Abstract
Gestational diabetes mellitus (GDM) is a temporary form of diabetes during pregnancy which influences the health of both mother and child. Both inflammation and oxidative stress have been implicated in the pathophysiology of GDM. Apocynin, acetophenone with anti-oxidative and anti-inflammation activities, has been shown to protect against insulin resistance. In the current study, the effects of apocynin on GDM symptoms, productive outcomes, oxidative stress, and inflammation were evaluated and the underlying mechanisms were explored. We administrated apocynin to GDM mice and monitored the GDM symptoms including body weight, serum levels of glucose, insulin, lipid profile, and the fetal outcomes in GDM mice. We also evaluated the effects of apocynin on placental oxidative stress, inflammation, and activation of TLR4/NF-κB signaling pathway in GDM mice. Here, we reported that apocynin treatment significantly reduced serum levels of glucose, cholesterol, triglyceride, and low-density lipoprotein in GDM mice, while significantly increased serum level of insulin and high-density lipoprotein. Apocynin improved fetal outcomes in GDM mice. Apocynin ameliorated placental oxidative stress and inflammation and inhibited TLR4/NF-κB signaling pathway activation in GDM mice. We concluded that apocynin suppressed oxidative stress and inflammation in GDM by inhibiting the TLR4/NF-κB signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gestational diabetes mellitus (GDM) is a type of diabetes in women that occurs during pregnancy and usually disappears shortly after giving birth. Symptoms which GDM manifests with include gestational hypertension, insulin resistance, and subclinical metabolic dysfunction [1]. Women with GDM are also more likely to develop type 2 diabetes mellitus (T2DM) and coronary heart disease post pregnancy. In addition, increased fetal-maternal morbidity is associated with GDM and the offspring of women with GDM are at increased risk of developing diabetes and metabolic syndrome [2].
Decreased maternal insulin sensitivity and altered maternal lipid metabolism have been implicated in GDM. In women with GDM, exaggeration of the physiological changes in insulin and lipids has been described. For example, in women with GDM, serum triglycerides and low-density lipoprotein cholesterol (LDL-C) were elevated while the high-density lipoprotein (HDL) cholesterol level was significantly decreased.
Several factors have been shown to contribute to the pathophysiology of GDM. When compared with normal pregnant women, increased oxidative stress has been found in women with GDM. The increased oxidative stress markers and decreased anti-oxidative defense are observed in GDM, which could be causative factors in increasing the risk of congenital anomalies [3, 4]. Toll-like receptor 4 (TLR4) is a member of the TLR family of receptors and a key mediator of pro-inflammatory responses. The activation of the TLR4 signaling pathway leads to the production of pro-inflammatory cytokines through the activation of several transcription factors, including NF-κB, activated protein 1 (AP-1), and interferon regulatory factors (IRFs) [5]. Enhanced expression of TLR4 has been observed in patients with T2DM and GDM [6, 7]. Enhanced expression/activation of the TLR4-MyD88-NF-kB pathway also occurs in GDM placenta [8]. Pro-inflammatory cytokines including interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-a) have been shown to interfere with insulin signaling and implicated in insulin resistance and GDM [4]. Therefore, targeting these factors which have been implicated in GDM could be used as a potential treatment to GDM.
Apocynin (4-hydroxy-3-methoxyacetophenone) is acetophenone isolated from Picrorhiza kurroa roots. Apocynin is a selective nicotine adenine dinucleotide phosphate (NADPH) oxidase inhibitor which has been shown to suppress superoxide production in various cell types [9, 10]. It has been shown that apocynin attenuated cerebral infarction by suppressing NADPH oxidase activity and reducing the production of superoxide [11]. Apocynin has also been described to attenuate traumatic brain injury and neuronal damage by suppressing TLR4/NF-κB signaling pathway–mediated inflammation [12]. Taking the anti-oxidative and anti-inflammation activities of apocynin into account, we hypothesized that apocynin could play protective effects on GDM. In the current study, we evaluated the effects of apocynin on GDM using a GDM mice model.
Methods and Materials
Animals and Study Design
Six- to eight-week-old C57BL/KsJ+/+ (wild type) and C57BLKsJdb/+ (db/+) mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA) and used as genetic GDM model as described previously [13, 14]. All mice were housed in a room with controlled temperature (22 °C), humidity (50%), and 12/12 h light cycle and fed with chow diet containing 29% protein, 48% carbohydrate, and 17% fat (Envigo Teklad, USA). Apocynin (Sigma, St Louis, MO, USA) was dissolved with dimethyl sulfoxide (DMSO). For experimental design, female mice were randomly divided into five experimental groups: wild type, ad libitum fed; db/+ pair-fed, food intake of the ad libitum-fed wild-type was measured daily, and the same amount of food was pair-fed to db/+ mice; db/+ pair-fed + 5 mg/kg/day apocynin; db/+ pair-fed + 20 mg/kg/day Apocynin; db/+ pair-fed + 50 mg/kg/day apocynin. Apocynin was administered to mice by oral gavage throughout the entire span of the study. The dose of apocynin used in the current study was followed using the previous description [15, 16]. Wild-type and db/+ pair-fed group mice were administrated with 100 μL corresponding DMSO in saline as control. At 10–12 weeks of age, the female mice were individually mated with males of the same genotype, and mating was confirmed by the presence of a copulatory plug the next morning, which was designated gestation day (GD) 0. Sixteen pregnant female mice from each group were selected for future study. All animal studies were approved by the Ethical Committee of the Affiliated Hospital of Medical School of Ningbo University.
Measurement of Body Weight, Serum Glucose, and Insulin
Maternal body weight, blood glucose, and serum insulin were measured on gestation day (GD) 18 in all 5 groups of mice. Body weight was measured on a top-loading balance. Non-fasting blood samples were obtained via tail venipuncture, and serum glucose level was measured using the glucometer (Roche, Indianapolis, IN, USA). Insulin Mouse ELISA Kit (Thermo Fisher, Waltham, MA, USA) was used to measure serum insulin level. The homeostasis model assessment for insulin resistance (HOMA-IR) was calculated by dividing the product of insulin (μU/mL) and glucose (mmol/L) by 22.5 as described previously [17]. The formula for HOMA-β (HOMA for β-cell function) was insulin (μ-units/ml) × 20/glucose(mmol/l) − 3.5 as described previously [18].
Lipid Profile Detection
On GD 18, the serum was collected from all 5 groups of mice, then the serum levels of total cholesterol (TCh), triglyceride (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) were detected using commercial kits from Cayman Chemical (Ann Arbor, MI, USA). Atherogenic index (AI) was calculated using the following equation: (total cholesterol − HDL-cholesterol)/HDL-cholesterol.
Fetal Analysis
On GD18, pregnant mice were anesthetized and euthanized. After cesarean section, the litter size was counted in combination with their location along the length of the respective uterine horn. Viable fetuses were identified by virtue of the ability to move, breathe, and weighed. Placentas from viable fetuses were weighed and then further analyzed.
ELISA
Malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione (GSH) in the placenta were measured by ELISA on GD18 using commercial ELISA kits purchased from Abcam (Shanghai, China) following manufacture’s protocols. Serum levels of IL-1β, IL-6, and TNF-α were measured using ELISA kits from R&D Systems, Inc. (Minneapolis, MN, USA) following manufacture’s protocols.
Quantitative Real-time PCR
Total RNA from the placenta was isolated using the RNeasy Mini kit (Qiagen, Germantown, MD, USA) according to the manufacturer’s protocols. SuperScript® III First-Strand Synthesis System (Thermo Fisher, USA) was used for RNA reverse-transcription. Real-time PCR was performed using the QuantiTect SYBR Green PCR Kit (Qiagen, USA) on a QuantStudio 5 Real-Time PCR System (Thermo Fisher, USA). The primers used in the current study were listed below: IL-6: Forward 5′-CCTCTGGTCTTCTGGAGTACC-3′, Reverse 5′-ACTCCTTCTGTGACTC CAGC -3′; IL-1β: Forward 5′-CCTTCCAGGATGAGGACATGA-3′, Reverse 5′-TGAGTCAC AGAGGATGGGCTC-3′; TNF-α: Forward 5′-TACTGAACTTCGGGGTG ATTGGTCC-3′, Reverse 5′-CAGCCTTGTCCCTTGAAGAGAACC-3′; TLR4: Forward 5′-CAGGTGGAA TTGTATCGCCT -3′, Reverse 5′-CGAGGCTTTTCCATCCAATA-3′; GAPDH was used as the internal control. Forward 5′-ACCACAGTCCATGCCATCAC-3′ Reverse 5′- CACCACCCTGTTG CTGTAGCC-3′.
Western Blot
Total proteins from the placenta were extracted using the ReadyPrep™ Protein Extraction Kit (Bio-Rad, Hercules, CA, USA), and protein concentration was measured using Bio-Rad Protein Assay (Bio-Rad, USA). A total of 30 μg proteins were loaded onto sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel and transferred to polyvinylidene fluoride membrane. The membranes were blocked with 5% non-fat milk at room temperature for 1 h and then incubated with primary antibodies overnight at 4 °C. The next day, membranes were washed with wash buffer (Thermo Fisher, USA) for 3 times and then incubated with corresponding HRP-conjugated secondary antibodies at room temperature for 1 h. Primary antibodies used in the current study were anti-TLR4 (Abcam, Cambridge, MA, USA), anti-phospho-NF-κB p65 (Abcam, USA), anti-NF-κB p65 (Abcam, USA), and anti-GAPDH (Abcam, USA). Immunoreactive proteins were detected by Clarity™ Western ECL Blotting Substrates (Bio-Rad, USA). The quantities of signals were analyzed by using GS-900™ Calibrated Densitometer (Bio-Rad, USA) and Image Lab software (Bio-Rad, USA).
Statistical Analysis
All data were presented as mean ± standard deviation (SD). Data were analyzed by one-way ANOVA test followed Tukey’s post hoc test. The statistical difference was considered as significant when p value is less than 0.05.
Results
Apocynin Alleviated Gestational Diabetes Mellitus Symptoms in GDM Mice
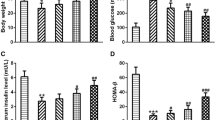
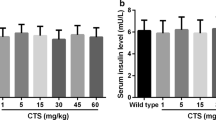
We did not observe any obvious side effects, including death and weight loss reported below, in the GDM mice treated with apocynin. First, we evaluated the effects of apocynin on GDM symptoms by monitoring body weight, blood glucose, and insulin level on GD18. As shown in Fig. 1a, on GD18, there was no significant difference in body weight among all 5 groups of mice, indicating apocynin treatment did not affect body weight at this time point. The GDM mice had significantly elevated blood glucose level when compared with wild-type mice on GD18 (Fig. 1b). Although 5 mg/kg apocynin treatment only slightly decreased the blood glucose level in GDM mice, both 20 and 50 mg/kg apocynin treatments significantly decreased the blood glucose level in GDM mice. In addition, 50 mg/kg treatment had a better effect of decreasing blood glucose level than 20 mg/kg. This result indicated that apocynin decreased blood glucose level of GDM mice in a dose-dependent manner. We detected significantly decreased serum insulin level in GDM mice when compared with wild-type mice (Fig. 1c). Five milligrams per kilogram apocynin treatment did not significantly increase the blood insulin level in GDM mice while both 20 mg/kg and 50 mg/kg apocynin treatment significantly increased blood insulin level. The HOMA-IR was significantly increased in GDM mice when compared with wild type mice (Fig. 1d). Treatment with 5, 20, and 50 mg/kg apocynin did not change the HOMA-IR. GDM mice had significantly decreased HOMA-β when compared with wild-type mice. In contrast, treatment with 20 and 50 mg/kg apocynin significantly increased the HOMA-β (Fig. 1e), indicating that apocynin treatment increased β cell function. It is worth noting that apocynin treatment did not affect the observed parameters above in the control mice in our pilot study (data not shown). Taken together, our data demonstrated that apocynin treatment decreased blood glucose level, increased blood insulin level, and increased β cell function, indicating that apocynin alleviated GDM symptoms in GDM mice.
Apocynin alleviated gestational diabetes mellitus symptoms in pregnant db/+ mice. Maternal body weight (a), blood glucose (b), and serum insulin (c) were measured on gestation day (GD) 18 among indicated groups. Homeostasis model assessment of insulin resistance (HOMA-IR) (d) and homeostasis model assessment of β-cell function (HOMA-β) (e) were calculated on GD 18. Data are presented as mean ± SD. *p < 0.05, **p < 0.01, and ***p < 0.001 between the indicated two groups
Apocynin Ameliorated Lipid Profile and Atherogenic Index in GDM Mice
We next evaluated the effects of apocynin on serum lipid profile in GDM mice. On GD18, blood serum levels of cholesterol (TCh), triglyceride (TG), serum high-density lipoprotein (HDL), and serum low-density lipoprotein (LDL) were measured. GDM mice had significantly increased blood levels of TCh (Fig. 2a), TG (Fig. 2b), and LDL (Fig. 2d) while had significantly decreased HDL (Fig. 2c). Five milligrams per kilogram apocynin treatment significantly decreased the blood level of TCh while did not obviously affect TG, HDL, and LDL levels in GDM mice. Both 20 mg/kg and 50 mg/kg apocynin treatments significantly decreased blood levels of TCh, TG, and LDL while significantly increased HDL level and these effects were in a dose-dependent manner. Correspondingly, the atherogenic index of GDM mice was significantly higher than that of wild-type mice (Fig. 2e). All 3 different concentrations of apocynin treatments significantly decreased the atherogenic index of GDM mice in a dose-dependent manner. Collectively, our data demonstrated that apocynin ameliorated lipid profile and atherogenic index in GDM mice by decreasing TCh, TG, and LDL levels and increasing HDL level.
Apocynin ameliorated biochemical indexes in gestational diabetes mellitus mice in the late stage of pregnancy. Total serum cholesterol (TCh) (a), serum triglyceride (TG) (b), serum high-density lipoprotein (HDL) (c), serum low-density lipoprotein (LDL) (d), and atherogenic index (e) were tested on GD 18 among indicated groups. Data are presented as mean ± SD. *p < 0.05, **p < 0.01, and ***p < 0.001 between the indicated two groups
Apocynin Improved Fetal Outcomes in GDM Mice
We continued to explore the potential effect of apocynin on fetal outcomes in GDM mice. The GDM mice had significantly decreased fetal alive rate (Fig. 3a), fetal weight (Fig. 3b), crown-rump length (Fig. 3c), and placenta weight (Fig. 3d) when compared with wild-type mice. Although 5 mg/kg apocynin treatment did not obviously affect fetus alive rate, fetal weight, and placental weight, it significantly increased crown rump length. Twenty milligrams per kilogram apocynin treatment did not obviously affect fetus alive rate while significantly increased fetal weight, crown rump length, and placental weight. Fifty milligrams per kilogram apocynin treatment significantly increased alive rate, fetal weight, crown rump length, and placental weight. Therefore, our data showed that apocynin improved fetal outcomes in GDM mice in a dose-dependent manner.
Apocynin Suppressed Placental Oxidative Stress in GDM Mice
As apocynin displayed the protective activities on GDM symptoms in GDM mice, we continued to explore whether apocynin affects placental oxidative stress, which has been implicated in GDM pathophysiology. We analyzed the levels of oxidative stress markers including MDA, a product of lipid peroxidation, SOD and GPx, the antioxidant enzymes, and GSH, a low molecular weight antioxidant in the placenta. As shown in Fig. 4, there were significantly increased MDA level (Fig. 4a) and significantly decreased levels of SOD (Fig. 4b), GPx (Fig. 4c), and GSH (Fig. 4d) in placenta of GDM mice when compared with that of wild type mice, indicating obvious placental oxidative stress in GDM mice. In contrast, 5, 20, and 50 mg/kg apocynin treatments significantly decreased MDA level and increased SOD, GPx, and GSH levels in the placenta of GDM mice in a dose-dependent manner, when compared with non-treated GDM mice. Taken together, our data demonstrated that apocynin suppressed placental oxidative stress in GDM mice.
Apocynin attenuated placental oxidative stress in gestational diabetes mellitus mice. ELISA was used to analyze the activities of MDA (a), SOD (b), GPx (c), and GSH (d) in the placenta on GD 18 among indicated groups. Data are presented as mean ± SD. *p < 0.05 and **p < 0.01 between the indicated two groups
Apocynin Attenuated Inflammation in GDM Mice
To evaluate the effect of apocynin on inflammation, we monitored the levels of inflammatory cytokines including IL-1β, IL-6, and TNF-α in both serum and placenta. We detected significantly increased serum levels of IL-1β (Fig. 5a), IL-6 (Fig. 5b), and TNF-α (Fig. 5c) in GDM mice when compared with wild-type mice. We also detected significantly increased mRNA levels of IL-1β (Fig. 5d), IL-6 (Fig. 5e), and TNF-α (Fig. 5f) in the placenta of GDM mice. Five, 20, and 50 mg/kg apocynin treatments significantly decreased both protein levels in serum and mRNA levels in the placenta of these inflammatory cytokines in a dose-dependent manner. Taken together, our data demonstrated that apocynin attenuated inflammation in GDM mice.
Apocynin suppressed serum and placental inflammation in gestational diabetes mellitus mice. ELISA was used to analyze the concentrations of IL-1β (a), IL-6 (b), and TNF-α (c) in serum from indicated mice on GD18. RT-PCR was used to analyzed the mRNA levels of IL-1β (d), IL-6 (e), and TNF-α (f) in the placenta from indicated mice on GD18. Data are presented as mean ± SD. *p < 0.05, **p < 0.01, and ***p < 0.001 between the indicated two groups
Apocynin Inhibited Activation of TLR4/NF-κB Signaling Pathway in GDM Mice
Finally, we explored the potential effects of apocynin on the TLR4/NF-κB signaling pathway, which had been also implicated in GDM [8]. We detected significantly increased protein and mRNA level of TLR4 in the placenta of GDM mice (Fig. 6a, c). Five, 20, and 50 mg/kg apocynin treatments significantly decreased both protein and mRNA levels of TLR4 in the placenta in a dose-dependent manner. In the placenta of GDM mice, the NF-κB p65 was activated as we detected significantly increased phosphor-NF-κB when compared with wild-type mice. Five, 20, and 50 mg/kg apocynin treatments significantly decreased NF-κB p65 phosphorylation in the placenta in a dose-dependent manner, while did not affect total NF-κB p65 protein (Fig. 6b) and mRNA level (Fig. 6d). Collectively, our data demonstrated that apocynin inhibited the activation of the TLR4/NF-κB signaling pathway in GDM mice.
TLR4/NF-κB signaling pathway participates in the protective effects of apocynin on gestational diabetes mellitus mice. Western blotting was used to assay the protein expressions of TLR4 (a) and p-p65 and p65 (b) in the placenta from indicated mice on GD18. GAPDH was used as a loading control and relative expressions were normalized to wild-type group. RT-PCR was used to analyze the mRNA level of TLR4 (C) and p65 (D) in the placenta from indicated mice on GD18. Data are presented as mean ± SD. *p < 0.05, **p < 0.01, and ***p < 0.001 between the indicated two groups
Discussion
Nowadays there are around 10% of pregnancies that are affected by GDM, which causes maternal diabetic symptoms and abnormal fetal development. GDM is linked to both short-term and long-term adverse health outcomes in women and their offspring and represents a growing health concern. For instance, women with GDM have an increased risk for gestational hypertension and pre-eclampsia during pregnancy and an exceptionally high risk for type 2 diabetes after pregnancy [19]. Children born after pregnancies with GDM are more likely to have birth defects, and there is evidence to suggest that they are also at higher risk of developing childhood obesity [20]. Therefore, prevention of GDM could be useful in curbing the obesity and diabetes epidemic in this and future generations.
In the current study, we evaluated the potential of apocynin for GDM treatment. Using a GDM mice model, we demonstrated that the administration of apocynin in GDM mice ameliorated GDM symptoms in GDM mice, including decreasing blood glucose level; increasing serum insulin level; decreasing the TCh, TG, and LDL levels; and increasing HDL level. In addition, apocynin improved fetal outcomes in GDM mice, including increasing fetal alive rate, fetal weight, crown-rump length, and placental weight. All these results indicated that apocynin was protective in GDM mice, suggesting apocynin could be used as a potential therapeutic agent to treat GDM.
Apocynin has been used as an efficient inhibitor of the complex NADPH-oxidase in many experimental models. The anti-oxidative and anti-inflammation activities of apocynin have been well studied [21, 22]. By exploring the mechanisms through which apocynin ameliorated GDM symptoms, we found that apocynin significantly suppressed placental oxidative stress and inflammatory cytokines (IL-1β, IL-6, and TNF-α) production in GDM mice. Furthermore, we found that apocynin inhibited the upregulation of TLR4 and activation of NF-κB in GDM mice.
Elevated oxidative stress is found in GDM women, which has been shown to be conducive to insulin resistance, β cell dysfunction, and glucose intolerance. In the current study, significantly decreased anti-oxidative factors including SOD, GPx, and GSH were found in GDM mice. In contrast, administration of apocynin in GDM mice significantly enhanced the levels of these oxidative factors. Our results were consistent with previous reports. Meng and colleagues demonstrated that in high-fat-diet (HFD) mice, apocynin treatment significantly lowered the serum level of MDA and increased serum level of SOD. Apocynin treatment also strengthened the anti-oxidative system with increased activity of GPx and content of reduced GSH. These activities contributed to the amelioration of insulin resistance in HFD-fed mice [22].
Placental inflammation has been observed in women with GDM and plays a central role in insulin resistance in these pregnancies [23]. Increased pro-inflammatory cytokines including IL-1β, IL-6, IL-8, TNF-α, and monocyte-chemoattractant-protein-1 (MCP-1) have been shown to be associated with GDM. As these cytokines could interfere with insulin signaling, these cytokines have also been implicated in insulin resistance in type 2 diabetes mellitus (T2DM). IL-1β has been shown to induce insulin resistance in adipocytes by downregulating insulin receptor substrated-1 expression [24]. TNF-α could inhibit the phosphorylation of the insulin receptor and decrease the glucose transporter-4 expression, which contributes to insulin resistance [25, 26]. IL-6 has been shown to inhibit insulin receptor (IR) signal transduction and insulin action in both mouse and the human hepatocytes [27]. In the current study, all of these three cytokines were significantly upregulated in GDM mice, while apocynin treatment significantly decreased these cytokines level. The anti-inflammation activity of apocynin was well studied too. Kim et al. demonstrated that apocynin inhibited the production of pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6 in experimental asthma mice [28]. Apocynin also inhibited the expression of TNF-α, IL-6, MCP-1, and leptin in adipose tissue, which contributed to the improvement of insulin resistance in HFD-fed mice [29].
TLR4 has been shown to be a potential molecule function in IR. Enhanced expression of TLR4 has been observed in T2DM as well as GDM [8]. A recent study suggested that TLR4 and downstream pathways (MAPK and NF-κB) are important in the pathogenesis of IR [30]. In the current study, we observed enhanced TLR4 expression and NF-κB activation in GDM mice. Apocynin has been shown to inhibit the TLR4/NF-κB signaling pathway. Nam and colleagues demonstrated that apocynin attenuated LPS-induced TLR4/ NF-κB activation and downstream inflammatory cytokines production [31]. Our study confirmed the inhibitory activities of apocynin on TLR4/NF-κB activation. In apocynin-treated GDM mice, the expression levels of TLR4 and active NF-κB were significantly reduced. The activation of TLR4/NF-κB resulted in inflammatory cytokines production. Therefore, the inhibition of TNF-α, IL-1β, and IL-6 by apocynin in GDM mice could be caused by the suppression of TLR4/NF-κB activation by apocynin.
One important point in the current study we would point out is that although apocynin suppressed inflammation in GDM mice, it did not totally prevent or abolish them as GDM mice still got higher levels of inflammation when compared with normal mice. One possibility is that the bioavailability of apocynin in the current study is not high. Careful analysis of apocynin pharmacokinetic should provide us useful information. Another point is the safety of apocynin. Now, the safety data of apocynin are scarce and limited studies showed the low toxicity and high stability of apocynin. The safety of apocynin in pregnancy is never described. It is still unknown whether apocynin could cross the placenta. The long-term effects of apocynin on the fetus still need to be explored.
Conclusion
Taken together, our current study demonstrated that apocynin suppressed oxidative stress and inflammation in GDM by inhibiting the TLR4/NF-κB signaling pathway.
References
Reece EA, Leguizamon G, Wiznitzer A. Gestational diabetes: the need for a common ground. Lancet. 2009;373:1789–97.
Nansseu JR, Ngo-Um SS, Balti EV. Incidence, prevalence and genetic determinants of neonatal diabetes mellitus: a systematic review and meta-analysis protocol. Syst Rev. 2016;5:188.
Zhu C, Yang H, Geng Q, et al. Association of oxidative stress biomarkers with gestational diabetes mellitus in pregnant women: a case-control study. PLoS One. 2015;10:e0126490.
Sudharshana Murthy KA, Bhandiwada A, Chandan SL, Gowda SL, Sindhusree G. Evaluation of oxidative stress and proinflammatory cytokines in gestational diabetes mellitus and their correlation with pregnancy outcome. Indian J Endocrinol Metab. 2018;22:79–84.
O’Neill LA, Golenbock D, Bowie AG. The history of toll-like receptors - redefining innate immunity. Nat Rev Immunol. 2013;13:453–60.
Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, et al. Lack of toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101:10679–84.
Xie BG, Jin S, Zhu WJ. Expression of toll-like receptor 4 in maternal monocytes of patients with gestational diabetes mellitus. Exp Ther Med. 2014;7:236–40.
Feng H, Su R, Song Y, et al. Positive correlation between enhanced expression of TLR4/MyD88/NF-kappaB with insulin resistance in placentae of gestational diabetes mellitus. PLoS One. 2016;11:e0157185.
Barbieri SS, Cavalca V, Eligini S, et al. Apocynin prevents cyclooxygenase 2 expression in human monocytes through NADPH oxidase and glutathione redox-dependent mechanisms. Free Radic Biol Med. 2004;37:156–65.
Vejrazka M, Micek R, Stipek S. Apocynin inhibits NADPH oxidase in phagocytes but stimulates ROS production in non-phagocytic cells. Biochim Biophys Acta. 2005;1722:143–7.
Tang LL, Ye K, Yang XF, Zheng JS. Apocynin attenuates cerebral infarction after transient focal ischaemia in rats. J Int Med Res. 2007;35:517–22.
Feng Y, Cui C, Liu X, Wu Q, Hu F, Zhang H, et al. Protective role of apocynin via suppression of neuronal autophagy and TLR4/NF-kappaB signaling pathway in a rat model of traumatic brain injury. Neurochem Res. 2017;42:3296–309.
Kaufmann RC, Amankwah KS, Dunaway G, Maroun L, Arbuthnot J, Roddick JW Jr. An animal model of gestational diabetes. Am J Obstet Gynecol. 1981;141:479–82.
Zou C, Zhang Q, Zhang S. Mogroside IIIE attenuates gestational diabetes mellitus through activating of AMPK signaling pathway in mice. J Pharmacol Sci. 2018;138:161–6.
Choi BY, Kim JH, Kho AR, et al. Inhibition of NADPH oxidase activation reduces EAE-induced white matter damage in mice. J Neuroinflammation. 2015;12:104.
Sun Z, Satomoto M, Adachi YU, et al. Apocynin preserves glutamatergic neurons in the basolateral amygdala in mice with neonatal sevoflurane exposure. Korean J Anesthesiol. 2017;70:335–40.
Fraulob JC, Ogg-Diamantino R, Fernandes-Santos C, Aguila MB, Mandarim-de-Lacerda CA. A mouse model of metabolic syndrome: insulin resistance, fatty liver and non-alcoholic fatty pancreas disease (NAFPD) in C57BL/6 mice fed a high fat diet. J Clin Biochem Nutr. 2010;46:212–23.
Singh B, Saxena A. Surrogate markers of insulin resistance: a review. World J Diabetes. 2010;1:36–47.
Bellamy L, Casas JP, Hingorani AD, et al. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773–9.
Hillier TA, Pedula KL, Schmidt MM, et al. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30:2287–92.
Hwang YJ, Lee SJ, Park JY, et al. Apocynin suppresses lipopolysaccharide-induced inflammatory responses through the inhibition of MAP kinase signaling pathway in RAW264.7 Cells. Drug Dev Res. 2016;77:271–7.
Meng R, Zhu DL, Bi Y, Yang DH, Wang YP. Anti-oxidative effect of apocynin on insulin resistance in high-fat diet mice. Ann Clin Lab Sci. 2011;41:236–43.
Kleiblova P, Dostalova I, Bartlova M, et al. Expression of adipokines and estrogen receptors in adipose tissue and placenta of patients with gestational diabetes mellitus. Mol Cell Endocrinol. 2010;314:150–6.
Jager J, Gremeaux T, Cormont M, et al. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology. 2007;148:241–51.
Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci U S A. 1994;91:4854–8.
Stephens JM, Lee J, Pilch PF. Tumor necrosis factor-alpha-induced insulin resistance in 3T3-L1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and GLUT4 expression without a loss of insulin receptor-mediated signal transduction. J Biol Chem. 1997;272:971–6.
Senn JJ, Klover PJ, Nowak IA, Mooney RA. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes. 2002;51:3391–9.
Kim SY, Moon KA, Jo HY, et al. Anti-inflammatory effects of apocynin, an inhibitor of NADPH oxidase, in airway inflammation. Immunol Cell Biol. 2012;90:441–8.
Meng R, Zhu DL, Bi Y, et al. Apocynin improves insulin resistance through suppressing inflammation in high-fat diet-induced obese mice. Mediat Inflamm. 2010;2010:858735.
Reyna SM, Ghosh S, Tantiwong P, et al. Elevated toll-like receptor 4 expression and signaling in muscle from insulin-resistant subjects. Diabetes. 2008;57:2595–602.
Nam YJ, Kim A, Sohn DS, et al. Apocynin inhibits toll-like receptor-4-mediated activation of NF-kappaB by suppressing the Akt and mTOR pathways. Naunyn Schmiedeberg's Arch Pharmacol. 2016;389:1267–77.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal studies were approved by the Ethical Committee of the Affiliated Hospital of Medical School of Ningbo University.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Liu, T., Zheng, W., Wang, L. et al. TLR4/NF-κB Signaling Pathway Participates in the Protective Effects of Apocynin on Gestational Diabetes Mellitus Induced Placental Oxidative Stress and Inflammation. Reprod. Sci. 27, 722–730 (2020). https://doi.org/10.1007/s43032-019-00078-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-019-00078-5