Abstract
Gestational diabetes mellitus (GDM) is a disease characterized by insufficient insulin secretion and glucose metabolic disorder during pregnancy. Tetramethylpyrazine has been reported to inhibit endoplasmic reticulum (ER) stress and high glucose-induced inflammation, which are closely associated with GDM. This study aimed to investigate the effects of tetramethylpyrazine on inflammatory responses, ER stress and oxidative stress of the placenta in a mouse model of GDM. Our results showed that tetramethylpyrazine treatment significantly alleviated the GDM symptoms characterized by low body weight and serum insulin levels, high blood glucose, and decreased β-cell function in pregnant C57BL/KsJdb/+ mice. In addition, tetramethylpyrazine reduced the level of malondialdehyde, and increased the levels of superoxide dismutase, glutathione peroxidase and glutathione. Moreover, tetramethylpyrazine decreased the total serum cholesterol, serum triglyceride, and serum low-density lipoprotein levels and increased the high-density lipoprotein level. Further, tetramethylpyrazine regulated the levels of serum and placental inflammatory factors and the expression of ER stress related proteins. Taken together, the present study demonstrated that tetramethylpyrazine attenuated placental oxidative stress, inflammatory responses and ER stress in GDM mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gestational diabetes mellitus (GDM) refers to diabetes in pregnant women with abnormal glucose metabolism or potential impaired glucose tolerance during pregnancy (Mishra et al. 2018). In recent years, with the improvement of living standards in China, the prevalence of GDM has also increased and has reached 1% to 3% (Poola-Kella et al. 2018). The quality of blood glucose control during pregnancy is directly related to the health and safety of pregnant women and fetuses (Miettinen et al. 2018). Poorly controlled GDM is likely to cause excessive amniotic fluid, increased incidence of pregnancy-induced hypertension and infection, which is an important cause of increased perinatal mortality (Feghali et al. 2018). Increasing studies have shown that the occurrence of GDM is closely related to the lipid metabolism disorder, genetic factors and placental vascular endothelial injury in pregnant women (Plows et al. 2018). It is not determined by a single factor, but caused by changes in molecular, tissue, organ, and system interactions with genetic factors and the environment (Dias et al. 2018). Among them, vascular endothelium lesions caused by oxidative stress, which leads to hypoperfusion of the placenta, blood supply to the placenta and umbilical cord, and insufficient oxygen supply, is the main cause of fetal distress and even fetal death in pregnant women (Lappas et al. 2011; Poulakos et al. 2015). Therefore, in-depth study of the molecular mechanisms and further exploration of better treatments for GDM are of great significance.
Tetramethylpyrazine, also known as ligustrazine, is a nitrogen-containing heterocyclic compound in which a methyl group is attached to each pyrazine ring carbon atom (Wu et al. 2019). Tetramethylpyrazine is the main active alkaloid component of the Chinese medicinal material Ligusticum wallichii, which exhibits the functions in dilating blood vessels, improving microcirculation and inhibiting platelet aggregation, and is widely used in clinical practice (Zhao et al. 2016). Tetramethylpyrazine has been reported to inhibit endoplasmic reticulum (ER) stress (Liu et al. 2018; Yang and Wu 2018), which been shown to be caused by GDM in the placenta (Yung et al. 2016). In addition, tetramethylpyrazine is also able to inhibit high glucose-induced inflammation (Yang et al. 2011; Chen et al. 2019). Therefore, tetramethylpyrazine might play an important role in GDM. There is currently no research on tetramethylpyrazine in GDM, and study is also limited with regard to the effect of tetramethylpyrazine on ER stress in the placenta. Therefore, this work focuses on the protective effects of tetramethylpyrazine in a mouse model of GDM from in terms of oxidative stress, inflammation and ER stress in the placenta.
Methods
Animals
All animal studies in this paper were approve by the Ethics Commitment of Xingtai People’s Hospital of Hebei Province. For generating GDM mice model, C57BL/KsJ+/+ (wild type) and C57BL/KsJdb/+ (db/+) mice were purchased from iBio Logistics Co. (Changping, Beijing, China). Up to six mice were placed in a ventilated SPF cage. Mice were housed under 22–26 °C and a 12/12-h light/dark cycle with adequate sterile food and water supply. In each group, 18 mice matched for successful pregnancy were selected for further experimental study. Different doses (0, 20, 40, and 60 mg / kg body weight) of tetramethylpyrazine (≥ 98%, W323713, Sigma-Aldrich, St. Louis, MO, USA) were administered once per day by intragastric administration from the time of successful pregnancy to the end of the experiment. The dose of tetramethylpyrazine used in the following experiments was determined according to our preliminary experiments, and no side effects were observed during the entire experimental period. The mice were sacrificed at gestation day (GD) 18 after the last administration.
Blood glucose and serum insulin level measurements
Blood samples were obtained from the tail vein of the mice. A Glucose Detection Kit (GOD-POD Microplate Method) was purchased from Leagene biotech.co. (Haidian, Beijing, China) to measure the blood glucose according to the manufacturers’ instructions. An Insulin Mouse Enzyme-Linked ImmunoSorbent Assay (ELISA) Kit (Invitrogen Life Technologies, Carlsbad, CA, USA) was used to determine the serum insulin levels of the mice. The pancreatic islet β-cell function in different groups was examined by homeostasis model assessment (HOMA-β), as follows: β-cell function = (serum insulin, μIU/mL × 20)/(serum glucose, mmol/L) − 3.5.
Serum lipid level measurement
Total serum cholesterol (TCh), serum triglyceride (TG), and serum low-density lipoprotein (LDL) levels, serum high-density lipoprotein (HDL) level in serum were analyzed by ILab Chemistry Analyzer 300 PLUS (Instrumentation Laboratory, Bedford, MA, USA).
Placental oxidative stress measurement
ELISA was used to analyze the activities of malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione (GSH) in placenta on GD 18 among indicated groups according to the previous described methods (Fu et al. 2012a, b).
RNA extraction and qRT-PCR
The total RNA in placenta were extracted by Trizol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA). RNA was reverse transcribed into cDNA using a reverse transcription kit (Fermentas, St. Leon-Rot, Germany). SYBR-Green Master mix (Life Technologies, Carlsbad, CA, USA) was used to quantify the expression levels.
Primers used in this paper were listed as follows:
Interleukin (IL)-6 forward 5′-TCCAGTTGCCTTCTTGGGAC-3′; IL-6 Reverse 5′-GTGTAATTAAGCCTCCGACTTG-3′;
Tumor necrosis factor (TNF)-α Forward 5′-CATCTTCTCAAAATTCGAGTGACAA-3′; TNF-α Reverse 5′-TGGGAGTAGACAAGGTACAACCC-3′;
Monocyte chemotactic protein 1 (MCP-1) Forward 5′-TTCACAGTTGCTGCCTGTAG-3′; MCP-1 Reverse 5′-TCTGATCTCACTTGGTTCTGG-3′;
GAPDH Forward 5′-TTCACCACCATGGAGAAGGC-3′; GAPDH Reverse 5′-GGCATGGACTGTGGTCATGA-3′.
Western blot
Western blot was performed with the standard method. The following antibodies were used: CCAAT/Enhancer-Binding Protein Homologous Protein (CHOP, sc-575, Santa Cruz, Dallas, TX, USA), glucose-regulated protein 78 (GRP78, ab21685, Abcam, Cambridge, UK), phospho-eukaryotic initiation factor 2 (eIF2a, #3398, Cell Signaling Technology, Shanghai, China), eIF2a (#5324, Cell Signaling Technology), and GAPDH (#5174, Cell Signaling Technology).
Statistical analysis
SPSS software version 19.0 was used in the data analysis. All data in the experiment was shown as mean ± standard deviation (SD). One-way ANOVA with a Tukey’s post hoc test was used to calculate the differences between each group.
Results
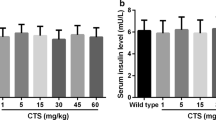
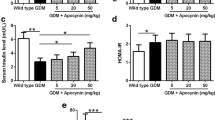
First, we conducted a tetramethylpyrazine safety verification and concentration selection experiment. Different concentrations of tetramethylpyrazine were found to have no significant effect on blood glucose and insulin concentrations in healthy pregnant mice (Fig. S1A and B). In addition, we treated the C57BL/KsJ+/+ (wild type) and C57BL/KsJdb/+ (db/+) mice with different concentrations of tetramethylpyrazine and detected the blood glucose and serum insulin levels. We found that blood glucose, which was increased in GDM mice, was decreased with administration of different doses of tetramethylpyrazine (Fig. S1C). Treatment of tetramethylpyrazine increased the serum insulin levels in GDM mice. Furthermore, 20, 40 and 60 mg/kg of tetramethylpyrazine were selected as the optimum concentrations and were used in the follow-up experiments. In addition, we also studied the toxicity of tetramethylpyrazine on the fetal mice. There was no significant change in fetus survival ratio and fetal weight, which indicated that tetramethylpyrazine had no obvious toxicity to fetal mice (data not shown). Next, maternal body weight, blood glucose and serum insulin were measured on GD 18 following administration of different doses of tetramethylpyrazine. We found that the body weight of in GDM mice was lower than that in wild type mice, and tetramethylpyrazine treatment significantly increased the body weight of GDM mice (Fig. 1a). In addition, our results showed that tetramethylpyrazine treatment rescued the altered blood glucose (Fig. 1b) and serum insulin (Fig. 1c) levels in GDM mice in a dose-dependent manner. Moreover, we examined the pancreatic islet β-cell function in different groups by HOMA-β). We found that tetramethylpyrazine treatment rescued the inhibitory effects on HOMA-β in GDM mice in a dose-dependent manner (Fig. 1d). Taken together, we found that tetramethylpyrazine treatment alleviated the GDM symptoms characterized by low body weight and serum insulin levels, high blood glucose, and decreased β-cell function in pregnant db/+ mice.
Tetramethylpyrazine alleviated GDM symptoms in pregnant db/+ mice. a–c Maternal body weight (a), blood glucose (b) and serum insulin (c) were measured on gestation day (GD) 18 among different doses of tetramethylpyrazine. d Homeostasis model assessment of β-cell function (HOMA-β) were calculated on GD 18. Data are presented as mean ± SD. *p < 0.05, **p < 0.01 and ***p < 0.001 compared to wild type group,#p < 0.05,##p < 0.01 and###p < 0.001 compared to GDM mice
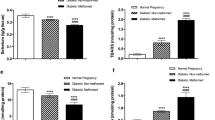
To further explore the effects of tetramethylpyrazine in GDM mice, various biochemical parameters were measured among different groups in the late stage of pregnancy. We found that TCh (Fig. 2a), TG (Fig. 2b), and serum LDL (Fig. 2c) levels were significantly upregulated in GDM mice, whereas tetramethylpyrazine treatment decreased this trend in a dose-dependent manner. In addition, tetramethylpyrazine treatment remarkably rescued the down-regulated serum HDL levels in GDM mice (Fig. 2d). Moreover, the atherogenic index, which was significantly elevated in GDM mice, gradually decreased with increasing doses of tetramethylpyrazine (Fig. 2e). Therefore, we confirmed that tetramethylpyrazine treatment ameliorated the biochemical indexes in GDM mice.
Tetramethylpyrazine ameliorated biochemical indexes in GDM mice in the late stage of pregnancy. a–e TCh (a), TG (b), LDL (c), HDL (d), and atherogenic index (e) were tested on GD 18 among indicated groups. Data are presented as mean ± SD. **p < 0.01 and ***p < 0.001 compared to wild type group,#p < 0.05,##p < 0.01 compared to GDM mice
It is reported that oxidative stress plays an important role in the pathogenesis of GDM (Liu et al. 2018). Thus, we detected the MDA content and the anti-oxidative enzyme activities in placenta tissues on GD 18 in different groups. We found that tetramethylpyrazine treatment reduced the MDA levels which were increased in GDM mice (Fig. 3a). In addition, we found that the activities of SOD, GPx and GSH were remarkably decreased in GDM mice. Following tetramethylpyrazine treatment, the activities of each anti-oxidative enzyme were enhanced in a dose-dependent manner (Fig. 3b–d). Thus, our data demonstrated that tetramethylpyrazine treatment attenuated the elevated placental oxidative stress in GDM mice.
Tetramethylpyrazine attenuated placental oxidative stress in GDM mice. a–d ELISA was used to analyze the activities of MDA (a), SOD (b), GPx (c) and GSH (d) in placenta on GD 18 among indicated groups. Data are presented as mean ± SD. **p < 0.01 compared to wild type group,#p < 0.05,##p < 0.01 compared to GDM mice
Previous studies have reported that tetramethylpyrazine possesses anti-inflammatory effects and could inhibit the inflammation responses caused by high glucose (Yang et al. 2011). Thus, we examined the levels of various inflammatory factors in the serum and placenta by ELISA and qRT-PCR, to identify the role of tetramethylpyrazine in inflammatory responses in GDM mice. Our results showed that the levels of IL-6, MCP-1 and TNF-α were significantly increased in GDM mice, whereas tetramethylpyrazine treatment inhibited this trend in a dose-dependent manner (Fig. 4a–i). Therefore, we speculated that tetramethylpyrazine might play a role in GDM by regulating the concentration of serum and placental inflammatory factors.
Tetramethylpyrazine attenuated serum and placental inflammation in GDM mice. a–c ELISA was used to analyze the levels of IL-6 (a), MCP-1 (b) and TNF-α (c) in serum from indicated mice on GD18. d–f ELISA was used to analyze the levels of IL-6 (d), MCP-1 (e) and TNF-α (f) in placenta from indicated mice on GD18. g–i QRT-PCR was used to analyzed the mRNA levels of IL-6 (g), MCP-1 (h) and TNF-α (i) in placenta from indicated mice on GD18. Data are presented as mean ± SD. *p < 0.05, **p < 0.01 and ***p < 0.001 compared to wild type group,#p < 0.05,##p < 0.01 compared to GDM mice
Previous studies have found that GDM could cause ER stress in the placenta (Yung et al. 2016). Therefore, we evaluated the effects of tetramethylpyrazine on ER stress by detecting the levels of various ER stress related proteins in the placenta. Our results revealed that tetramethylpyrazine treatment decreased the expression of GRP78, CHOP and p-eIF2/eIF2a, which were significantly elevated in GDM mice (Fig. 5a–d). Taken together, our data suggested that tetramethylpyrazine treatment might attenuate the placental ER stress in GDM mice by down-regulating the expression of GRP78, CHOP and p-eIF2a/eIF2a.
Tetramethylpyrazine attenuated placental endoplasmic reticulum stress in GDM mice. aWestern blotting was used to analyze the protein expression of placental GRP78, CHOP, p-eIF2α, eIF2α and relative expression. b–d Quantification of the band intensity from (a). Data are presented as mean ± SD. ***p < 0.001 compared to wild type group,#p < 0.05,##p < 0.01 compared to GDM mice
Discussion
GDM is one of the common complications during pregnancy. In recent years, with the development of medical diagnostic techniques and living standards, the incidence of GDM has been gradually increasing. GDM has many adverse effects on pregnant women and fetuses (Silva et al. 2017). For instance, GDM women are prone to complications such as pregnancy-induced hypertension syndrome, polyhydramnios, infection, ketoacidosis, and the possibility of diabetes and metabolic syndrome after delivery is higher than health pregnant women (Johns et al. 2018). If GDM patients have poor long-term control of blood glucose, it may cause fetal chronic intrauterine hypoxia, abnormal growth and development, malformation, and neonatal hyperbilirubinemia (Prakash et al. 2017). After leaving the maternal hyperglycemic environment, neonates often have hypoglycemia. Because fetal hyperinsulinemia affects the synthesis of alveolar surfactants, fetal lung development retardation, the prevalence of neonatal respiratory distress syndrome increases (Badon et al. 2017). The neonates of GDM patients are more susceptible to obesity, impaired glucose tolerance, and even diabetes in adolescence (Li et al. 2017). Therefore, it is imperative to further explore the pathogenesis of GDM and find new treatments against GDM.
Tetramethylpyrazine is the main active ingredient of traditional Chinese medicine Chuanxiong. It has the functions in activating blood circulation, improving blood supply to organs, thrombolysis, and scavenging free radicals (Hu et al. 2013). Recent studies have found that tetramethylpyrazine could inhibit ER stress and inflammation caused by high glucose (Yang et al. 2011; Liu et al. 2018; Yang and Wu 2018; Chen et al. 2019). Here, we reported that tetramethylpyrazine alleviated the GDM symptoms in our mouse model. Tetramethylpyrazine attenuated the GDM-induced alterations in body weight, blood glucose, serum insulin and lipid levels. Therefore, tetramethylpyrazine may contribute to alleviating the symptoms of GDM.
The pathogenesis of GDM is still unclear. Researchers believe that its mechanism is similar to that of type II diabetes. Insulin resistance and islet β-cell dysfunction are central to the pathogenesis of GDM (Li et al. 2018). Studies have shown that oxidative stress plays an important role in insulin resistance and islet β-cell dysfunction, leading to impaired glucose regulation and elevated blood glucose (Kaneto et al. 2006). Therefore, oxidative stress may be one of the important pathogenic factors of GDM. Some scholars believe that pregnancy is in a state of persistent low oxidative stress (Coughlan et al. 2004). Compared with non-pregnant women, serum lipid peroxidation levels in normal pregnant women are elevated and peak during mid-pregnancy, and fall to levels comparable to early pregnancy during late pregnancy (Chen and Scholl 2005). During normal pregnancy, as soon as the maternal fetal blood circulation is established, the concentration of oxygen in the placenta increases rapidly (Zhang et al. 2019). This phenomenon is similar to the ischemia-reperfusion process and produces a large amount of reactive oxygen species (ROS) (Sudharshana Murthy et al. 2018). During normal pregnancy, low levels of ROS in the body play an important role in embryo development and implantation, placental formation and function, and childbirth (Schliefsteiner et al. 2017). As the pregnancy progresses, in order to maintain normal pregnancy, the body’s anti-oxidant level is also increased, maintaining a relatively balanced oxidation/anti-oxidation effect (Schliefsteiner et al. 2017). Studies have shown that in GDM placenta, maternal and fetal oxidative stress response is enhanced, suggesting that oxidative stress is not only involved in the occurrence and development of GDM, but may also be involved in the occurrence of GDM complications such as fetal complications. Previous studies have shown that patients with GDM have elevated plasma MDA levels, decreased levels of anti-oxidant markers, and decreased insulin secretion and sensitivity (Rueangdetnarong et al. 2018). MDA is a major factor affecting islet β-cell function, suggesting that islet β-cell dysfunction in patients with GDM may be associated with increased oxidative stress in the body (Kotani et al. 2015). Since the content and activity of anti-oxidative enzymes in islet β-cells are relatively low, it is considered to be one of the main targets of oxidative stress-mediated damage (Kotani et al. 2015). In the present study, we demonstrated that tetramethylpyrazine treatment reduced the GDM-induced MDA levels in mice. In addition, we found that following tetramethylpyrazine treatment, the activities of anti-oxidative enzymes, including SOD, GPx and GSH, were enhanced in a dose-dependent manner. Further, we observed that tetramethylpyrazine could ameliorate islet β-cell dysfunction. Therefore, our research provides a new idea for the treatment of GDM and its complications.
ILs are involved in inflammatory response, and regulating the total amount of fat and muscle tissues by controlling apoptosis (Kuzmicki et al. 2009). Studies have shown that IL-6 is involved in the pathogenesis of GDM (Kuzmicki et al. 2009). In the early stages of diabetes, IL-6 promotes insulin secretion, leading to hyperinsulinemia (Kim et al. 2008). When IL-6 is increased to a certain extent, it inhibits insulin secretion and damages islet β-cells, further aggravating diabetes (Kim et al. 2008). Besides, studies have shown that TNF gene polymorphism is associated with the pathogenesis of GDM (Gao et al. 2008), by increasing plasma TNF levels in GDM pregnant women and causing insulin resistance. The mechanism by which TNF induces insulin resistance may be through its promotion of lipolysis and the resulted increase in free fatty acid levels (Friedman et al. 2008). TNF inhibits the tyrosine kinase activity of the insulin receptor in muscle tissue and suppresses the expression of the serine phosphorylation transporter 4 of the insulin receptor, which can alter the catalytic activity of the insulin receptor (Kuzmicki et al. 2006). In this paper, we found that the levels of IL-6, MCP-1 and TNF-α were significantly increased in GDM mice, whereas tetramethylpyrazine treatment inhibited this trend in a dose-dependent manner. Thus, our results suggested that tetramethylpyrazine might play a role in GDM by regulating the serum and placental levels of inflammatory factors, including IL-6, MCP-1 and TNF-α.
A variety of factors such as hyperglycemia, hyperlipidemia, viral infection, exotoxin, pro-inflammatory cytokines and mutant protein expression may interfere with the homeostasis of the ER, triggering a series of reactions within the cell by activating the corresponding signaling pathway called ER stress (Flamment et al. 2012). Upon ER stress, eIF2α is phosphorylated to inhibit the initiation of protein translations, therefore maintaining the homeostasis of the ER (Kim et al. 2006; Chambers et al. 2008). Previous studies found that the expression of ER chaperones GRP78 and CHOP in islet cells increased significantly during high glucose stress (Yung et al. 2016). It is believed that the ER stress caused by high glucose is due to excessive synthesis of proteins such as insulin in β-cells (Rueangdetnarong et al. 2018). ER stress hinders the downstream signaling of insulin receptor activation, thereby affecting the physiological function of insulin and triggering insulin resistance (Pirot et al. 2007). Therefore, we speculate that tetramethylpyrazine may affect the levels of blood glucose and insulin in GDM by regulating ER stress. Our results showed that tetramethylpyrazine treatment decreased the expression of GRP78, CHOP and the phosphorylation of eIF2a, indicating that tetramethylpyrazine exerted inhibitory effect on ER stress. When ER stress persists or the reaction is too strong, expression of CHOP, an ER stress-specific protein (Oyadomari and Mori 2004), is increased to initiate the relevant apoptosis program, causing apoptosis of islet β-cells and diabetes (Oyadomari and Mori 2004; Pirot et al. 2007). Therefore, tetramethylpyrazine treatment might modulate the blood glucose and insulin levels by affecting ER stress, inhibiting CHOP and apoptosis of islet β-cells.
In conclusion, we reported that tetramethylpyrazine treatment alleviated the GDM symptoms characterized by low body weight and serum insulin levels, high blood glucose, and decreased β-cell function in pregnant db/+ mice. In addition, tetramethylpyrazine reduced the serum lipid levels and increased the activities of anti-oxidative enzymes. Furthermore, tetramethylpyrazine regulated the concentration of serum and placental inflammatory factors and the expression of ER stress related proteins. Our results revealed the protective mechanism of tetramethylpyrazine against GDM, which could be utilized as novel agents for the clinical treatment of GDM.
References
Badon SE, Enquobahrie DA, Wartko PD, Miller RS, Qiu C, Gelaye B, Sorensen TK, Williams MA (2017) Healthy lifestyle during early pregnancy and risk of gestational diabetes mellitus. Am J Epidemiol 186:326–333. https://doi.org/10.1093/aje/kwx095
Chambers KT, Unverferth JA, Weber SM, Wek RC, Urano F, Corbett JA (2008) The role of nitric oxide and the unfolded protein response in cytokine-induced beta-cell death. Diabetes 57:124–132. https://doi.org/10.2337/db07-0944
Chen X, Scholl TO (2005) Oxidative stress: changes in pregnancy and with gestational diabetes mellitus. Curr Diab Rep 5:282–288
Chen B, Ma Y, Xue X, Wei J, Hu G, Lin Y (2019) Tetramethylpyrazine reduces inflammation in the livers of mice fed a high fat diet. Mol Med Rep 19:2561–2568. https://doi.org/10.3892/mmr.2019.9928
Coughlan MT, Vervaart PP, Permezel M, Georgiou HM, Rice GE (2004) Altered placental oxidative stress status in gestational diabetes mellitus. Placenta 25:78–84. https://doi.org/10.1016/S0143-4004(03)00183-8
Dias S, Pheiffer C, Abrahams Y, Rheeder P, Adam S (2018) Molecular biomarkers for gestational diabetes mellitus. Int J Mol Sci. https://doi.org/10.3390/ijms19102926
Feghali MN, Abebe KZ, Comer DM, Caritis S, Catov JM, Scifres CM (2018) Pregnancy outcomes in women with an early diagnosis of gestational diabetes mellitus. Diabetes Res Clin Pract 138:177–186. https://doi.org/10.1016/j.diabres.2018.02.004
Flamment M, Hajduch E, Ferre P, Foufelle F (2012) New insights into ER stress-induced insulin resistance. Trends Endocrinol Metab 23:381–390. https://doi.org/10.1016/j.tem.2012.06.003
Friedman JE, Kirwan JP, Jing M, Presley L, Catalano PM (2008) Increased skeletal muscle tumor necrosis factor-alpha and impaired insulin signaling persist in obese women with gestational diabetes mellitus 1 year postpartum. Diabetes 57:606–613. https://doi.org/10.2337/db07-1356
Fu J, Fu J, Yuan J, Zhang N, Gao B, Fu G, Tu Y, Zhang Y (2012a) Anti-diabetic activities of Acanthopanax senticosus polysaccharide (ASP) in combination with metformin. Int J Biol Macromol 50:619–623. https://doi.org/10.1016/j.ijbiomac.2012.01.034
Fu JF, Yuan J, Tu YY, Fu JF, Zhang NY, Gao B, Fu GQ, Zhang YS (2012b) A polysaccharide from Acanthopanax senticosus improves the antioxidant status in alloxan-induced diabetic mice. Carbohydr Polym 88:517–521. https://doi.org/10.1016/j.carbpol.2011.12.037
Gao XL, Yang HX, Zhao Y (2008) Variations of tumor necrosis factor-alpha, leptin and adiponectin in mid-trimester of gestational diabetes mellitus. Chin Med J 121:701–705. https://doi.org/10.1097/00029330-200804020-00008
Hu JZ, Huang JH, Xiao ZM, Li JH, Li XM, Lu HB (2013) Tetramethylpyrazine accelerates the function recovery of traumatic spinal cord in rat model by attenuating inflammation. J Neurol Sci 324:94–99. https://doi.org/10.1016/j.jns.2012.10.009
Johns EC, Denison FC, Norman JE, Reynolds RM (2018) Gestational diabetes mellitus: mechanisms, treatment, and complications. Trends Endocrinol Metab 29:743–754. https://doi.org/10.1016/j.tem.2018.09.004
Kaneto H, Nakatani Y, Kawamori D, Miyatsuka T, Matsuoka TA, Matsuhisa M, Yamasaki Y (2006) Role of oxidative stress, endoplasmic reticulum stress, and c-Jun N-terminal kinase in pancreatic beta-cell dysfunction and insulin resistance. Int J Biochem Cell Biol 38:782–793
Kim R, Emi M, Tanabe K, Murakami S (2006) Role of the unfolded protein response in cell death. Apoptosis 11:5–13. https://doi.org/10.1007/s10495-005-3088-0
Kim C, Cheng YJ, Beckles GL (2008) Inflammation among women with a history of gestational diabetes mellitus and diagnosed diabetes in the Third National Health and Nutrition Examination Survey. Diabetes Care 31:1386–1388. https://doi.org/10.2337/dc07-2362
Kotani K, Tashiro J, Yamazaki K, Nakamura Y, Miyazaki A, Bujo H, Saito Y, Kanno T, Maekawa M (2015) Investigation of MDA-LDL (malondialdehyde-modified low-density lipoprotein) as a prognostic marker for coronary artery disease in patients with type 2 diabetes mellitus. Clin Chim Acta 450:145–150. https://doi.org/10.1016/j.cca.2015.08.003
Kuzmicki M, Szamatowicz J, Kretowski A, Kuc P, Kretowski M, Wawrusiewicz N, Okruszko A, Leroith D, Gorska M (2006) Evaluation of adiponectin and TNFalpha genes expression in women with gestational diabetes. Preliminary results. Ginekol Pol 77:930–936
Kuzmicki M, Telejko B, Szamatowicz J, Zonenberg A, Nikolajuk A, Kretowski A, Gorska M (2009) High resistin and interleukin-6 levels are associated with gestational diabetes mellitus. Gynecol Endocrinol 25:258–263. https://doi.org/10.1080/09513590802653825
Lappas M, Hiden U, Desoye G, Froehlich J, Hauguel-De Mouzon S, Jawerbaum A (2011) The role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxid Redox Signal 15:3061–3100. https://doi.org/10.1089/ars.2010.3765
Li S, Zhu Y, Yeung E, Chavarro JE, Yuan C, Field AE, Missmer SA, Mills JL, Hu FB, Zhang C (2017) Offspring risk of obesity in childhood, adolescence and adulthood in relation to gestational diabetes mellitus: a sex-specific association. Int J Epidemiol 46:2104. https://doi.org/10.1093/ije/dyx211
Li J, Leng J, Li W, Zhang C, Feng L, Wang P, Chan JCN, Hu G, Yu Z, Yang X (2018) Roles of insulin resistance and beta cell dysfunction in macrosomia among Chinese women with gestational diabetes mellitus. Prim Care Diabetes 12:565–573. https://doi.org/10.1016/j.pcd.2018.07.010
Liu W, Liu K, Zhang S, Shan L, Tang J (2018) Tetramethylpyrazine showed therapeutic effects on sepsis-induced acute lung injury in rats by inhibiting endoplasmic reticulum stress protein kinase RNA-like endoplasmic reticulum kinase (PERK) signaling-induced apoptosis of pulmonary microvascular endothelial cells. Med Sci Monit 24:1225–1231. https://doi.org/10.12659/msm.908616
Miettinen HE, Rono K, Koivusalo SB, Eriksson JG, Gylling H (2018) Effect of gestational diabetes mellitus on newborn cholesterol metabolism. Atherosclerosis 275:346–351. https://doi.org/10.1016/j.atherosclerosis.2018.06.879
Mishra S, Bhadoria AS, Kishore S, Kumar R (2018) Gestational diabetes mellitus 2018 guidelines: an update. J Fam Med Prim Care 7:1169–1172. https://doi.org/10.4103/jfmpc.jfmpc_178_18
Oyadomari S, Mori M (2004) Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11:381–389. https://doi.org/10.1038/sj.cdd.4401373
Pirot P, Ortis F, Cnop M, Ma YJ, Hendershot LM, Eizirik DL, Cardozo AK (2007) Transcriptional regulation of the endoplasmic reticulum stress gene chop in pancreatic insulin-producing cells. Diabetes 56:1069–1077. https://doi.org/10.2337/db06-1253
Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH (2018) The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. https://doi.org/10.3390/ijms19113342
Poola-Kella S, Steinman RA, Mesmar B, Malek R (2018) Gestational diabetes mellitus: post-partum risk and follow up. Rev Recent Clin Trials 13:5–14. https://doi.org/10.2174/1574887112666170911124806
Poulakos P, Mintziori G, Tsirou E, Taousani E, Savvaki D, Harizopoulou V, Goulis DG (2015) Comments on gestational diabetes mellitus: from pathophysiology to clinical practice. Hormones 14:335–344. https://doi.org/10.14310/horm.2002.1570
Prakash GT, Das AK, Habeebullah S, Bhat V, Shamanna SB (2017) Maternal and neonatal outcome in mothers with gestational diabetes mellitus. Indian J Endocrinol Metab 21:854–858. https://doi.org/10.4103/ijem.IJEM_66_17
Rueangdetnarong H, Sekararithi R, Jaiwongkam T, Kumfu S, Chattipakorn N, Tongsong T, Jatavan P (2018) Comparisons of the oxidative stress biomarkers levels in gestational diabetes mellitus (GDM) and non-GDM among Thai population: cohort study. Endocr Connect 7:681–687. https://doi.org/10.1530/EC-18-0093
Schliefsteiner C, Hirschmugl B, Kopp S, Curcic S, Bernhart EM, Marsche G, Lang U, Desoye G, Wadsack C (2017) Maternal gestational diabetes mellitus increases placental and foetal lipoprotein-associated phospholipase A2 which might exert protective functions against oxidative stress. Sci Rep 7:12628. https://doi.org/10.1038/s41598-017-13051-6
Silva AL, Amaral AR, Oliveira DS, Martins L, Silva MR, Silva JC (2017) Neonatal outcomes according to different therapies for gestational diabetes mellitus. J Pediatr 93:87–93. https://doi.org/10.1016/j.jped.2016.04.004
Sudharshana Murthy KA, Bhandiwada A, Chandan SL, Gowda SL, Sindhusree G (2018) Evaluation of oxidative stress and proinflammatory cytokines in gestational diabetes mellitus and their correlation with pregnancy outcome. Indian J Endocrinol Metab 22:79–84. https://doi.org/10.4103/ijem.IJEM_232_16
Wu Y, Xu Z, Yang Y, Qiu J, Yang M, Wu C, Lai Z, Tang M, Ge J, Yu K, Zhuang J (2019) Tetramethylpyrazine (TMP) ameliorates corneal neovascularization via regulating cell infiltration into cornea after alkali burn. Biomed Pharmacother 109:1041–1051. https://doi.org/10.1016/j.biopha.2018.10.091
Yang HL, Wu SK (2018) Ligustrazine attenuates renal damage by inhibiting endoplasmic reticulum stress in diabetic nephropathy by inactivating MAPK pathways. RSC Adv 8:21816–21822. https://doi.org/10.1039/c8ra01674g
Yang QH, Liang Y, Xu Q, Zhang Y, Xiao L, Si LY (2011) Protective effect of tetramethylpyrazine isolated from Ligusticum chuanxiong on nephropathy in rats with streptozotocin-induced diabetes. Phytomedicine 18:1148–1152. https://doi.org/10.1016/j.phymed.2011.05.003
Yung HW, Alnaes-Katjavivi P, Jones CJ, El-Bacha T, Golic M, Staff AC, Burton GJ (2016) Placental endoplasmic reticulum stress in gestational diabetes: the potential for therapeutic intervention with chemical chaperones and antioxidants. Diabetologia 59:2240–2250. https://doi.org/10.1007/s00125-016-4040-2
Zhang C, Yang Y, Chen R, Wei Y, Feng Y, Zheng W, Liao H, Zhang Z (2019) Aberrant expression of oxidative stress related proteins affects the pregnancy outcome of gestational diabetes mellitus patients. Am J Transl Res 11:269–279
Zhao Y, Liu Y, Chen K (2016) Mechanisms and clinical application of tetramethylpyrazine (an interesting natural compound isolated from Ligusticum wallichii): current status and perspective. Oxid Med Cell Longev 2016:2124638. https://doi.org/10.1155/2016/2124638
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiao, Y., Zhang, S., Zhang, J. et al. Tetramethylpyrazine attenuates placental oxidative stress, inflammatory responses and endoplasmic reticulum stress in a mouse model of gestational diabetes mellitus. Arch. Pharm. Res. 42, 1092–1100 (2019). https://doi.org/10.1007/s12272-019-01197-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-019-01197-y