Abstract
Crop residue ashes exhibit a significant capacity to adsorb pesticides and can affect their adsorption behaviour in soils. The effect of sugarcane trash ash (STA), the third most important crop whose residues are burnt in fields in India, on atrazine (ATR) and fipronil (FIP) kinetics and adsorption behaviour was studied in three sugarcane growing soils. The kinetics and adsorption of pesticides in STA-mixed soils were studied using the batch adsorption method. Pesticide residues were analysed using high-performance liquid chromatography (HPLC). The kinetics study suggested that adsorption of pesticides in the soil/soil + STA (0.2%) was best explained by the pseudo-second-order model. The STA (0.1 and 0.2%) showed maximum effect on ATR adsorption in the silty clay loam, while for FIP, the effect was maximum in the sandy clay loam soil. Adsorption was well explained by the Freundlich isotherm. The adsorption was nonlinear as the Freundlich constant 1/n values were < 1. The STA decreased the amount of pesticides desorbed. High surface area unburnt carbon in STA was responsible for increase in adsorption and decrease in desorption of pesticides. Adsorption and desorption constants correlated well with STA content in the soils. ATR and FIP adsorption in the sugarcane soils was controlled by the physical adsorption, including partition and surface adsorption, and STA affected the phenomenon of adsorption. Results suggested that STA increased adsorption and decreased desorption of both pesticides used in the sugarcane cultivation. This study has implications in identifying the role of crop residue burning on the fate of the pesticides applied in soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
India is an agrarian country that generates large quantities of agricultural wastes leading to the challenge of disposal of a considerable amount of crop residues. Farmers opt for in situ burning of surplus residues because it is a quick and easy way to manage the substantial quantities of crop residues and prepare the field for the next crop well in time. Burning of crop residues after harvest for land clearing is a major issue as burning leads to air pollution, threating people’s lives. Sugarcane is the third important crop, after rice and wheat, whose residues are burnt in fields in India; Uttar Pradesh is the greatest contributor to the burning of sugarcane trash followed by Karnataka (Jain et al., 2014). Sugarcane trash (or cane trash) is the dried leaves that are left in the field after the sugarcane is harvested. Trash is burnt by farmers as it resists biodegradation, is highly indigestible as fodder, has a low density voluminous biomass, and is difficult to transport. After burning, the sugarcane trash ash (STA) is mixed in the soil before sowing the next crop. This ash-mixing changes the physicochemical properties of soil including decrease in bulk density, improved soil porosity, and water retention (Pandey and Singh, 2010). The ashes are alkaline in nature and result in some agronomic benefit like buffering the soil pH, improving the soil nutrient status, and increasing plant growth and nutrient uptake (Trivedi et al., 2016). The ashes also have significant pesticide adsorption potential (Deokar et al., 2016a, 2016b; Kumar et al., 2019) and therefore, affect the fate of pesticide applied in soils (Yang and Sheng, 2003; Sheng et al., 2005; Hiller et al., 2007, 2009). Yang and Sheng (2003) reported that wheat ash showed > 600–10,000 times higher diuron adsorption than the soil (Sheng et al., 2005). Hiller et al. (2007, 2009) reported that 1% ash increased 2-methyl-4-chlorophenoxyacetic acid by 8–16 times than no ash control soils, reduced desorption by ~ 20%, and reduced degradation substantially (Cui et al., 2009). However, all these studies were performed at very high levels of ash that are not realistic in the field situation. Recently, Kumar and Singh (2020) reported that wheat and the rice straw ashes, even at 0.1–0.5% levels, increased pretilachlor and sulfosulfuron adsorption, respectively; however, the effect varied with the ash content and soil type. High surface area unburnt carbon contents in the ashes contributed to enhanced adsorption and reduced desorption. The pH of soil/soil + ash mixtures affected herbicide adsorption, but the effect was significant for pretilachlor. However, the effect of the ashes on pesticide adsorption might differ with the nature of soil and pesticide.
Atrazine (ATR) and fipronil (FIP) are two commonly used pesticides in sugarcane cultivation in India. ATR is a selective, systemic, pre- and post-emergent herbicide used to control annual grasses and many broad-leaved weeds in sugarcane (Turner, 2015). FIP is a broad-spectrum insecticide that belongs to the phenylpyrazole group and is used for control of early shoot borer and termites in sugarcane. Recently, the authors have reported that STA exhibited very high adsorption capacity for FIP and ATR with partition coefficient (Kd) values of 1267.5–3321.1 and 137.0–1445.3 mL g−1, respectively (Yadav and Singh, 2020). Burning sugarcane trash in fields generates a significant amount of STA that is mixed in the soils, which can substantially increase adsorption of pesticides applied in the sugarcane crop. Higher pesticide adsorption in STA-mixed soils can affect their degradation and leaching behaviour as adsorption decreases availability of pesticide in soil solution. However, absolutely no information is available on the effect of burning sugarcane trash on adsorption behaviour of pesticides used in the sugarcane crop in the Indian tropical conditions, where sugarcane residues are mainly burnt on fields. Therefore, the present study reported the effect of sugarcane trash ash (STA) on the sorption behaviour of ATR and FIP in three sugarcane soils of northern India. The role of the physiochemical properties of STA-soil mixtures on pesticides’ adsorption was worked out.
2 Materials and Methods
2.1 Chemicals
Analytical grade ATR (purity 98.9%, octanol-water partition coefficient (log Kow) 2.82, water solubility 33 mg L−1 (pH 7)) and FIP (purity 97.5%, log Kow 4.0, water solubility 1.9 mg L−1 (pH 7)) were purchased from the Sigma-Aldrich, India. Analytical or gradient grade solvents used in the study were purchased locally.
2.2 Soils
Three soils from sugarcane growing regions of Uttar Pradesh were used in the study. Soils included a silty clay loam (Indian Institute of Sugarcane Research, Lucknow, 26.56° N and 80.52° E), sandy clay loam (G.B. Pant University of Agriculture and Technology, Pantnagar, 29° N and 79° E), and loam (Baraut, 29.10° N, 77.26° E). Soil samples were collected from the surface 0–15 cm, air dried, and stored at room temperature. Soils were characterized for the pH, organic carbon (OC) content, electrical conductivity (EC), and mechanical fractions (clay, silt and sand) using standard analytical methods (Table 1).
2.3 Sugarcane Trash Ash
The STA used in the study was prepared as per the method reported earlier (Yadav and Singh, 2020). Briefly, the sugarcane trash was dried in sun for 2 days and burnt on field. Burnt residues (ash) were collected, ground, and sieved and 60 British Standards Society (BSS) fraction (< 250 μm) was stored in air-tight PTFE containers at room temperature. The STA was characterized for pH, OC content, and EC using standard methods (Supplementary Table A). The carbon (C), hydrogen (H), nitrogen (N), and oxygen (O) contents were determined using CHNS (O) analyser (Thermo Finnigan, FLASH EA 1112 Series, Italy). The OC content was estimated using TOC analyser (Elementar, Vario TOC Cube). The specific surface area (SSA) and pore volume were estimated using the Brunauer, Emmett, and Teller (BET) nitrogen adsorption technique at 77 K, using an automated manometric gas adsorption apparatus (Quantachrome NOVA 10.01, Quantachrome Instruments, Florida, USA). Fourier transform-infrared absorbance (FT-IR) spectrum of STA was recorded on Bruker ALPHA, FT-IR system (typically 24 scans, resolution 4 cm−1) at wave numbers from 360 to 4000 cm−1. Scanning electron microscopy (SEM) was used to characterize the surface morphology of STA. X-ray diffraction (XRD) analysis was carried out using a Philips PW 1710 X-ray diffractometer, equipped with copper Kα radiation (generator voltage 40 kV, tube current 20 mA) as the X-ray source in 2θ ranges 3–70°.
2.4 Kinetics Studies
The effect of the 0.2% STA on ATR and FIP adsorption kinetics was studied in the silty clay loam soil using the batch method. Kinetics study was conducted only in the silty clay loam soil as, among the three soils used in the study, it contained the highest clay content and was supposed to take maximum time to attain equilibrium. Soil or soil + STA (5 g, oven dry basis) and 0.01 M CaCl2 solution of ATR (10 mL of 5 mg L−1) or FIP (20 mL of 1 mg L−1) in glass centrifuge tubes were equilibrated on a horizontal shaker for 0.5, 1, 2, 4, 6, 8, 12, 24, and 48 h. Two controls (pesticide solution without soil/soil + STA and soil/soil + STA with 0.01 M CaCl2 solution without pesticide) were maintained to observe any interference from the soil/soil + STA or degradation of pesticides during experimentation and each treatment had three replicates. After desired equilibration time, samples from each treatment were centrifuged using a Sigma 3-16 KL centrifuge at 3139g for 20 min. The pesticides were quantified in the supernatant using high-performance liquid chromatography (HPLC). The amount of pesticide adsorbed by the soil/soil + STA was calculated from the difference of the initial and final concentration of the pesticide in the supernatant. During the equilibration period, pesticides were stable as no decrease in their concentration was observed in the control samples.
2.5 Adsorption-Desorption Studies
Batch adsorption studies as per Organisation for Economic Cooperation and Development (OECD) guidelines were planned to study the effect of STA on ATR/FIP adsorption behaviour in three sugarcane soils. Sorption studies for ATR and FIP were performed at 1:2 and 1:4 soil:solution ratio, respectively. Soil (0, 0.1, and 0.2% STA) samples (5 g) and 0.01 M CaCl2 solution of ATR (10 mL) or FIP (20 mL) were taken in stoppered test tubes. The STA levels were worked out based on the fact that a maximum of 15 t ha−1 trash is generated during sugarcane cultivation and at 6.7% trash-to-ash conversion efficiency, STA obtained would correspond to ~ 1 t ha−1 (0.05%), when thoroughly mixed in the surface 6 cm soil profile. Practically, uniform mixing of STA in soil is not possible in the field; therefore, higher doses of 2 and 4 t ha−1 (equivalent to 0.1 and 0.2%) were selected for the study.
The initial concentration of the pesticides used was within the limits of their aqueous solubility. The concentrations used were 1, 2, 3, 4, and 5 μg mL−1 for ATR and 0.2, 0.4, 0.6, 0.8, and 1.0 μg mL−1 for FIP. Controls were maintained and each treatment was replicated thrice. Samples were equilibrated for 24 h and 6 h at room temperature for ATR and FIP, respectively. After equilibration, soil-water suspension was centrifuged (3139g for 20 min) and pesticide concentration in the supernatant was quantified using HPLC.
After the adsorption study was over, desorption was performed at the lowest and highest concentration used for adsorption. After adsorption was over, 5 mL (ATR) or 10 mL (FIP) of the supernatant was removed from each tube and was replaced with equal volume of fresh 0.01 M aqueous CaCl2 solution. The tubes were equilibrated again to attain the state of equilibrium; soil suspension was centrifuged and the above mentioned process was repeated. A total of 3 desorption cycles were performed for each sample. The ATR/FIP was quantified in the supernatant after each desorption cycle and amount desorbed was calculated from the difference of pesticide concentration before and after equilibration.
2.6 Analysis
Aqueous samples were analysed for pesticides by directly injecting filtered samples (0.45 μm) in HPLC (Varian, Prostar) equipped with quaternary pump, UV detector, and Rheodyne injection system using LiChrospher C-18 stainless steel column (250 mm × 4 mm (i.d.)). Conditions for ATR analysis included acetonitrile:0.1% aqueous o-phosphoric acid (70:30) at 1 mL min−1 flow and wave length of 222 nm. The conditions for FIP were acetonitrile:0.1% aqueous o-phosphoric acid (80:20) at 1 mL min−1 flow and wave length of 222 nm. The recovery of ATR from water at 0.1, 1, and 10 μg mL−1 fortification levels was 92.8, 93.1, and 97.4%, respectively. The limit of detection (LOD) of the method used was 0.01 μg mL−1 while the corresponding limit of quantification (LOQ) was 0.05 μg mL−1. The standard curve for ATR was linear in the range of 0.05–15 μg mL−1. The recovery of FIP from water at 0.1, 0.5, and 1 μg mL−1 fortification levels was 91.5, 92.1, and 9.5.2%, respectively. The LOD of the method used was 0.01 μg mL−1 while the corresponding LOQ was 0.03 μg mL−1. The standard curve for FIP was linear in the range of 0.05–5 μg mL−1.
2.7 Kinetics Models
Kinetics data was modelled using the pseudo-first-order (PFO), pseudo-second-order (PSO), Elovich, and the intraparticle diffusion (IPD) equations mentioned below:
where qe is the amount of pesticide adsorbed at equilibrium (μg g−1), qt is the amount of pesticide adsorbed (μg g−1) at time t (min), k1 is the pseudo-first-order rate constant (min−1), k2 is the pseudo-second-order rate constant (g (μg min)−1), α is the initial sorption rate constant (μg g−1 min−1), β is the desorption constant (g μg−1), and k is the IPD constant (μg (g min0.5)−1).
2.8 Adsorption Models
ATR and FIP adsorption data was modelled using the Freundlich, the Langmuir, and the Temkin isotherms:
where qe is the amount of pesticide adsorbed at equilibrium (μg g−1), Ce is the concentration of pesticide in solution at equilibrium (μg mL−1), KF is the Freundlich adsorption constant (μg1−nF g−1 mLnF), 1/n is the dimensionless constant expressing ease of sorption, bL is the Langmuir adsorption constant (mL μg−1), Qo is the maximum monolayer coverage capacity (μg g−1), ATem is the Temkin isotherm equilibrium binding constant (mL μg−1), and B is the constant related to heat of adsorption (J mol−1).
3 Results
3.1 Characterization of STA
The STA was characterized for its physicochemical characteristics (Supplementary Table A). The trash-to-ash conversion ratio was 6.7%. The STA was alkaline in nature (pH 10.5) due to the presence of oxides of Na, K, and Ca, which are alkaline in nature (Trivedi et al., 2016). Elemental analysis showed that the STA contained C 13.31%, H 0.44%, N 0.17%, and O 3.65%. The OC content of STA was 8.31% and can be assigned to the unburned carbon (Deokar et al., 2016a); thus, it contained a significant amount of inorganic C (5%) and it can be due to the presence of carbonates of alkali and alkaline earth metal carbonates (Deokar et al., 2016a; Kumar et al., 2019). The loss of ignition was 9.12%. The elemental ratios H/C and O/C can be used to explain the degree of aromaticity and polarity, respectively. The degree of aromaticity was 0.40 while polarity was 0.21. The specific surface area of STA was 27.23 m2 g−1 while porosity was 0.0596 cm3 g−1. Due to its high surface area and porosity, STA can be a very effective adsorbent for the pesticides.
The SEM photograph (Supplementary Fig. A(a)) suggested that original cell/leaf structure was intact in STA and showed porous structures and channel type repeated units. The FT-IR spectrum of STA (Supplementary Fig. A(b)) showed major peaks at 620, 784, 874, 1049, and 1260–1660 cm−1. These peaks indicated the presence of carbonate (CO3−2) (broad peak at 1260–1660 and 874 cm−1), phosphate (PO4−3), and silicate or quartz (SiO2) components (broad peaks at 1049 cm−1 along with 620 cm−1).
The XRD pattern of STA (Supplementary Fig. A(c)) showed a broad peak between 15 and 18° 2θ, a pattern corresponding to amorphous silica, while peaks at 2θ of 20.9° and 26.7° indicated the presence of quartz (crystalline silica). An intense sharp peak at 2θ ~ 27.5°, 38.5, and 47.2 was assigned to the calcite (CaCO3) (Fungaro and Silva, 2014). The spectrum suggested the presence of calcite as the main crystalline compound with quartz (SiO2) as minor compounds.
3.2 Kinetics Study
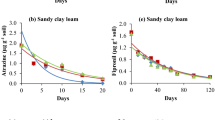
The kinetics study indicated that adsorption of ATR/FIP in the silty clay loam soil (without and with STA) increased with increase in the equilibration time (Supplementary Fig. B). The STA greatly affected rate of adsorption of both pesticides and it was faster in STA-mixed soil than the control soil. After 24 h, compared to 38.77% (ATR) and 47.71% (FIP) adsorption in the control soils, adsorption in the 0.2% STA-mixed soils was 51.07% (ATR) and 86.68% (FIP). There was no appreciable change in the amount of ATR and FIP adsorbed after 24 and 6 h, respectively; therefore, they were chosen as the equilibration time for studying the adsorption-desorption behaviour of ATR and FIP, respectively. The kinetics study suggested that the adsorption equilibrium for FIP was attained faster (6 h) than for ATR (24 h); therefore, FIP will be adsorbed faster than ATR in soils/STA-mixed soils.
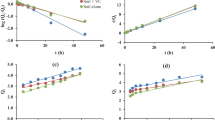
The kinetics data was fitted to the pseudo-first-order (PFO), the pseudo-second-order (PSO), and the Elovich models and the constants were calculated (Fig. 1a–c, e–g; Table 2). The best fitted kinetics model was judged by the highest adjusted R2 (R2Adj) value and observed versus predicted adsorption values. Among the three models, the R2Adj values for both the pesticides in the control and the 0.2% STA-mixed soils were the highest for the PSO model. The calculated amount of pesticide adsorbed (qe,calc) using the PSO model was nearly the same as the observed value (qe,exp) (Table 2). Thus, the PSO model was the best model to explain adsorption of both pesticides in the soil/soil + STA. The IPD model is used to indicate the contribution of diffusion phenomenon on adsorption kinetics. The IPD graphs for both the pesticides (Fig. 1d, h) showed two straight lines, representing two stages. Stage I corresponded to liquid film diffusion, while stage II explained pore diffusion. The IPD graph did not pass through the origin indicating that pore diffusion was not the sole rate-limiting step. The value of the rate constant kI (stage I) for both the pesticides in the control and STA-mixed soil was higher than the kII values (stage II), suggesting that liquid film diffusion contributed more to the adsorption process. Compared to the control soil, the kI values were higher in STA-mixed soil for both the pesticides. Similarly, kI values for FIP were higher than those for ATR. The intercept (C) of the graph reflects the boundary layer effect or surface adsorption and the higher the value, the higher will be the contribution. The intercept (CII) values in stage II were higher than the intercept (CI) values in stage I for both the pesticides. Similarly, in general, C values were higher in STA-mixed soil suggesting higher contribution of boundary layer effect/surface adsorption (Pholosi et al., 2020).
Plots of pseudo-first-order (PFO), pseudo-second-order (PSO), Elovich, and intraparticle diffusion (IPD) models for a–d atrazine and e–h fipronil in the control and 0.2% sugarcane trash ash (STA)–mixed silty clay loam soil. The qe is amount of pesticide adsorbed at equilibrium (µg g-1), qt is amount of pesticide adsorbed (µg mL-1) at time t (h)
3.3 Adsorption-Desorption Studies
Adsorption results suggested that soils showed varying capacity to adsorb ATR and FIP and adsorption of both the pesticides in all three soils decreased with increase in the pesticides’ concentration in the soil solution. The STA enhanced adsorption of both the pesticides; however, the effect varied with soil types and STA content. The STA showed maximum effect on ATR adsorption in the silty clay loam soil, where, compared to the control (36.41–46.17%), adsorption in the 0.1 and 0.2% STA-mixed soils was 43.85–56.20% and 53.80–73.27%, respectively. The maximum effect of STA on FIP adsorption was observed in the sandy clay loam soil (control = 31.08–45.94%; STA (0.1%) = 54.77–66.53; STA (0.2%) = 64.80–68.47).
ATR/FIP adsorption data in the soil/soil + STA was fitted to three adsorption isotherms (the Freundlich, the Langmuir, and the Temkin; Supplementary Figs. C and D) and adsorption constants were calculated (Tables 3 and 4). Among the three isotherms, the R2Adj values for ATR adsorption were nearly the same for the Freundlich and the Langmuir isotherms. However, among the Freundlich (KFads) and the Langmuir (qm) adsorption constants, only the KFads values for all the treatments were in agreement with the observed Kd values that increased with increase in STA content in the soils. Therefore, the Freundlich isotherm was chosen as the best isotherm to explain the effect of STA on ATR and FIP adsorption in the sugarcane soils.
The Freundlich constant KFads, a measure of adsorption at 1 μg mL−1, for ATR and FIP in all three soils increased in STA-mixed soils. The KFads values for ATR in the control (no ash), 0.1, and 0.2% STA-mixed silty clay loam, sandy clay loam, and loam soils were 1.49, 2.16, and 3.18; 2.01, 2.40, and 2.94; and 2.65, 2.93, and 3.78, respectively. The maximum effect of STA was observed in the silty loam soil. The KFads values for FIP in the control, 0.1, and 0.2% STA-mixed silty clay loam, sandy clay loam, and loam soils were 1.95, 2.36, and 2.63; 1.66, 4.08, and 6.55; and 2.37, 5.74, and 5.68, respectively. Thus, among the two pesticides, FIP was more adsorbed in the soils. It can be attributed to its lower water solubility and higher Kow as adsorption is inversely related to water solubility and directly correlated to Kow (Giori et al., 2014).
The Freundlich constant 1/nads is a measure of the intensity of adsorption as well as the effect of the pesticide concentration on sorption. In general, 1/nads values were < 1 suggesting that adsorption of ATR (0.56–0.77) and FIP (0.32–1.01) in soil/soil +S TA followed the L-type of adsorption isotherm.
Reversibility of adsorption helps in assessing the net amount adsorbed and the amount available for leaching and degradation. Desorption of ATR and FIP in all three sugarcane growing soils was studied at the lowest and the highest concentration after adsorption. The STA reduced desorption of both pesticides; however, the effect varied with the type of soil and the concentration and nature of pesticide. No FIP desorption was observed at the lower concentration, both from the control and STA-mixed soils. The 0.2% STA reduced the amount of FIP desorbed at the higher concentration by ~ 70%. Compared to FIP, ATR was more desorbed, and desorption was more at the higher ATR concentration. Except loam soil, where ATR desorption was more in the 0.2% STA-mixed soil, higher STA levels reduced ATR desorption. The higher ATR desorption can be attributed to its higher aqueous solubility and lower Kow than FIP.
The desorption data fitted well to the Freundlich isotherm (Table 5; Figs. 2 and 3) and the KFdes values for ATR and FIP in all three soils were higher in STA-mixed soils and increased with increase in STA content. The KFdes values for ATR were higher for desorption at the higher ATR concentration than at the lower concentration. These results suggested that the net amount of ATR retained in the soil/soil + STA was more at the higher concentration as well as in soils mixed with 0.2% STA. The 1/ndes values were lower than the 1/nads values in the respective treatments, indicating that the rate of desorption was less than the rate of adsorption.
Freundlich isotherms for atrazine adsorption-desorption in the control, 0.1, and 0.2% sugarcane trash ash (STA)–mixed a–c silty clay loam soil, d–f sandy clay loam, and g–i loam soils. The qe the amount of pesticide adsorbed at equilibrium (µg g-1), Ce is the the concentration of pesticide in solution at equilibrium (µg mL-1)
Freundlich isotherms for fipronil adsorption-desorption in the control, 0.1, and 0.2% sugarcane trash ash (STA)–mixed a–c silty clay loam soil, d–f sandy clay loam, and g–i loam soils. The qe is the amount of pesticide adsorbed at equilibrium (µg g-1), Ce is the concentration of pesticide in solution at equilibrium (µg mL-1)
3.4 Thermodynamic Studies
The effect of STA on change in the free energy (ΔG) for ATR and FIP adsorption was calculated using the equation ΔG = − RT lnKF. The ΔG values for ATR and FIP varied in the range of − 0.424 to − 1.408 and − 0.538 to − 1.988 KJ mol−1, respectively (Supplementary Table C). The negative ΔG values suggested that the process was exothermic and spontaneous. In general, values were higher for FIP suggesting faster adsorption of FIP than ATR. The values increased with increase in STA content in all soils, suggesting that adsorption was more favourable in STA-mixed soils and ease of adsorption increased with increase in STA content.
4 Discussion
Sugarcane is the third most important crop that contributes to on-farm burning of crop residues in India and an estimated 16.8–28.2 Mt per year sugarcane trash is burnt (Jain et al., 2014). The ash generated in the field as a post-harvest cleaning technique of land has alkaline pH due to the presence of carbonates of alkali and alkaline earth metals. The STA is highly porous and has high surface area fractions and might affect the adsorption of the pesticides that are applied in the sugarcane crop grown in fields after burning of trash. The PSO model best explained the adsorption kinetics suggesting that adsorption was limited by the availability of adsorption sites (Liu, 2008) and STA provided additional sites for pesticide adsorption. The IPD graph suggested that the pore diffusion was not the sole rate-limiting step and surface adsorption too attributed to the adsorption kinetics. Mass transfer predominated during the first stage while intraparticle diffusion was the rate-controlling step during the second stage.
Mixing STA in three sugarcane growing soils enhanced adsorption of both pesticides; however, the extent of increase varied. The Freundlich adsorption isotherm best explained the adsorption of ATR and FIP in STA-mixed soils. ATR adsorption in STA-mixed soils followed the order silty clay loam > loam > sandy clay loam. Compared to control silty clay loam soils, STA resulted in nearly twofold increase in the KFads for ATR. However, FIP adsorption in STA-mixed soils followed the order sandy clay loam > loam > silty clay loam. The KFads in STA-mixed sandy clay loam and loam, compared to the control, showed 4- and 2.5-fold increase, respectively. The effect of STA was more on FIP adsorption than on ATR and the KFads-FIP in the sandy clay loam and loam soils was nearly 1.5 to 2.2 times higher than the KFads-ATR in respective soil. The 1/nads values for ATR in STA-mixed soils decreased with increase in the pesticide concentration, suggesting that adsorption was limited by the availability of adsorption sites. However, 1/nads values for FIP increased with increase in the concentration, suggesting multilayer pesticide adsorption. The STA decreased desorption of both pesticides, indicating hysteresis (H = 1/ndes/1/nads). The H < 1 indicates that the rate of desorption is slower than the rate of adsorption (positive hysteresis), while H > 1 indicates negative hysteresis. The H values in the silty clay loam, sandy clay loam, and loam soils varied between 0.13 and 0.47, 0.21 and 1.21, and 0.01 and 0.55, respectively. Thus, except control sandy clay loam soil, where negative hysteresis was observed, positive hysteresis was observed in the rest of the treatments. Decrease in the rate of desorption in STA-mixed soils can be explained by the entrapment of the pesticide molecules in micropores present in the unburnt carbon fractions of STA.
The OC content is an important soil component that affects pesticide adsorption. Previous reports suggested that the OC content greatly affected ATR and FIP adsorption (Celis et al., 1997; Ying and Kookana, 2001; Spomer and Kamble, 2010; Singh et al., 2016). If OC content is the sole factor responsible for the adsorption, the Koc values among different treatments should be the same. However, the Koc values among different treatments in all three soils varied (Tables 3 and 4). The maximum increase in the Koc was observed in the silty clay loam for ATR and the sandy clay loam for FIP, where, compared to the control, the Koc in the 0.2% STA-mixed soils increased by ~ 2 (ATR) to 5 (FIP) times. Probably it is not the OC content, but the increase in the surface area and porosity in STA-mixed soils might have resulted in the higher ATR and FIP adsorption. Except for the loam soil, both adsorption (KFads) and desorption (KFdes) constants for ATR and FIP correlated well with STA content in the soils (Supplementary Table D). Variation in the extent of STA’s effect on adsorption-desorption behaviour of pesticides in soils might be due to their varying properties (pH, EC, clay content, etc.). Mixed reports are available for the effect of pH on ATR adsorption. Some reports suggested decrease in ATR adsorption with increase in pH (Liu et al., 1995; Yue et al., 2017), while Clay et al. (1988) observed no effect of soil pH. Singh et al. (2016) observed no effect of soil pH on FIP adsorption, while increase in soil EC reduced adsorption. Earlier reports suggested that the ashes increased pesticide adsorption in soils and attributed it to the high surface area and porous unburnt carbon fraction content (Yang and Sheng, 2003; Hiller et al., 2007, 2009; Cui et al., 2009; Loganathan et al., 2009; Giori et al., 2014; Garba et al., 2018; Kumar and Singh, 2020). ATR can interact both specifically (H-bonding and charge-transfer interactions) and non-specifically (hydrophobic-like interactions and partition) with humic substances/carbonaceous materials (Mandal et al., 2017). However, no information is available on FIP adsorption mechanism, the interaction between the functional groups in carbonaceous surface and functional groups (amino, sulfonyl, cyano) of FIP, as well as the hydrophobic nonpolar fractions, might be paying a role in adsorption.
Earlier, Carter et al. (1995) suggested that physical adsorption and partition are the predominant mechanism of adsorption, if the ΔG is > 40 KJ mol−1, while surface adsorption is predominant at < 40 KJ mol−1. The ΔG values for ATR and FIP are < 2 KJ mol−1, suggesting that surface adsorption was the major adsorption mechanism. Contribution of the partition and surface adsorption to ATR and FIP adsorption in the soil/soil + STA in all three soils was quantified as suggested by Zhu and Chen (2000), following Eqs. (8)–(10):
where QT (μg g−1) is the total amount of pesticide adsorbed; QP (μg g−1) is the pesticide adsorption amount generated by partition; QA (μg g−1) is the adsorption amount of pesticide generated by the surface adsorption; KOC (mL g−1) is the partition coefficient normalized to the OC content. The graphs between QT, QP, and QA with the Ce for ATR (Fig. 4) and FIP (Fig. 5) suggested that both surface adsorption and partition processes attributed to the adsorption in the soil/soil + STA; however, the contribution varied among the treatments. At lower equilibrium concentration, the ATR adsorption process in the soils was dominated by the surface adsorption; however, with increase in the equilibrium concentration, surface adsorption reached saturation and the adsorption process was dominant by the partition. The STA did not show any effect on adsorption process mechanism. Compared to ATR adsorption, the surface adsorption played an important role in FIP adsorption and variation among the soils was observed. FIP adsorption in the silty clay loam soil was mainly attributed to surface adsorption and its role increased in STA-mixed soils. It was followed in the loam and the sandy loam soil as far as dominance of surface adsorption on FIP is concerned. Effect was maximum at lower concentrations and partition mechanism became dominating with increase in the pesticide concentration and STA content.
The contribution of partition and surface adsorption to atrazine adsorption in the control and 0.1 and 0.2% sugarcane trash ash (STA)–mixed a-c silty clay loam, d-f sandy clay loam, and g-i loam soils. The Ce is the concentration of atrazine in solution at equilibrium (µg mL-1), Qt is the total adsorption amount of atrazine, Qp is the atrazine adsorption amount generated by partition, and Qa is the atrazine adsorption amount generated by surface adsorption
The contribution of partition and surface adsorption to fipronil adsorption in the control and 0.1 and 0.2% sugarcane trash ash (STA)–mixed a-c silty clay loam, d-f sandy clay loam, and g-i loam soils. The Ce is the concentration of pesticide in solution at equilibrium (µg mL-1), Qt is the total adsorption amount of fipronil, Qp is the fipronil adsorption amount generated by partition, and Qa is the fipronil adsorption amount generated by surface adsorption
5 Conclusions
Burning of crop residues for land clearing after harvesting the crops is a common practice followed by farmers. Crop residue ashes contain high surface area unburnt carbon; therefore, they have the ability to adsorb pesticides and mixing these ashes in the soils can affect the fate of pesticides applied after burning. The present study evaluated the effect of sugarcane trash ash (STA) on atrazine (ATR) and fipronil (FIP) adsorption kinetics and adsorption behaviour in three sugarcane growing soils. The STA increased adsorption and decreased desorption of ATR and FIP in all three soils; however, the effect varied with the soil type and amount of STA mixed. Results indicated enhanced retention of ATR and FIP in STA-mixed soils; therefore, it might affect the subsequent degradation and leaching behaviour of both pesticides in the soils. The study has implications in assessing the fate of these pesticides applied in sugarcane fields, where trash is burnt year after year and STA is mixed in the soil. This process might have cumulative effect on the fate of pesticides applied in such soils. However, findings of the present study cannot be extended to results obtained in the field situation; therefore, a field study may provide a better estimate.
References
Carter MC, Kilduff JE, Weber WJ (1995) Site energy distribution analysis of preloaded adsorbents. Environ Sci Technol 29:1773–1780. https://doi.org/10.1021/es00007a013
Celis R, Cornejo J, Hermosin A, Koskinen WC (1997) Sorption-desorption of atrazine and simazine by model soil colloid components. Soil Sci Soc Am J 61:436–443. https://doi.org/10.2136/sssaj1997.03615995006100020010x
Clay SA, Koskinen WC, Allmaras RR, Dowdy RH (1988) Differences in herbicide adsorption on soil using several soil pH modification techniques. J Environ Sci Health B23:559–573. https://doi.org/10.1080/03601238809372626
Cui XY, Wang HL, Lou LP, Chen YX, Yu YL, Shi JY, Xu L, Khan MI (2009) Sorption and genotoxicity of sediment-associated pentachlorophenol and pyrene influenced by crop residue ash. J Soil Sediments 9:604–612. https://doi.org/10.1007/s11368-009-0124-7
Deokar SK, Mandavgane SA, Kulkarni BD (2016a) Agro-industrial waste: a low cost adsorbent for effective removal of 4-chloro-2-methylphenoxyacetic acid herbicide in batch and packed bed modes. Environ Sci Pollut Res 23:16164–16175. https://doi.org/10.1007/s11356-016-6769-z
Deokar SK, Singh D, Modak S, Mandavgane SA, Kulkarni BD (2016b) Adsorptive removal of diuron on biomass ashes: a comparative study using rice husk ash and bagasse fly ash as adsorbents. Desalin Water Treat 57:22378–22391. https://doi.org/10.1080/19443994.2015.1132394
Fungaro DA, Silva MVD (2014) Utilization of water treatment plant sludge and coal fly ash in brick manufacturing. American J Environ Protect 2:83–88. https://doi.org/10.12691/env-2-5-2
Garba J, Samsuri AW, Othman R, Hamdani MSA (2018) Adsorption-desorption and leaching potential of glyphosate and aminomethylphosphonic acid in acidic Malaysian soil amended with cow dung and rice husk ash. Environ Monit Assess 190:676–691. https://doi.org/10.1007/s10661-018-7034-3
Giori FG, Tornisielo VL, Regitano JB (2014) The role of sugarcane residues in the sorption and leaching of herbicides in two tropical soils. Water Air Soil Pollut 225:1935–1944. https://doi.org/10.1007/s11270-014-1935-8
Hiller E, Fargasova A, Zemanova L, Bartal M (2007) Influence of wheat ash on the MCPA immobilization in agricultural soils. Bull Environ Contam Toxicol 78:345–348. https://doi.org/10.1007/s00128-008-9400-2
Hiller E, Barta M, Milicka J, Cernansky S (2009) Environmental fate of the herbicide MCPA in two soils as affected by the presence of wheat ash. Water Air Soil Pollut 197:395–402. https://doi.org/10.1007/s11270-008-9820-y
Jain N, Bhatia A, Pathak H (2014) Emission of air pollutants from crop residue burning in India. Aerosol Air Qual Res 14:422–430. https://doi.org/10.4209/aaqr.2013.01.0031
Kumar A, Singh N (2020) Crop residue ashes reduce leaching, persistence and bioavailability of sulfosulfuron and pretilachlor used in the succeeding crop. Soil Res 58:551–560. https://doi.org/10.1071/SR20142
Kumar A, Mandal A, Singh N (2019) Rice and wheat straw ashes: characterization and modeling of pretilachlor sorption kinetics and adsorption isotherm. J Environ Sci Health B 54:303–312. https://doi.org/10.1080/03601234.2018.1561059
Liu Y (2008) New insights into pseudo-second-order kinetic equation for adsorption. Colloids Surf Physicochem Eng Aspects 320:275–278. https://doi.org/10.1016/j.colsurfa.2008.01.032
Liu Z, Clay SA, Clay DE, Harper SS (1995) Ammonia impacts on atrazine leaching through undisturbed soil columns. J Environ Qual 24:1170–1173. https://doi.org/10.2134/jeq1995.00472425002400060017x
Loganathan VA, Feng Y, Sheng GD, Clement TP (2009) Crop-residue-derived char influences sorption, desorption and bioavailability of atrazine in soils. Soil Sci Soc Am J 73:967–974. https://doi.org/10.2136/sssaj2008.0208
Mandal A, Singh N, Purakayastha TJ (2017) Characterization of pesticide sorption behaviour of slow pyrolysis biochars as low cost adsorbent for atrazine and imidacloprid removal. Sci Total Environ 577:376–385. https://doi.org/10.1016/j.scitotenv.2016.10.204
Pandey VC, Singh N (2010) Impact of fly ash incorporation in soil systems. Agric Ecosys Environ 136:16–27. https://doi.org/10.1016/j.agee.2009.11.013
Turner JA (2015) Pesticide Manual. British Crop Protection Council, Worcestershire
Pholosi A, Naidoo EB, Ofomaja AE (2020) Intraparticle diffusion of Cr (VI) through biomass and magnetite coated biomass: a comparative kinetic and diffusion study. South African J Chem Engin 32:39–55. https://doi.org/10.1016/j.sajce.2020.01.005
Sheng G, Yang Y, Huang M, Yang K (2005) Influence of pH on pesticide sorption by soil containing wheat residue-derived char. Environ Poll 134:457–463. https://doi.org/10.1016/j.envpol.2004.09.009
Singh A, Srivastava A, Srivastava PC (2016) Sorption–desorption of fipronil in some soils, as influenced by ionic strength, pH and temperature. Pest Manag Sci 72:1491–1499. https://doi.org/10.1002/ps.4173
Spomer NA, Kamble ST (2010) Sorption and desorption of fipronil in Midwestern soils. Bull Environ Contam Toxicol 84:264–268. https://doi.org/10.1007/s00128-009-9915-1
Trivedi NS, Mandavgane SA, Kulkarni BD (2016) Mustard plant ash: a source of micronutrient and an adsorbent for removal of 2, 4-dichlorophenoxyacetic acid. Environ Sci Pollut Res 23:20087–20099. https://doi.org/10.1007/s11356-016-6202-7
Yadav S, Singh N (2020) Sugarcane trash ash: a low cost adsorbent for atrazine and fipronil removal from water. Ind J Chem Technol 27:319–325 http://nopr.niscair.res.in/handle/123456789/55490
Yang Y, Sheng G (2003) Enhanced pesticide sorption by soils containing particulate matter from crop residue burns. Environ Sci Technol 37:3635–3639. https://doi.org/10.1021/es034006a
Ying GG, Kookana RS (2001) Sorption of fipronil and its metabolites on soils from South Australia. J Environ Sci Health B 36:545–558. https://doi.org/10.1081/PFC-100106184
Yue L, JunGe C, Feng D, Yu H, Deng H, Fu B (2017) Adsorption–desorption behavior of atrazine on agricultural soils in China. J Environ Sci 57:180–189. https://doi.org/10.1016/j.jes.2016.11.002
Zhu L, Chen B (2000) Sorption behavior of p-nitrophenol on the interface between anion− cation organobentonite and water. Environ Sci Technol 34:2997–3002. https://doi.org/10.1021/es991460z
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 530 kb)
Rights and permissions
About this article
Cite this article
Yadav, S., Singh, N. Increased Sorption of Atrazine and Fipronil in the Sugarcane Trash Ash–Mixed Soils of Northern India. J Soil Sci Plant Nutr 21, 1263–1276 (2021). https://doi.org/10.1007/s42729-021-00438-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-021-00438-8