping systems can substantially enhance crop diversification [4, 5].
Water stresses (drought and flooding stress)
The sustainability of legume production is significantly influenced by various external factors, with water availability being the most critical environmental determinant. Water is an essential resource not only for drinking, industrial processes, and various economic activities, but also for agriculture. In 2018, agricultural usage constituted about two-thirds of the global freshwater consumption [6]. Unfortunately, the availability of water for agricultural purposes is expected to be disrupted by the effects of climate change, heightening the risks of drought and surface runoffs or flooding, and limiting agricultural productivity [7].
Drought stress in the soil, characterized by a decrease in soil moisture, leads to plant dehydration due to an imbalance between the rates of transpiration and water uptake [8]. On the other hand, flooding stress rapidly reduces the oxygen content in the soil [9]. Both drought and flooding have profound impacts on the yield of major crops [10,11,12]. Notably, there is a growing recognition of the nonlinear relationship between the severity of drought and yield loss, with the risk of yield loss expected to rise [13].
Root system architecture (RSA)
Historically, breeders and scientists have primarily focused on improving aboveground parameters such as shoot biomass, number of branches, seed number, and seed size while overlooking the significance of roots in enhancing crop yield [14,15,16,17]. Root systems play pivotal roles in a multitude of plant processes that encompass nutrient and water uptake, interactions with rhizospheric microbes, soil anchorage, and responses to various stresses [18]. Only recently has the impact of the root system on yield gained substantial attention in legumes such as soybean (Glycine max), common bean (Phaseolus vulgaris), pea (Pisum sativum L.), lentil (Lens culinaris Medik.), and mungbean (Vigna radiata L.) [19,20,21,22,23,24,25,26]. Root system architecture (RSA) refers to the spatial distribution of the root system within the soil matrix, encompassing multiple traits including root biomass, root length, root diameter, root angle, and root volume (Fig. 1) [27]. The anatomical composition of the root encompasses the epidermis, cortex, endodermis, and vascular system (Fig. 1). RSA profoundly influences the capacity of roots to explore the soil environment, interact with rhizospheric microbes, and react to soil cues, and is therefore a major determinant of plant productivity [27, 28].
Typical root system architecture and anatomical features (cross-section) of the legume root. The legume root consists of the primary root, lateral roots, and specialized nodules. For some legume species, basal roots and adventitious roots are also developed and form a large proportion of the whole root system. Root system architecture (RSA) is a composite of the root growth angle, root diameter, primary root length, total root length, total root surface area, total root volume, lateral root number, and root biomass. The anatomical features of the mature region of a soybean root are shown in the cross-section. This figure was generated using unpublished original photos and the software Adobe Illustrator
RSA varies depending on the plant species and the soil composition, and is a result of the interplay between genetic and environmental influences. As dicots, the root systems of legumes commonly adhere to the structure of tap roots, consisting of primary roots and lateral roots (Fig. 1). A distinctive characteristic of legume roots is their ability to form nitrogen-fixing nodules through symbiosis with rhizobia (Fig. 1) [29]. Furthermore, legumes have developed a range of strategies to cope with water stress, with a principal one involving adaptations in the RSA. Roots, being subterranean organs, are often the initial structures to perceive and react to environmental stimuli. This underscores their significance as sensors and responders to environmental conditions [30, 31].
RSA as a potential breeding target
Recognizing the pivotal role of root systems, plant breeders and researchers have lately identified RSA traits as crucial targets for crop breeding and enhancement, particularly in the context of adapting to diverse environmental stresses [28, 32,33,34]. Agronomic traits are derived from the interplay between genetic and environmental factors. Genetic elements underpinning these traits can be discerned using methodologies such as linkage mapping and association mapping. Root traits, characterized by their complexity, are often governed by multiple genes (polygenes) and are highly influenced by environmental conditions, making the quantification of the association between these genes and the corresponding phenotypic traits challenging [35,36,37]. Endeavors have been dedicated to identifying quantitative trait loci (QTLs) responsible for various root traits that display strong associations with drought or flooding stress. The insights gleaned from such QTL identification can be harnessed by breeding programs to develop new plant varieties harboring favorable root traits for sustaining crop yields in the face of environmental stressors [38, 39].
To characterize RSA-related traits, various methods have been used to delineate and quantify root shapes according to their architectural features [40,41,42,43]. One of the foremost challenges is to devise non-destructive root phenotyping techniques capable of precisely gauging RSA across diverse developmental stages. While certain advanced 2-D and 3-D techniques have been devised to accommodate distinct experimental conditions, there is an urgent need to establish cost-effective, field-deployable, and high-throughput protocols required for the efficient examination of extensive genetic populations within breeding programs.
To facilitate the breeding of legume crops with improved tolerance to water stresses, genetic mapping and association studies have been pivotal in identifying gene targets from diversified germplasm resources. However, most of these studies only focused on the responses of aboveground traits, but not root traits, to water stresses [44,45,46], even though a thorough understanding of root traits is essential for developing optimal RSA for better water-stress adaptations in legumes. To bridge this gap, this review offers a comprehensive perspective on the genetic mapping and association studies on the adaptability of legume roots to drought and flooding stresses, as well as the phenotypic alterations in the RSA of various legumes in response to water stress. In addition, the technical challenges, and progress made, in developing root phenotyping methodologies for evaluating RSA were also discussed in detail. This review highlighted the importance of RSA in legume adaptations to water stress and its potential role as a new breeding target.
Responses of root system architecture (RSA) to water stress in legumes
Responses of legume RSA to drought stress
Lynch introduced the concept of a ‘steep, cheap, and deep’ ideotype to optimize water uptake efficiency in the maize root system [47]. This idea aligns with the understanding that improved drought tolerance in legumes is often associated with specific root traits. These traits include a larger root angle [48, 49], deeply penetrating roots [48, 50,51,52], increased total root length (TRL) [53,54,55] and larger root diameter [48, 56]. For instance, legumes possessing deeper root systems are believed to be able to access water from deeper soil layers, therefore able to supply more water for shoot growth. This deeper root system is thought to confer greater drought resistance and maintain a more stable yield under drought stress, particularly when the topsoil dries out. Consequently, the development of an optimal root type has emerged as a key strategy for enhancing drought tolerance in legumes [57, 58].
While optimizing RSA according to the ideotype can theoretically enhance drought tolerance in legumes, it is important to note that drought stress can also alter the legume root morphology [59, 60]. Although drought stress was found to inhibit root growth and development in legumes under controlled laboratory conditions [53, 61, 62], it was observed to increase root branching in both drought-tolerant and drought-sensitive soybean cultivars in the field, likely as the plants adapted by exploring a larger area for soil water to maintain normal growth [48]. Similarly, in chickpea (Cicer arietinum L.), drought stress resulted in elevated root length, root density and root biomass, and decreased root diameter in field conditions [55]. Similar results have also been reported with polyethylene glycol (PEG)-induced simulated drought stress [54]. In any case, these studies imply that genotypes with a greater overall root system tend to exhibit higher drought tolerance. Drought-tolerant genotypes often demonstrate the ability to adjust their RSA to effectively contend with low soil moisture, allowing for increased water absorption to support above-ground growth [48, 56].
Various root anatomical traits, such as the cortex-to-stele area ratio, xylem-to-stele area ratio, and aerenchyma-to-cortex area ratio, play a key role in determining a plant's ability to adapt to different soil water levels [63]. For instance, certain legumes have adopted strategies such as changing the metaxylem morphology and increasing the number of metaxylem elements to improve water uptake efficiency and root conductance during drought, ultimately minimizing yield loss [64, 65]. Additionally, the width of the cortex and the ratio of cortex width to vascular bundle width have been observed to increase significantly in drought-stressed soybean plants compared to non-stressed controls [66]. These anatomical adaptations further emphasize the significance of root traits in enabling plants to respond effectively to varying soil water conditions.
In addition to the root system itself, recent research has highlighted the significance of the symbiotic relationship between legumes and rhizobia in enhancing nutrient transport to legume plants and bolstering their resilience against drought stress. Notably, a positive correlation between soybean nodule size and seed yield was observed under drought conditions [48]. For instance, the soybean cultivar 'Prima 2000', despite having an intermediate root phenotype, displayed abundant nodule formation, substantial shoot biomass accumulation, and higher grain yield under drought stress compared to deep-rooted cultivars [48]. Similarly, Chinese milk vetch plants (Astragalus sinicus L.) with active nodules exhibited greater drought tolerance than those with low-activity nodules or no nodules [67]. Furthermore, the introduction of rhizobia into the soybean rhizosphere has been shown to enhance plant growth during drought stress, by influencing antioxidant enzyme activities and proline metabolism [68]. Nevertheless, recent studies have also uncovered instances where drought stress led to reduced nodule numbers and nodule weights in soybean [48, 68], alfalfa (Medicago sativa L.) [69] and guar (Cyamopsis tetragonoloba [L.] Taub.) [70]. Furthermore, symbiotic nitrogen fixation (SNF) in legumes is highly affected by drought stress [71, 72]. SNF-related traits, such as percentage of nitrogen derived from the atmosphere (%Ndfa) and total nitrogen fixed in the seed, were severely inhibited in soybean by drought stress [73]. These findings underline the complexity of legume-rhizobium interactions and their impact on drought tolerance, reflecting that the role of nodulation in drought tolerance varies across different legume species.
Flooding stress and RSA of legumes
In contrast to drought stress, flooding stress in agricultural fields often arises from heavy and prolonged rainfall. The presence of excess water in the field diminishes the availability of oxygen to the roots, leading to the inhibition of aerobic processes. Generally, flooding stress can be categorized into two types: waterlogging stress and submergence stress [74]. Waterlogging occurs when only the root system of the crop is submerged in water, while submergence describes a situation where a significant portion of the aerial parts of the plant is also underwater. Flooding events frequently have detrimental effects on crop growth and can seriously jeopardize crop yield. Given the increasing unpredictability of precipitation patterns as a result of climate change, it becomes more important to uncover the mechanisms that important crops employ to respond to flooding conditions in order to enhance their survival.
Different crops have evolved various strategies to cope with flooding stress, including the escape strategy, which can be beneficial in shallow submergence conditions, and the quiescence strategy, which is adaptive in temporary deep submergence conditions [75,76,77]. In soybean, flooding leads to decreased primary root growth but increased numbers of adventitious roots near the soil surface [78,79,80]. Similar phenotypes have also been observed in other legumes. Prolonged flooding stress inhibits root extension and significantly reduces the overall root system size in crops including faba bean (Vicia faba L.), white lupin (Lupinus albus L.), pea (Pisum sativum), lentil (Lens culinaris), mungbean (Vigna radiata [L.] Wilczek), and blackgram (Vigna mungo [L.] Hepper) [81,82,83], with the more tolerant varieties being less affected [84]. Two major structural adaptations to counteract hypoxic conditions during flooding include the development of shallow adventitious roots and aerenchyma in root tissues [80, 83, 85]. The former enhances gaseous exchange by increasing the surface area and reducing the distance from the water surface, while the latter facilitates gas diffusion across tissues.
In legumes, SNF in nodules is highly dependent on gaseous exchange in the rhizosphere. Flooding treatment of soybean led to reduced nodule weights, and the development of most nodules on the adventitious roots rather than on the primary and lateral roots [78, 79]. Additionally, it has been observed that the aerenchyma structure can form on the surface of soybean nodules under flooding conditions [86]. In mungbean and blackgram, nodules were also found near the soil surface under waterlogging stress. Moreover, nodules formed on deep roots appeared white in color, indicating a reduction or even loss of function in these nodules [83]. However, in contrast to the observations in soybean, no nodules were formed on the adventitious roots of mungbean and blackgram under waterlogging conditions [83].
Genetic mapping and association studies of drought-adaptive RSA variations in legumes
The RSA is crucial for the adaptation to drought stress by crops. Genetic variations in RSA have been reported among different legume genotypes. Therefore, studies investigating the genetic factors that control RSA are important for improving legume crop performance under drought conditions (Table 1).
Common bean (Phaseolus vulgaris L.)
Common bean (Phaseolus vulgaris L.) is a significant legume that provides humans with high-quality nutrients, but its yield is severely affected by drought stress [94]. Different genetic populations have been used to identify the genetic loci related to root trait responses to drought stress.
A recombinant inbred (RI) population derived from the cross between a high-yield commercial line (DOR364) and a deep-rooted line (BAT477) was employed to identify the quantitative trait loci (QTLs) for rooting pattern traits under both water-stressed and well-watered conditions. QTLs for traits such as total root length, root volume, and root biomass were found to co-localize on linkage group b11 under both well-watered and water-stressed conditions. This suggests that these QTLs contribute to drought adaptations through the constitutive expression of genes related to these RSA-related traits. Analyses of the additive effects of QTLs revealed that the positive alleles for most of these QTLs originated from the deep-rooted parent BAT477, confirming the significance of the deep-root genotype in drought adaptations [87].
In a genome-wide association study (GWAS), a natural population comprising 438 common bean accessions was subjected to drought and normal conditions, and 196 root trait-related loci significantly associated with drought stress were identified [88]. These studies have enhanced our understanding of the genetic controls of root traits during drought stress in common bean.
Pea (Pisum sativum L.)
A QTL analysis of drought tolerance-related root traits in pea revealed three QTLs (rwclF-2, rwcsF-2, and audpc_rwcs-2) explaining 11.37–19.64% of the phenotypic variance, and they were discovered to co-localize within the same genomic region as the QTL (rl3) associated with longer root length, along with the QTLs for Didymella pinodes resistance previously identified in another study [89]. This discovery implies that modifying root traits could result in several simultaneous advantageous outcomes in pea plants.
Lentil (Lens culinaris Medik.)
In lentil, six QTLs linked to root traits (dry root biomass, lateral root number, root surface area, root-to-shoot ratio, and specific root length) were consistently identified under progressive drought stress across two growing seasons [90]. These QTLs were estimated to account for 5–28.9% of the phenotypic variance. Notably, the QTLs for dry root biomass, lateral root number, and root surface area were found to co-localize within a QTL hotspot region along with the QTLs associated with shoot length and dry shoot weight [90].
Chickpea (Cicer arietinum L.)
Chickpea is an important cool-season legume which is also severely affected by drought stress [39]. To dissect the genomic loci for root trait responses to drought, different genetic populations have been evaluated. Two intra-specific RI populations ICCRIL03 and ICCRIL04 were used to identify genetic loci associated with drought tolerance-related traits. A QTL hotspot was found on CaLG04 which contained QTLs for root length density (RLD), ratio between root dry weight and total plant dry weight (RTR) and other shoot traits [91]. Later, this QTL-hotspot region was refined to a narrower distance with 14 centimorgan (cM) in genetic map, and candidate genes related to drought responses, such as dehydration-responsive element-binding protein (DREB), were identified [92]. Similarly, another study used an RI population generated by crossing a drought-tolerant genotype and a drought-sensitive one to tease out the QTLs related to root traits under drought stress and non-stressed conditions [93]. A total of seven QTLs on chromosomes 2, 4, 5, 6, and 7, were evaluated for root-to-shoot ratio (RSR), root length density (RLD), root dry weight (RDW), and the ratio of root dry weight to total plant dry weight (RDW/TDW). Notably, the QTL related to root dry weight was co-located with the QTLs for shoot traits, such as yield and harvest index. The discovery of QTL hotspots indicated that the root-related traits under drought stress in chickpea were highly genetically linked to shoot traits.
Soybean
In soybean, the identification of QTLs related to RSA and their effects on drought resistance has been quite limited. However, the examination of QTL co-localization can indirectly reveal the potential impacts of RSA-related QTLs on drought resistance. In a QTL mapping study, five QTLs associated with soybean fibrous roots were detected on chromosomes Gm01, Gm03, Gm04, Gm08, and Gm20 [95]. Some of these QTLs were in close proximity or overlapped with QTLs associated with drought tolerance, suggesting that QTLs related to fibrous roots could contribute to drought resistance in soybean [95].
Primary root length is widely regarded as a critical parameter for drought resistance. Various studies have pinpointed different QTLs for root length using different genetic populations [38, 96,97,98] For instance, a major QTL controlling primary root length was identified in recombinant inbred lines (RILs) derived from the cross between ‘K099’ (with short primary roots) and ‘Fendou 16’ (with long primary roots) [98]. Located on chromosome 16, this major QTL explained approximately 30% of the phenotypic variation and its validity was confirmed across different genetic populations [98].
More recently, an extensive study detected eight QTL clusters associated with various RSA-related traits in soybean, including primary root length, lateral root number, and root biomass [38]. This comprehensive analysis has provided a wealth of genetic insights into soybean RSA development, which can further contribute to soybean breeding for enhanced drought resistance. However, there still exists a gap in the identification of candidate genes within drought stress-related RSA QTLs.
Genome-wide association studies (GWAS) have emerged as a popular tool for detecting single nucleotide polymorphisms (SNPs), or genes linked to complex traits like RSA. By evaluating RSA traits in a natural soybean population using GWAS, a significant locus on chromosome 16 was found to be closely linked to lateral root number. Notably, soybean genotypes carrying the “G” and “A” variants in Glyma.16G141800 exhibited distinct root cortical cell properties and lateral root numbers, and demonstrated superior yield protection in water-limiting conditions [99]. Similarly, another study utilizing a panel of 137 Canadian soybean core collection identified ten QTL regions associated with total root length and root diameter. Within these regions, Glyma.03g06570 and Glyma.07g096000 were pinpointed as the candidate genes for total root length and primary root diameter, respectively [100]. It is important to note that further functional validation of these candidate genes is necessary to confirm their regulatory roles in soybean RSA and their effects on water stress responses.
In soybean, drought stress is a major factor restricting SNF in nodules [72]. A recent study investigated SNF-related traits under drought condition by utilizing a diverse panel of 103 early-maturity Canadian soybean cultivars [73]. After conducting GWAS analyses, five QTL regions associated with %Ndfa and drought tolerance were identified [73].
Genetic mapping and association studies of flooding-adaptive RSA variations in legumes
Compared to drought stress, fewer studies have delved into the genetic underpinnings of plant RSA in response to flooding stress using techniques such as QTL mapping and GWAS. The latest findings in the genetic foundation of RSA responses under flooding stress in various legume species were summarized below (Table 2).
Soybean
Utilizing an RI population from the cross between a hypoxia-sensitive cultivar Tachinagaha and a hypoxia-tolerant landrace Iyodaizu, Van Nguyen and colleagues successfully identified ten QTLs linked to six root-related traits (root length, root length development, root surface area, root surface area development, root diameter, change in average root diameter) on chromosomes 11, 12, 13, and 14 during the seedling stage under hypoxic conditions [101]. These QTLs accounted for 11% to 23% of the overall phenotypic variance, with logarithm of the odds (LOD) scores ranging from 2.60 to 6.15 [101].
In a separate study, the fine mapping of a prominent waterlogging tolerance QTL, qWT_Gm03, narrowed it down to a 380-kb segment on chromosome 3. The tolerant allele of qWT_Gm03 was found to regulate soybean RSA and root plasticity in waterlogged conditions within field settings, enhancing waterlogging stress tolerance [102].
Furthermore, a total of 15 significant SNPs associated with root length during the germination stage under flooding conditions were discovered through a GWAS involving 34,718 SNPs in a population comprising 243 plant introductions originating from 22 countries [103].
Common bean
In a recent GWAS, the Middle-American Diversity Panel, comprising 272 common bean genotypes, was subjected to flooding stress under greenhouse conditions [104]. A region on Pv08/1.6Mb exhibited a strong association with root weight under flooding stress. This region was found to be syntenic with the simple sequence repeat (SSR) marker Sat_064, the flooding-associated marker in soybean, through synteny analyses. This finding implies an evolutionarily conserved flooding response mechanism shared between common bean and soybean [104]. Similarly, another GWAS on the genetic basis of flooding tolerance in common bean, utilizing the Andean Diversity Panel of 277 genotypes, led to the identification of two significant QTLs located on Pv09/13.5 Mb and Pv08/62.3 Mb which were associated with root weight, and four QTLs on Pv07/28.7 Mb, Pv08/62.3 Mb, Pv09/13.5 Mb and Pv09/20.2 Mb governing the formation of adventitious roots under flooding conditions [105].
Techniques used in legume root phenotyping
Climate change-induced water stress is posing a global threat to crop yields. In response, research into modifications in RSA under water stress can offer potential solutions for stabilizing crop production. While significant progress has been made in understanding the physiological basis of RSA traits through root phenotyping, accurately assessing RSA remains challenging due to the absence of precise, high-throughput, and labor-efficient technologies.
RSA is an intricate three-dimensional (3-D) structure with specific spatial and temporal configurations within the soil. Unlike shoot-related traits, RSA-related traits are influenced by complex interactions between genetics and the environment. Thus, root phenotyping techniques need to accurately quantify root traits, dynamically depict the 3-D distribution of roots in soil and be suitable for population-level measurements. Presently, various platforms have been successfully established for investigating root phenotypes in both laboratory and field settings (Fig. 2). Based on the root distribution pattern in the environment and data acquisition methods, root phenotyping techniques can be broadly categorized into two-dimensional (2-D) versus three-dimensional (3-D) approaches [106, 107]. Here we presented an overview of the common techniques employed in root phenotyping (Fig. 2; Table 3).
Technologies used in root phenotyping studies. Two-dimensional (2-D) technologies include agar medium, paper pouch, semi-hydroponic system, rhizoboxes, minirhizotron, and shovelomics. Three-dimensional (3-D) technologies include X-ray computerized tomography (CT), neutron radiography, magnetic resonance imaging (MRI) and digital imaging of root traits 3D (DIRT/3D). This figure was generated using unpublished original photos and the software Adobe Illustrator
Two-dimensional (2-D) root phenotyping technologies
Two-dimensional phenotyping involves the assessment of root parameters based on 2-D images. These techniques can be applied in both soil-based and soil-free environments, including setups such as rhizotrons, hydroponic systems, agar gel, and paper pouches. Agar is a suitable medium for supporting root growth in transparent containers to facilitate observation. Agar plates have long been used to study Arabidopsis root morphology. Moreover, vessels such as polypropylene containers or transparent pots have been employed for plants with larger seeds and seedlings, such as maize, barley, and beans [108, 109]. Recent advances have refined agar-based systems to cater to various experimental requirements. The GrowScreen-Agar system was developed to enable the dynamic studies of Arabidopsis roots on agar plates [110]. This system has also been used in legumes, such as pea, to allow the monitoring of root phenotypes from seedlings to mature plants in high-throughput phenotyping [111]. Another innovative approach involves embedding fluidic channels in the agar medium to investigate root development in different nutrient solutions [108]. Additionally, a sterile microcosm featuring an agar medium was established to observe the interaction between the legume plant Medicago truncatula and the rhizobium Sinorhizobium meliloti, focusing on root and nodule developments [40].
A paper pouch provides a simple and cost-effective method for root observation, allowing roots to grow vertically on germination paper. This approach has been utilized for root screening across various plant species, including wheat (Triticum turgidum) [112], sorghum (Sorghum bicolor) [113], maize (Zea mays) [114], and mung bean (Vigna radiata) [115].
A 2-D semi-hydroponic system has been successfully developed for high-throughput root phenotyping [116], offering rapid, economical and non-destructive measurements with minimal disturbance to root growth. It has found wide applications in legumes such as soybean (Wang et al. 2022; Liu et al. 2021a; Salim et al. 2022), narrow-leafed lupin (Lupinus angustifolius L.) [119] and chickpea (Cicer arietinum L.) [42]. The consistent rankings of genotypes with contrasting root characteristics across different growth media highlight the capacity of the semi-hydroponic system to simulate diverse growth conditions [129, 130]. Root samples obtained from this soil-free system are then flattened and their images captured using a camera or scanner. The images are subsequently assessed using software such as WinRhizo [131] and IJ_Rhizo [132].
All the above platforms offer a straightforward operation for root phenotyping studies, but the growth conditions they provide are artificial and may not faithfully replicate real-life soil conditions. Furthermore, soil-free systems are often unsuitable for simulating water stress, particularly drought. In light of this, chemical substances such as polyethylene glycol (PEG) and mannitol are frequently used to induce osmotic stress in plants and mimic drought conditions [133].
To incorporate realistic soil environments in their root studies, researchers often opt to extract roots from actual field soils. A technique called ‘shovelomics’ was introduced to visually assess the root systems of common bean (Phaseolus vulgaris L.) and cowpea (Vigna unguiculata) in the field, which is flexible, rapid, and cost-effective [43]. Nevertheless, this method may substantially disrupt root tissues in the soil, particularly for plants with deeper and more extensive root systems. In contrast, cone systems, utilizing PVC tubes and plastic pots filled with soil as the growth medium for examining root growth under drought and flooding conditions, have gained popularity [134,135,136]. To minimize root disturbance, some soil-based systems are equipped with cameras to enable real-time, in-situ observations for the dynamic studies of roots.
A classic laboratory-based system for non-destructive root observation is the rhizotron. It features a soil-filled compartment for plant growth, with a transparent glass panel on one side for root observations [137]. In recent times, rhizotrons have been widely employed for visualizing root systems across various plant species, such as soybean, tomato, and maize [41, 120, 121]. The minirhizotron system operates on a similar principle to the rhizotron but differs in that plants are grown in the field or in regular pots. A transparent tube housing a camera is inserted directly into the soil to capture root images [138]. Various imaging systems have been developed for the minirhizotron, including SoilCam [122] and EnRoot [123]. One such rhizotron system, the GROWSCREEN-Rhizo phenotyping platform, was used to analyze the root phenotype of faba bean germplasms, enabling dynamic phenotyping across multiple time points [139].
Recently, researchers have employed both semi-hydroponic systems and rhizoboxes to assess root traits in various soybean germplasms [41, 117]. The majority of the root traits measured, including total root length, exhibited significant positive correlations between the two setups, underscoring the dependability of these phenotyping methods. However, certain root traits, such as root diameter, did not display consistency between the two systems, highlighting the greater sensitivity of certain root traits to variations in growth conditions.
Three-dimensional (3-D) root phenotyping technologies
While 2-D techniques have successfully enabled high-throughput root phenotyping, certain parameters, such as root arrangement, cannot be precisely determined in two dimensions as root systems are inherently 3-D in nature. To address this limitation, 3-D phenotyping approaches have been developed, capable of capturing the intricate root architecture. In laboratory settings, methods such as X-ray computer tomography (CT), magnetic resonance imaging (MRI), and neutron radiography have been employed to document root systems within growth containers [124,125,126]. However, these techniques are confined to the laboratory environment due to their low-throughput, time-consuming nature, and their reliance on expensive and specialized equipment. In contrast, the Digital Imaging of Root Traits (DIRT)/3D, a 3-D root phenotyping platform, enables automated, time-efficient, and high-throughput imaging directly in the field [127, 128]. Recent applications of DIRT/3D in characterizing 18 maize root traits under field conditions have validated its accuracy at both the individual and crown levels [127]. It is noteworthy that, compared to cereals, the utilization of 3-D root phenotyping platforms in legumes remains relatively limited.
To compare the efficacy of various methods for root trait phenotyping, a study employed three distinct techniques: hydroponics, rhizotron, and neutron tomography, to analyze root traits in grapevine (Vitis vinifera L.) [140]. The investigation revealed that the hydroponic system offered the simplest and quickest means of observing root traits, but it posed challenges in precisely quantifying these traits due to root tangling within the liquid medium. On the other hand, both the rhizotron and neutron tomography proved more adept at quantifying root traits, particularly adventitious root length and root volume. However, it is important to note that these two latter methods are notably more intricate to operate compared to the hydroponic system.
Phenotyping root anatomical features has traditionally necessitated manual sample sectioning followed by microscopy. To speed up the process, an innovative 3-D rapid anatomical phenotyping platform utilizing laser ablation tomography (LAT) has recently been introduced [141]. This technology offers high-throughput spatial scanning with micron-level resolution in full color [141]. LAT is particularly well-suited for visualizing root anatomy, allowing for the characterization of root structure and the assessment of damage caused by soil pests and pathogens [142]. In a recent study, LAT was employed to compare between the root anatomy of maize landraces and that of teosinte, revealing the impact of artificial selection on maize root traits during its domestication from teosinte [143].
Despite these advancements, the root phenotyping process mostly remains time-consuming, primarily due to the lack of a high-throughput system for analyzing large plant populations and the absence of suitable field technologies for non-destructive root measurements, particularly for water stress conditions.
Challenges and perspectives
Global climate change is driving scientists to explore strategies for enhancing crop survival and maintaining yield through the fluctuating environment, while practicing sustainable agriculture. Concealed within the soil, the root system plays pivotal roles in helping crops adapt to water stress. Resilient plants can alter their RSA in response to changes in the soil environment, ensuring better growth and survival. Despite the significance of root systems, selecting legume genotypes with optimal RSA for various water stress conditions remains challenging.
First of all, the growth environment for the root system is intricate, influenced by numerous factors. Besides water stress, root growth and structure are affected by multiple external stimuli, including the availability of soil nutrients, temperature, and soil composition. Additionally, root-rhizospheric microbe interactions can enhance plant stress tolerance and are often influenced by abiotic stresses in return [144].
Secondly, the lack of high-throughput root phenotyping platforms presents a bottleneck, impeding the direct selection of a promising RSA for water stress adaptation. Though advanced techniques exist, affordable high-throughput methods for field applications are limited. To circumvent this problem, due to the strong correlation between shoot and root traits and the ease of shoot trait observation, selecting for desirable shoot traits may serve as a proxy for root trait selection. It has been suggested that selecting for high shoot biomass could indirectly select for high root biomass [145]. QTL mapping has shown strong genetic linkage between shoot traits and RSA traits in soybean [38]. There are also other above-ground traits or related genes that are closely linked to RSA. For example, a study on a large teosinte-maize population found that flowering time-related QTLs were associated with around half of the genetic variations in nodal root number. Flowering time-regulating genes also co-controlled nodal root number in maize, indicating that flowering time selection could influence nodal root number during maize domestication [146]. In soybean, RSA-related QTLs were found to overlap with the flowering-time QTL E1, and the knockdown mutant of the E1 family showed a diminished root system compared to wild type [38].
Thirdly, breeding crops with optimal root architecture using genetic and molecular approaches can face significant challenges. Root traits are intricate, regulated by multiple genes and environmental factors. While numerous genetic loci controlling water stress-responsive RSA traits have been identified, the functional characterization of causal genes within these loci is still crucial for accelerating breeding efforts. However, identifying a decisive key gene for water stress tolerance in legumes remains elusive. Marker-assisted selection (MAS) has been proven to be valuable in breeding desirable traits governed by major loci, but not for complex traits such as root structure which is controlled by multiple small-effect loci. In this regard, genomic selection (GS) and stacking of beneficial alleles are better suited to breeding optimal root traits. For instance, an upland rice cultivar achieved higher yield by the introgression of four root length-related QTLs into its genome [147].
However, uncovering a universal root ideotype for drought and flooding tolerance seems unlikely. Developing plants capable of autonomously adjusting their RSA towards drought and flooding might not be currently feasible. Instead, seeding appropriate cultivars with desired RSA based on climate model predictions could be a more practical solution. Integrating advanced field monitoring systems and agronomic practices is also crucial for maintaining legume yield under unpredictable climate conditions [148].
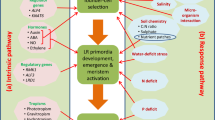
In conclusion, root systems are key factors of healthy legume growth and development, fundamental to agriculture and food security, and therefore should be the new focus for crop breeding. The combination of root phenotyping, molecular technologies, and genetic methods will aid in selecting RSAs adapted to diverse scenarios, ensuring that legume crop productivity and yield can be sustained under drought and flooding stresses (Fig. 3).
A conceptual framework for using root system architecture (RSA) as a prospective target for legume breeding for resistance to water stress. The red and blue arrows indicate negative and positive effects of water stresses on root development, respectively. Root phenotyping and genetic methodologies can be developed by the collaborative efforts of breeders to select and breed the optimal root system architecture to adapt to water stress. This figure was generated using unpublished original photos and the software Adobe Illustrator
Availability of data and materials
Not applicable.
References
Dutta A, Trivedi A, Nath CP, Sen Gupta D, Hazra KK. A comprehensive review on grain legumes as climate-smart crops: challenges and prospects. Environ Challenges. 2022;2021(7):100479.
Maphosa Y, Jideani AV. The role of legumes in human nutrition. In: Functional Food — Improve Health through Adequate Food. London: IntechOpen; 2017.
Vasantha Kumari P, Sangeetha N. Nutritional significance of cereals and legumes based food mix- a review. Int J Agric Life Sci. 2017;3:115–22.
Stagnari F, Maggio A, Galieni A, Pisante M. Multiple benefits of legumes for agriculture sustainability: an overview. Chem Biol Technol Agric. 2017;4:1–13.
Wacker TS, Dresbøll DB. Checking the pulse: perspectives on grain legume production. Trends Plant Sci. 2023;28:991–4.
School WS. Irrigation Water Use. United States Geological Survey. 2018. https://www.usgs.gov/special-topics/water-science-school/science/irrigation-water-use.
Tao F, Yokozawa M, Hayashi Y, Lin E. Future climate change, the agricultural water cycle, and agricultural production in China. Agric Ecosyst Environ. 2003;95:203–15.
Lipiec J, Doussan C, Nosalewicz A, Kondracka K. Effect of drought and heat stresses on plant growth and yield: a review. Int Agrophysics. 2013;27:463–77.
Kozlowski TT. Plant responses to flooding of soil. Bioscience. 1984;34:162–7.
Ray RL, Fares A, Risch E. Effects of drought on crop production and cropping areas in Texas. Agric Environ Lett. 2018;3:170037.
Shirzaei M, Khoshmanesh M, Ojha C, Werth S, Kerner H, Carlson G, et al. Persistent impact of spring floods on crop loss in U.S. Midwest. Weather Clim Extrem. 2021;34 January:100392.
Lesk C, Rowhani P, Ramankutty N. Influence of extreme weather disasters on global crop production. Nature. 2016;529:84–7.
Leng G, Hall J. Crop yield sensitivity of global major agricultural countries to droughts and the projected changes in the future. Sci Total Environ. 2019;654:811–21.
Wang C, Wu T, Sun S, Xu R, Ren J, Wu C, et al. Seventy-five years of improvement of yield and agronomic traits of soybean cultivars released in the yellow-huai-hai river valley. Crop Sci. 2016;56:2354–64.
Mathan J, Bhattacharya J, Ranjan A. Enhancing crop yield by optimizing plant developmental features. Dev. 2016;143:3283–94.
Qin X, Feng F, Li D, Herbert SJ, Liao Y, Siddique KHM. Changes in yield and agronomic traits of soybean cultivars released in China in the last 60 years. Crop Pasture Sci. 2017;68:973–84.
Sun Z, Su C, Yun J, Jiang Q, Wang L, Wang Y, et al. Genetic improvement of the shoot architecture and yield in soya bean plants via the manipulation of GmmiR156b. Plant Biotechnol J. 2019;17:50–62.
Hodge A, Berta G, Doussan C, Merchan F, Crespi M. Plant root growth, architecture and function. Plant Soil. 2009;321:153–87.
El Hassouni K, Alahmad S, Belkadi B, Filali-Maltouf A, Hickey LT, Bassi FM. Root system architecture and its association with yield under different water regimes in Durum wheat. Crop Sci. 2018;58:2331–46.
Kitomi Y, Hanzawa E, Kuya N, Inoue H, Hara N, Kawai S, et al. Root angle modifications by the DRO1 homolog improve rice yields in saline paddy fields. Proc Natl Acad Sci U S A. 2020;117:21242–50.
Shao H, Xia T, Wu D, Chen F, Mi G. Root growth and root system architecture of field-grown maize in response to high planting density. Plant Soil. 2018;430:395–411.
Fried HG, Narayanan S, Fallen B. Evaluation of soybean [Glycine max (L.) Merr.] genotypes for yield, water use efficiency, and root traits. PLoS One. 2019;14:1–18.
Strock CF, Burridge J, Massas ASF, Beaver J, Beebe S, Camilo SA, et al. Seedling root architecture and its relationship with seed yield across diverse environments in Phaseolus vulgaris. F Crop Res. 2019;237:53–64.
Liao Q, Chebotarov D, Islam MS, Quintana MR, Natividad MA, De Ocampo M, et al. Aus rice root architecture variation contributing to grain yield under drought suggests a key role of nodal root diameter class. Plant Cell Environ. 2022;45:854–70.
Bourgault M, Lamb P, McPhee K, McGee RJ, Vandenberg A, Warkentin T. Genotypic variability in root length in pea (Pisum sativum L.) and lentil (Lens culinaris Medik.) cultivars in a semi-arid environment based on mini-rhizotron image capture. Plant Phenome J. 2022;5:1–14.
Singh V, Bell M. Genotypic variability in architectural development of mungbean (Vigna radiata L.) root systems and physiological relationships with shoot growth dynamics. Front Plant Sci. 2021;12 August:1–13.
Lynch J. Root architecture and plant productivity. Plant Physiol. 1995;109:7–13.
Tracy SR, Nagel KA, Postma JA, Fassbender H, Wasson A, Watt M. Crop Improvement from phenotyping roots: highlights reveal expanding opportunities. Trends Plant Sci. 2020;25:105–18.
Gonzalez-Rizzo S, Laporte P, Crespi M, Frugier F. Legume root architecture: a peculiar root system. In: Annual Plant Reviews online. 2018. p. 239–87.
Chen Y, Djalovic I, Siddique KHM. Advances in understanding grain legume physiology: understanding root architecture, nutrient uptake and response to abiotic stress. Achiev Sustain Cultiv grain Legum. 2018;1:11–28.
Chen Y, Wang Z, Ye H, Liu S, Nguyen HT, Lam HM, et al. Root physiology and morphology of soybean in relation to stress tolerance. In: Advances in Botanical Research, vol. 102. New York: Academic Press; 2022. p. 77–103.
Paez-Garcia A, Motes CM, Scheible WR, Chen R, Blancaflor EB, Monteros MJ. Root traits and phenotyping strategies for plant improvement. Plants. 2015;4:334–55.
Fradgley N, Evans G, Biernaskie JM, Cockram J, Marr EC, Oliver AG, et al. Effects of breeding history and crop management on the root architecture of wheat. Plant Soil. 2020;452:587–600.
Liu Z, Qin T, Atienza M, Zhao Y, Nguyen H, Sheng H, et al. Constitutive basis of root system architecture: uncovering a promising trait for breeding nutrient- and drought-resilient crops. aBIOTECH. 2023;4:315–31.
Slovak R, Ogura T, Satbhai SB, Ristova D, Busch W. Genetic control of root growth: from genes to networks. Ann Bot. 2016;117:9–24.
Coudert Y, Périn C, Courtois B, Khong NG, Gantet P. Genetic control of root development in rice, the model cereal. Trends Plant Sci. 2010;15:219–26.
Meister R, Rajani MS, Ruzicka D, Schachtman DP. Challenges of modifying root traits in crops for agriculture. Trends Plant Sci. 2014;19:779–88.
Wang Z, Huang C, Niu Y, Yung WS, Xiao Z, Wong FL, et al. QTL analyses of soybean root system architecture revealed genetic relationships with shoot-related traits. Theor Appl Genet. 2022;135:4507–22.
Kashiwagi J, Krishnamurthy L, Purushothaman R, Upadhyaya HD, Gaur PM, Gowda CLL, et al. Scope for improvement of yield under drought through the root traits in chickpea (Cicer arietinum L.). F Crop Res. 2015;170:47–54.
Jones KM, Mendis HC, Queiroux C. Single-plant, sterile microcosms for nodulation and growth of the legume plant Medicago truncatula with the rhizobial symbiont Sinorhizobium meliloti. J Vis Exp. 2013;80:e50916.
Salim M, Chen Y, Ye H, Nguyen HT, Solaiman ZM, Siddique KHM. Screening of soybean genotypes based on root morphology and shoot traits using the semi-hydroponic phenotyping platform and rhizobox technique. Agronomy. 2022;12:56.
Chen Y, Ghanem ME, Siddique KHM. Characterising root trait variability in chickpea (Cicer arietinum L.) germplasm. J Exp Bot. 2017;68:1987–99.
Burridge J, Jochua CN, Bucksch A, Lynch JP. Legume shovelomics: high-throughput phenotyping of common bean (Phaseolus vulgaris L.) and cowpea (Vigna unguiculata subsp, unguiculata) root architecture in the field. F Crop Res. 2016;192:21–32.
Saleem A, Roldán-Ruiz I, Aper J, Muylle H. Genetic control of tolerance to drought stress in soybean. BMC Plant Biol. 2022;22:1–19.
Jiang W, Liu Y, Zhang C, Pan L, Wang W, Zhao C, et al. Identification of major QTLs for drought tolerance in soybean, together with a novel candidate gene, GmUAA6. J Exp Bot. 2024. https://doi.org/10.1093/jxb/erad483.
Mutari B, Sibiya J, Shayanowako A, Chidzanga C, Matova PM, Gasura E. Genome-wide association mapping for component traits of drought tolerance in dry beans (Phaseolus vulgaris L.). 2023;18:e0278500.
Lynch JP. Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann Bot. 2013;112:347–57.
Fenta BA, Beebe SE, Kunert KJ, Burridge JD, Barlow KM, Lynch JP, et al. Field phenotyping of soybean roots for drought stress tolerance. Agronomy. 2014;4:418–35.
Ribeiro T, Silva DA, de Esteves JAF, Azevedo CVG, Gonçalves JGR, Carbonell SAM, et al. Evaluation of common bean genotypes for drought tolerance. Bragantia. 2019;78:1–11.
Sponchiadof BBN, White JW, Castillo JA, Jones PG. Root growth of four common bean cultivars in relation to drought tolerance in environments with contrasting soil types. Expl Agric. 1989;25:249–57.
Tatsumi Y, Murakami S, Ishibashi Y, Iwaya-Inoue M. Characteristics for deep root system of a drought tolerant cowpea cultivar. Cryobiol Cryotechnol. 2019;65:31–6.
Ho MD, Rosas JC, Brown KM, Lynch JP. Root architectural tradeoffs for water and phosphorus acquisition. Funct Plant Biol. 2005;32:737–48.
Priya S, Bansal R, Kumar G, Dikshit HK, Kumari J, Pandey R, et al. Root trait variation in lentil (Lens culinaris medikus) germplasm under drought stress. Plants. 2021;10:1–11.
Wang G, Zhou Q, He M, Zhong X, Tang G. Wilting index and root morphological characteristics used as drought-tolerance variety selection at the seedling stage in soybean (Glycine max L.). Plant Growth Regul. 2020;92:29–42.
Ramamoorthy P, Lakshmanan K, Upadhyaya HD, Vadez V, Varshney RK. Root traits confer grain yield advantages under terminal drought in chickpea (Cicer arietinum L.). F Crop Res. 2017;201:146–61.
Bhaskarla V, Zinta G, Ford R, Jain M, Varshney RK, Mantri N. Comparative root transcriptomics provide insights into drought adaptation strategies in chickpea (Cicer arietinum L.). Int J Mol Sci. 2020;21:21.
Belachew KY, Nagel KA, Fiorani F, Stoddard FL. Diversity in root growth responses to moisture deficit in young faba bean (Vicia faba L.) plants. PeerJ. 2018;6:e4401.
Polania J, Rao IM, Cajiao C, Grajales M, Rivera M, Velasquez F, et al. Shoot and root traits contribute to drought resistance in recombinant inbred lines of MD 23–24 × SEA 5 of common bean. Front Plant Sci. 2017;8:296.
Xiong R, Liu S, Considine MJ, Siddique KHM, Lam HM, Chen Y. Root system architecture, physiological and transcriptional traits of soybean (Glycine max L.) in response to water deficit: a review. Physiol Plant. 2021;172:405–18.
Ye H, Roorkiwal M, Valliyodan B, Zhou L, Chen P, Varshney RK, et al. Genetic diversity of root system architecture in response to drought stress in grain legumes. J Exp Bot. 2018;69:3267–77.
Chun HC, Lee S, Choi YD, Gong DH, Jung KY. Effects of drought stress on root morphology and spatial distribution of soybean and adzuki bean. J Integr Agric. 2021;20:2639–51.
Sofi PA, Djanaguiraman M, Siddique KHM, Prasad PVV. Reproductive fitness in common bean (Phaseolus vulgaris L.) under drought stress is associated with root length and volume. Indian J Plant Physiol. 2018;23:796–809.
Yamauchi T, Pedersen O, Nakazono M, Tsutsumi N. Key root traits of Poaceae for adaptation to soil water gradients. New Phytol. 2021;229:3133–40.
Strock CF, Burridge JD, Niemiec MD, Brown KM, Lynch JP. Root metaxylem and architecture phenotypes integrate to regulate water use under drought stress. Plant Cell Environ. 2021;44:49–67.
Prince SJ, Murphy M, Mutava RN, Durnell LA, Valliyodan B, Grover Shannon J, et al. Root xylem plasticity to improve water use and yield in water-stressed soybean. J Exp Bot. 2017;68:2027–36.
Makbul S, Saruhan Güler N, Durmuş N, Güven S. Changes in anatomical and physiological parameters of soybean under drought stress. Turk J Botany. 2011;35:369–77.
Liu Y, Guo Z, Shi H. Rhizobium symbiosis leads to increased drought tolerance in Chinese Milk Vetch (Astragalus sinicus L.). Agronomy. 2022;12:725.
Sheteiwy MS, Ali DFI, Xiong YC, Brestic M, Skalicky M, Hamoud YA, et al. Physiological and biochemical responses of soybean plants inoculated with Arbuscular mycorrhizal fungi and Bradyrhizobium under drought stress. BMC Plant Biol. 2021;21:1–21.
Soba D, Zhou B, Arrese-igor C, Munn S. Physiological, hormonal and metabolic responses of two alfalfa cultivars with contrasting responses to drought. Int J Mol Sci. 2019;20:5099.
Shrestha R, Adams CB, Rajan N. Does the drought tolerance of guar [Cyamopsis tetragonoloba (L.) Taub.] extend belowground to root nodules? J Agron Crop Sci. 2021;208:599–608.
Serraj R, Sinclair TR, Purcell LC. Symbiotic N2 fixation response to drought. J Exp Bot. 1999;50:143–55.
Serraj R. Effects of drought stress on legume symbiotic nitrogen fixation: physiological mechanisms. Indian J Exp Biol. 2003;41:1136–41.
Liyanage DK, Torkamaneh D, Belzile F, Balasubramanian P, Hill B, Thilakarathna MS. The genotypic variability among short-season soybean cultivars for nitrogen fixation under drought stress. Plants. 2023;12:1–14.
Sasidharan R, Bailey-Serres J, Ashikari M, Atwell BJ, Colmer TD, Fagerstedt K, et al. Community recommendations on terminology and procedures used in flooding and low oxygen stress research. New Phytol. 2017;214:1403–7.
Striker GG, Izaguirre RF, Manzur ME, Grimoldi AA. Different strategies of Lotus japonicus, L. corniculatus and L. tenuis to deal with complete submergence at seedling stage. Plant Biol. 2012;14:50–5.
El-Hendawy S, Sone C, Ito O, Sakagami JI. Traits associated with the escape strategy are responsible for flash flooding tolerance of rice during the emergence and seedling stages. Cereal Res Commun. 2015;43:525–36.
Chen XS, Li YF, Cai YH, Xie YH, Deng ZM, Li F, et al. Differential strategies to tolerate flooding in Polygonum hydropiper plants originating from low-and high-elevation habitats. Front Plant Sci. 2019;9:1–7.
Henshaw TL, Gilbert RA, Scholberg JMS, Sinclair TR. Soya bean (Glycine max L. Merr.) genotype response to early-season flooding: I. Root and nodule development. J Agron Crop Sci. 2007;193:177–88.
Hattori R, Matsumura A, Yamawaki K, Tarui A, Daimon H. Effects of flooding on arbuscular mycorrhizal colonization and root-nodule formation in different roots of soybeans. Agric Sci. 2013;04:673–7.
Bacanamwo M, Purcell LC. Soybean root morphological and anatomical traits associated with acclimation to flooding. Crop Sci. 1999;39:143–9.
Pampana S, Masoni A, Arduini I. Response of cool-season grain legumes to waterlogging at flowering. Can J Plant Sci. 2016;96:597–603.
Malik AI, Ailewe TI, Erskine W. Tolerance of three grain legume species to transient waterlogging. AoB Plants. 2015;7:1–11.
Kyu KL, Malik AI, Colmer TD, Siddique KHM, Erskine W. Response of Mungbean (cvs. Celera II-AU and Jade-AU) and Blackgram (cv. Onyx-AU) to transient waterlogging. Front Plant Sci. 2021;12:1–13.
Sakazono S, Nagata T, Matsuo R, Mochizuki T, Kajihara S, Watanabe M, et al. Variation in root development response to flooding among 92 soybean lines during early growth stages. Plant Prod Sci. 2014;17:228–36.
Butsayawarapat P, Juntawong P, Khamsuk O, Somta P. Comparative transcriptome analysis of waterlogging-sensitive and tolerant Zombi pea (Vigna vexillata) reveals energy conservation and root plasticity controlling waterlogging tolerance. Plants. 2019;8:268.
Shimamura S, Mochizuki T, Nada Y, Fukuyama M. Secondary aerenchyma formation and its relation to nitrogen fixation in root nodules of soybean plants (Glycine max) grown under flooded conditions. Plant Prod Sci. 2002;5:294–300.
Asfaw A, Blair MW. Quantitative trait loci for rooting pattern traits of common beans grown under drought stress versus non-stress conditions. Mol Breed. 2012;30:681–95.
Wu L, Chang Y, Wang L, Wu J, Wang S. Genetic dissection of drought resistance based on root traits at the bud stage in common bean. Theor Appl Genet. 2021;134:1047–61.
Iglesias-García R, Prats E, Fondevilla S, Satovic Z, Rubiales D. Quantitative trait loci associated to drought adaptation in Pea (Pisum sativum L.). Plant Mol Biol Rep. 2015;33:1768–78.
Idrissi O, Udupa SM, De Keyser E, McGee RJ, Coyne CJ, Saha GC, et al. Identification of quantitative trait loci controlling root and shoot traits associated with drought tolerance in a lentil (Lens culinaris medik.) recombinant inbred line population. Front Plant Sci. 2016;7:1174.
Varshney RK, Thudi M, Nayak SN, Gaur PM, Kashiwagi J, Krishnamurthy L, et al. Genetic dissection of drought tolerance in chickpea (Cicer arietinum L.). Theor Appl Genet. 2014;127:445–62.
Jaganathan D, Thudi M, Kale S, Azam S, Roorkiwal M, Gaur PM, et al. Genotyping-by-sequencing based intra-specific genetic map refines a ‘‘QTL-hotspot” region for drought tolerance in chickpea. Mol Genet Genomics. 2015;290:559–71.
Kushwah A, Bhatia D, Barmukh R, Singh I, Singh G, Bindra S, et al. Genetic mapping of QTLs for drought tolerance in chickpea (Cicer arietinum L.). Front Genet. 2022;13:1–15.
Losa A, Vorster J, Cominelli E, Sparvoli F, Paolo D, Sala T, et al. Drought and heat affect common bean minerals and human diet—What we know and where to go. Food Energy Secur. 2022;11:1–28.
Abdel-Haleem H, Lee GJ, Boerma RH. Identification of QTL for increased fibrous roots in soybean. Theor Appl Genet. 2011;122:935–46.
Prince SJ, Vuong TD, Wu X, Bai Y, Lu F, Kumpatla SP, et al. Mapping quantitative trait loci for soybean seedling shoot and root architecture traits in an inter-specific genetic population. Front Plant Sci. 2020;11:1–13.
Manavalan LP, Prince SJ, Musket TA, Chaky J, Deshmukh R, Vuong TD, et al. Identification of novel QTL governing root architectural traits in an interspecific soybean population. PLoS ONE. 2015;10:1–18.
Chen H, Kumawat G, Yan Y, Fan B, Xu D. Mapping and validation of a major QTL for primary root length of soybean seedlings grown in hydroponic conditions. BMC Genomics. 2021;22:1–9.
Prince SJ, Valliyodan B, Ye H, Yang M, Tai S, Hu W, et al. Understanding genetic control of root system architecture in soybean: insights into the genetic basis of lateral root number. Plant Cell Environ. 2019;42:212–29.
Seck W, Torkamaneh D, Belzile F. Comprehensive genome-wide association analysis reveals the genetic basis of root system architecture in soybean. Front Plant Sci. 2020;11:1–10.
Van Nguyen L, Takahashi R, Githiri SM, Rodriguez TO, Tsutsumi N, Kajihara S, et al. Mapping quantitative trait loci for root development under hypoxia conditions in soybean (Glycine max L. Merr.). Theor Appl Genet. 2017;130:743–55.
Ye H, Song L, Chen H, Valliyodan B, Cheng P, Ali L, et al. A major natural genetic variation associated with root system architecture and plasticity improves waterlogging tolerance and yield in soybean. Plant Cell Environ. 2018;41:2169–82.
Sharmin RA, Karikari B, Chang F, Al Amin GM, Bhuiyan MR, Hina A, et al. Genome-wide association study uncovers major genetic loci associated with seed flooding tolerance in soybean. BMC Plant Biol. 2021;21:1–17.
Soltani A, MafiMoghaddam S, Walter K, Restrepo-Montoya D, Mamidi S, Schroder S, et al. Genetic architecture of flooding tolerance in the dry bean middle-American diversity panel. Front Plant Sci. 2017;8 July:1–15.
Soltani A, Mafimoghaddam S, Oladzad-Abbasabadi A, Walter K, Kearns PJ, Vasquez-Guzman J, et al. Genetic analysis of flooding tolerance in an andean diversity panel of dry bean (Phaseolus vulgaris L.). Front. Plant Sci. 2018;9:767.
Atkinson JA, Pound MP, Bennett MJ, Wells DM. Uncovering the hidden half of plants using new advances in root phenotyping. Curr Opin Biotechnol. 2019;55:1–8.
Chen Y, Djalovic I, Rengel Z. Phenotyping for root traits. In: Phenomics in crop plants: trends, options and limitations. New Delhi: Springer-Verlag Berlin Heidelberg; 2015. p. 102–28.
Aziz AA, Lim KB, Rahman EKA, Nurmawati MH, Zuruzi AS. Agar with embedded channels to study root growth. Sci Rep. 2020;10:1–12.
Shi R, Junker A, Seiler C, Altmann T. Phenotyping roots in darkness: disturbance-free root imaging with near infrared illumination. Funct Plant Biol. 2018;45:400–11.
Nagel KA, Lenz H, Kastenholz B, Gilmer F, Averesch A, Putz A, et al. The platform GrowScreen-Agar enables identification of phenotypic diversity in root and shoot growth traits of agar grown plants. Plant Methods. 2020;16:1–17.
Zhao J, Bodner G, Rewald B, Leitner D, Nagel KA, Nakhforoosh A. Root architecture simulation improves the inference from seedling root phenotyping towards mature root systems. J Exp Bot. 2017;68:965–82.
Adeleke E, Millas R, McNeal W, Faris J, Taheri A. Variation analysis of root system development in wheat seedlings using root phenotyping system. Agronomy. 2020;10:1–18.
Tishchenko V, Wang M, Xin Z, Harrison M. Development of root phenotyping platforms for identification of root architecture mutations in EMS-induced and low-path-sequenced sorghum mutant population. Am J Plant Sci. 2020;11:838–50.
Salungyu J, Thaitad S, Bucksch A, Kengkanna J, Saengwilai PJ. From lab to field: Open tools facilitating the translation of maize root traits. F Crop Res. 2020;255 February:107872.
Chiteri KO, Jubery TZ, Dutta S, Ganapathysubramanian B, Cannon S, Singh A. Dissecting the root phenotypic and genotypic variability of the iowa Mung Bean diversity panel. Front Plant Sci. 2022;12:808001.
Chen YL, Dunbabin VM, Diggle AJ, Siddique KHM, Rengel Z. Development of a novel semi-hydroponic phenotyping system for studying root architecture. Funct Plant Biol. 2011;38:355–63.
Liu S, Begum N, An T, Zhao T, Xu B, Zhang S, et al. Characterization of root system architecture traits in diverse soybean genotypes using a semi-hydroponic system. Plants. 2021;10: 2781.
Qiao S, Fang Y, Wu A, Xu B, Zhang S, Deng X, et al. Dissecting root trait variability in maize genotypes using the semi-hydroponic phenotyping platform. Plant Soil. 2019;439:75–90.
Chen YL, Dunbabin VM, Diggle AJ, Siddique KHM, Rengel Z. Assessing variability in root traits of wild Lupinus angustifolius germplasm: basis for modelling root system structure. Plant Soil. 2012;354:141–55.
Putra FP, Ismoyojati R. Monitoring of maize root growth on N, P, and K fertilization using rhizotron. J Ilm Pertan. 2021;17:74–9.
Gandullo J, Ahmad S, Darwish E, Karlova R, Testerink C. Phenotyping tomato root developmental plasticity in response to salinity in soil rhizotrons. Plant Phenomics. 2021;2021:2760532.
Rahman G, Sohag H, Chowdhury R, Wahid KA, Dinh A, Arcand M, et al. Soilcam: a fully automated minirhizotron using multispectral imaging for root activity monitoring. Sensors (Switzerland). 2020;20:1–17.
Arnaud M, Baird AJ, Morris PJ, Harris A, Huck JJ. EnRoot: a narrow-diameter, inexpensive and partially 3D-printable minirhizotron for imaging fine root production. Plant Methods. 2019;15:1–9.
Moradi AB, Conesa HM, Robinson B, Lehmann E, Kuehne G, Kaestner A, et al. Neutron radiography as a tool for revealing root development in soil: capabilities and limitations. Plant Soil. 2009;318:243–55.
Pflugfelder D, Metzner R, Dusschoten D, Reichel R, Jahnke S, Koller R. Non-invasive imaging of plant roots in different soils using magnetic resonance imaging (MRI). Plant Methods. 2017;13:1–9.
Teramoto S, Takayasu S, Kitomi Y, Arai-Sanoh Y, Tanabata T, Uga Y. High-throughput three-dimensional visualization of root system architecture of rice using X-ray computed tomography. Plant Methods. 2020;16:1–14.
Liu S, Sherard Barrow C, Hanlon M, Lynch JP, Bucksch A. DIRT/3D: 3D root phenotyping for field-grown maize (Zea mays). Plant Physiol. 2021;187:739–57.
Das A, Schneider H, Burridge J, Ascanio AKM, Wojciechowski T, Topp CN, et al. Digital imaging of root traits (DIRT): a high-throughput computing and collaboration platform for field-based root phenomics. Plant Methods. 2015;11:1–12.
Chen YL, Dunbabin VM, Postma JA, Diggle AJ, Palta JA, Lynch JP, et al. Phenotypic variability and modelling of root structure of wild Lupinus angustifolius genotypes. Plant Soil. 2011;348:345–64.
Figueroa-Bustos V, Palta JA, Chen Y, Siddique KHM. Characterization of root and shoot traits in wheat cultivars with putative differences in root system size. Agronomy. 2018;8:1–14.
Abdel-Ghani A, Sanchez D, Kumar B, Lubberstedt T. Paper roll culture and assessment of maize root parameters. Bio-Protoc. 2016;6:e1926.
Pierret A, Gonkhamdee S, Jourdan C, Maeght JL. IJ_Rhizo: An open-source software to measure scanned images of root samples. Plant Soil. 2013;373:531–9.
Leonova T, Shumilina J, Kim A, Frolova N, Wessjohann L, Bilova T, et al. Agar-based polyethylene glycol (PEG) infusion model for pea (Pisum sativum L.) — perspectives of translation to legume crop plants. Biol Commun. 2022;67:236–44.
Prince SJ, Song L, Qiu D, Maldonado dos Santos JV, Chai C, Joshi T, et al. Genetic variants in root architecture-related genes in a Glycine soja accession, a potential resource to improve cultivated soybean. BMC Genomics. 2015;16:1–20.
Abenavoli MR, Leone M, Sunseri F, Bacchi M, Sorgonà A. Root phenotyping for drought tolerance in bean landraces from Calabria (Italy). J Agron Crop Sci. 2016;202:1–12.
Oram NJ, Sun Y, Abalos D, van Groenigen JW, Hartley S, De Deyn GB. Plant traits of grass and legume species for flood resilience and N2O mitigation. Funct Ecol. 2021;35:2205–18.
Klepper B, Kaspar TC. Rhizotrons: Their development and use in agricultural research. Agron J. 1994;86:745–53.
Rajurkar AB, McCoy SM, Ruhter J, Mulcrone J, Freyfogle L, Leakey ADB. Installation and imaging of thousands of minirhizotrons to phenotype root systems of field-grown plants. Plant Methods. 2022;18:1–12.
Belachew KY, Nagel KA, Fiorani F, Stoddard FL. Diversity in root growth responses to moisture deficit in young faba bean (Vicia faba L.) plants. PeerJ. 2018;2018:1–20.
Krzyzaniak Y, Cointault F, Loupiac C, Bernaud E, Ott F, Salon C, et al. In situ phenotyping of grapevine root system architecture by 2D or 3D imaging: advantages and limits of three cultivation methods. Front Plant Sci. 2021;12:1–15.
Hall B, Lanba A. Three-dimensional analysis of biological systems via a novel laser ablation technique. J Laser Appl. 2019;31:022602.
Strock CF, Schneider HM, Galindo-Castañeda T, Hall BT, Van Gansbeke B, Mather DE, et al. Laser ablation tomography for visualization of root colonization by edaphic organisms. J Exp Bot. 2019;70:5327–42.
Lopez-Valdivia I, Perkins AC, Schneider HM, Vallebueno-Estrada M, Burridge JD, Gonzalez-Orozco E, et al. Gradual domestication of root traits in the earliest maize from Tehuacán. Proc Natl Acad Sci U S A. 2022;119:e2110245119.
Petrushin IS, Vasilev IA, Markova YA. Drought tolerance of legumes: physiology and the role of the microbiome. Curr Issues Mol Biol. 2023;45:6311–24.
Maqbool S, Hassan MA, Xia X, York LM, Rasheed A, He Z. Root system architecture in cereals: progress, challenges and perspective. Plant J. 2022;110:23–42.
Zhang Z, Zhang X, Lin Z, Wang J, Xu M, Lai J, et al. The genetic architecture of nodal root number in maize. Plant J. 2018;93:1032–44.
Steele KA, Price AH, Witcombe JR, Shrestha R, Singh BN, Gibbons JM, et al. QTLs associated with root traits increase yield in upland rice when transferred through marker-assisted selection. Theor Appl Genet. 2013;126:101–8.
Lo Presti D, Di Tocco J, Massaroni C, Cimini S, De Gara L, Singh S, et al. Current understanding, challenges and perspective on portable systems applied to plant monitoring and precision agriculture. Biosens Bioelectron. 2022;2023(222):115005.
Acknowledgements
We would like to thank Ms. Jee Yan Chu for copy-editing this manuscript. Any opinions, findings, conclusions, or recommendations expressed in this publication do not reflect the views of the Government of the Hong Kong Special Administrative Region or the Innovation and Technology Commission.
Funding
This work was supported by Science, Technology and Innovation Commission of Shenzhen Municipality (Shenzhen-Hong Kong-Macau Science and Technology Program, Category C, SGDX20210823103535007), the Hong Kong Research Grants Council Area of Excellence Scheme (AoE/M‐403/16) and Lo Kwee-Seong Biomedical Research Fund awarded to H-ML, Australian Research Council Grant (FT210100902) to YC, and UWA Research Collaboration Awards (2022/GR000814) to YC and H-ML. CH was supported by the Science and Technology Innovation Program of Hunan Province (2023RC3153) and the Natural Science Foundation for Distinguished Young Scholars of Changsha City (kq2209016).
Author information
Authors and Affiliations
Contributions
ZW, M-WL, and H-ML conceptualized the manuscript. ZW, W-SY, and CH wrote the first draft. ZW, W-SY, YG, XZ, YC, M-WL, and H-ML reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Z., Yung, WS., Gao, Y. et al. From phenotyping to genetic mapping: identifying water-stress adaptations in legume root traits. BMC Plant Biol 24, 749 (2024). https://doi.org/10.1186/s12870-024-05477-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-024-05477-8