Abstract
This study was conducted to evaluate the environmental fate processes of the herbicide (4-chloro-2-methylphenoxy)acetic acid (MCPA) in agricultural soils in the presence and absence of ash originating from the burning of wheat residue. The ash-amended soils (1% ash by weight) were approximately 8–16 times more effective than the ash-free soils in sorbing MCPA. The desorption results showed that 40–78% of initially sorbed MCPA were desorbed in both soils, depending on the initial MCPA concentration in solution. Addition of ash to soils decreased the desorption of MCPA by approximately 20%. Degradation of MCPA was substantially reduced in the presence of the ash. A 6-week incubation resulted in 50–85% of MCPA microbially degraded in ash-amended soils, as compared to >85–100% in ash-free soils under the same conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Herbicides have received increasing attention globally in the last two decades with regard to their potential to negatively impact soil and groundwater quality. Degradation and sorption are key processes affecting the environmental fate and transport of weak acid herbicides in soils (Cox et al. 2001; Celis et al. 2005; Sørensen et al. 2006). Sorption, which controls herbicide concentrations in the soil solution, is often found to limit herbicide degradation in soil by reducing its concentrations in the aqueous solution (Jensen et al. 2004; Dyson et al. 2002). It has been suggested that herbicides in solution are much more bioaccessible to soil degrading microorganisms than sorbed herbicides (Guo et al. 1999, 2000; Park et al. 2003). Degradation is a fundamental attenuation process for herbicides in soil (Bending et al. 2006). Degradation process may follow complex pathways and it is affected by many factors related to microbial, soil physical and chemical conditions (Gaultier et al. 2008). Sorption and desorption of weak acid herbicides in soil are similarly key in controlling their transport, transformation and bioaccumulation processes (Socías-Viciana et al. 1999; Gaultier et al. 2008). The sorption and desorption of weak acid herbicides in soil are dependent on the soil properties, such as soil organic carbon content, soil pH and the properties of the herbicide (Bekbölet et al. 1999; Haberhauer et al. 2000; Hiller et al. 2006). However, recent studies have shown that ashes, a form of black carbon, originating from the burning of vegetation may also potentially affect the sorption of organic chemicals in soils (Chun et al. 2004), and thus their degradation. Burning of crop residues in the fields is a routine post-harvest practice worldwide for land clearing and results in a direct accumulation of ashes in soils. The ashes usually contain high-surface-area carbonaceous materials due to combustive carbonization. It has been shown that soil ashes effectively sorb applied nonpolar pesticides in a fashion similar to that of activated carbon and that they are responsible for the high sorptivity of ash-amended soils (Yang and Sheng 2003; Yang et al. 2006). One consequence of the high sorptivity of crop-residue-derived ashes may be reduced degradation and desorption of herbicides in soils. Up to now, only Zhang et al. (2004) and Yang et al. (2006) have reported the reduction in degradation of benzonitrile and diuron in one soil in the presence of wheat ash. However, it is still not clear whether the presence of ash in soil has also a similar effect on herbicide desorption.
Given the common practice of field burning of crop residues and extensive use of (4-chloro-2-methylphenoxy) acetic acid (MCPA) in Slovakia, the objectives of this study were to evaluate the impact of wheat ash on MCPA sorption and desorption for two common soils as well as the impact of wheat ash on MCPA degradation.
2 Materials and Methods
2.1 Sorbents
Wheat (Triticum aestivum L.) straw washed well with deionized water was used in this study. Air-dried straw (1 kg) was burned on a steel plate (50 × 40 cm) in a garden under natural conditions at the end of April. Totally, 48 g of wheat ash was obtained by burning of 1 kg of air-dried wheat straw. The ash contains 11.8% elemental carbon and 21.0% silicon. The pH measured in suspension of ash was 10.7. The specific surface area of the wheat ash was not measured. However, Yang and Sheng (2003) reported that the specific surface area of the wheat ash was 10.1 m2 g-1. The ash was used either pure or mixed with soils.
Two surface soils (0–20 cm) from agricultural fields of Western Slovakia were used. The soils were Arenic Fluvi-Gleyic Phaeozem (denoted as soil I in the text) and Arenic Regosol (denoted as soil II in the text) collected from the fields near Stupava and Lozorno, respectively. The soil samples were air-dried, crushed and sieved through a 2 mm mesh. The main physico-chemical properties of the soils were determined in accordance with the Slovak Soil Science Research Institute’s procedures (Fiala et al. 1999) and are shown in Table 1. The silicate clay mineralogy of the soil clay fraction was determined by X-ray diffraction analysis on oriented specimens treated with ethylene glycol at 60°C for 8 h. Ash-amended soils with the ash content of 1% (by weight) were prepared by thoroughly mixing soils with accurately weighed wheat ash.
2.2 Herbicide
MCPA, a weak acid herbicide, was used as a model pesticide in this study. Analytical grade MCPA was purchased from Dr Ehrenstorfer GmbH (Germany). MCPA exhibits relatively high water solubility (825 mg L−1 at 25°C) and has a pK a value of 3.07. MCPA is mostly neutral at pH < 3.07 and becomes negatively charged at pH > 3.07.
2.3 Sorption and Desorption
Sorption isotherms for MCPA in pure soils, pure wheat ash and ash-amended soils were obtained by a batch equilibration procedure. Primary stock solution of MCPA containing 0.01 M CaCl2 and 10–4 M NaN3 to keep ionic strength constant and to inhibit microbial activity, respectively, was diluted using a Milli-Q+ water (Millipore) to produce five initial MCPA concentrations ranging from 1.66 to 56.6 mg L−1. A predetermined weight of each sorbent (2 g of pure soil, 0.25 g of ash-amended soil, and 0.05 g of pure ash) and 10 mL of 0.01 M CaCl2 and 10–4 M NaN3 solution containing various amounts of MCPA were mixed in a series of 15 mL glass centrifuge tubes covered with aluminium foil to prevent photodegradation of MCPA. The suspensions were rotated end over end for 24 h. After the establishment of sorption equilibria, sorbents and aqueous phases were separated by centrifugation at 3,000 rpm for 15 min. Preliminary measurements on MCPA sorption kinetics have shown that sorption of MCPA by both the wheat ash and the ash-amended soil I reached equilibrium in 24 h since there were no significant differences in MCPA sorption between 24 h and longer equilibration periods (Fig. 1). The MCPA concentrations in supernatants were analyzed by a reversed-phase high-performance liquid chromatography. The amount of MCPA sorbed was calculated by the difference between the amount initially added and that remaining in equilibrium solution. Sorption tests for MCPA–pure soil combinations were made in duplicate. Measurements with ash-amended soils and pure ash were made in triplicate. The measurements with control samples containing only MCPA showed that there were no losses of MCPA due to adsorption onto the surface of the tubes, volatilization or microbial activity.
Desorption was determined on the same samples as used for the sorption test with initial MCPA concentrations of 5.7 and 56.6 mg L−1. The supernatants were carefully removed and replaced with fresh 0.01 M CaCl2 and 10–4 M NaN3 solution in the same amount as removed. The tubes were rotated end over end for 24 h. At 24 h, duplicate or triplicate samples were centrifuged, and the supernatants were analyzed for MCPA concentrations as described below.
2.4 Degradation
Two weeks before the start of degradation experiment a set of 20 g portions for each soil and ash-amended soil was placed in the dark at 23°C and at a soil moisture content of 15% by weight, in batches covered with perforated aluminium foil. Then, MCPA in 0.01 M CaCl2 solution (1 mL) was added to each 20 g portion of soil samples to give an initial concentration of ∼8 mg MCPA per 1 kg of soil. The soils were allowed to equilibrate for 2 h at 23°C and consequently thoroughly mixed. Wet soil samples were stored in the dark at 23°C and soil moisture content was maintained at the initial level of 20% by weight. Incubation periods were 0, 1, 6, 8, 10, 15, 22, 30 and 40 days. Duplicate samples were taken at each time point to measure remaining concentrations of MCPA in the soil. For MCPA determination, each soil sample was dried by flowing air, and then 0.5 g of soil sample was extracted with 1 mL of acetonitrile–acetic acid solution (0.5% acetic acid) in a ratio of 50:50 (v/v) using an ultrasonic system for 2 h. After centrifugation at 10,000 rpm for 10 min, an aliquot of the extract was used for the analysis of MCPA concentration. Extraction recoveries of this procedure were 86.4%, 78.8%, 92.6% and 90.9% for soil I, soil II, ash-amended soil I and ash-amended soil II, respectively, based on duplicates of freshly spiked samples. MCPA concentrations were adjusted for the recoveries.
2.5 Analytical Procedure
MCPA was analyzed by a reversed-phase high-performance liquid chromatography (Hewlett–Packard model 1100) using a Lichrosphere-100 RP column (4.6 × 125 mm, 5 μm) and a Hewlett–Packard 1046A fluorescence detector (excitation at 232 nm and emission at 314 nm). External solution standards were used to establish linear calibration curves for a fluorescence detector. The mobile phase was a mixture of acetonitrile and 0.03 M acetic acid solution containing 5% acetonitrile in a ratio of 50:50 (v/v) at a flow rate of 1.0 mL min−1 (isocratic elution). The sample injection volume was 20 μL. The average uncertainty for the measured concentrations was about ±5% and the limit of quantification was 0.02 mg L−1.
3 Results
3.1 Sorption and Desorption
Figure 2 shows the sorption isotherms of MCPA with pure soils, ash-amended soils and pure wheat ash. Since no commonly used sorption isotherm models, such as linear, Freundlich or Langmuir, provided adequate fits for the sorption data, the difference in MCPA sorption among the sorbents investigated was indicated by calculating the sorption coefficient (K d ) at an equilibrium concentration of about 1.5 mg L−1 (Table 2). The organic carbon sorption coefficients (K oc) were also calculated in order to compare MCPA sorption among the sorbents used. The curves were drawn to only assist in presentation and comparison of the sorption data. It is obvious that MCPA sorption increased in the order: soil II < soil I « ash-amended soil II < ash-amended soil I « wheat ash. Wheat ash was 90–390 times and 230–1,490 times more effective as a sorbent for MCPA than soil I and II, respectively. Compared to ash-free soils, the ash-amended soils I and II were approximately eight and 16 times more effective than the ash-free soils I and II in sorbing MCPA, respectively.
Isotherms for sorption of MCPA by soils, 1% ash-amended soils and wheat ash (open symbols). Full symbols denote desorption data points and desorption paths from the corresponding equilibrium sorption points are indicated by arrows. Error bars represent standard deviations, n = 3 for wheat ash and ash-amended soil, and n = 2 for soil
Desorption of MCPA from the soils, ash-amended soils and wheat ash was expressed as a percentage of the total sorbed MCPA that was removed within 24 h of the desorption test (Table 2). Generally, MCPA desorption decreased in the order: soil I ≈ soil II > ash-amended soil I ≥ ash-amended soil II ≈ wheat ash. The results show that the presence of wheat ash in soils significantly affected MCPA desorption.
3.2 Degradation
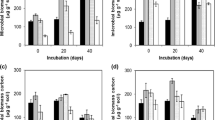
Percentage of MCPA remaining in soils and ash-amended soils versus time are shown in Fig. 3a,b. The degradation data were fitted satisfactorily to a first-order kinetic according to equation:
where C is the MCPA concentration in the soil at a given time t (milligram per kilogram), C 0 is the initial MCPA concentration added to the soil (milligram per kilogram) and k is the first-order rate constant (per day). The first-order rate constants and the calculated half-lives (t 1/2 = ln2/k) for MCPA in all soil samples are given in Table 3. The results clearly indicate that the degradation of MCPA in the ash-amended soils was significantly reduced as compared with the ash-free soils. It appears that MCPA degradation in the soils was primarily microbially mediated since total MCPA loss in the controls with no microbial activity (silica sand) was only 0.6% of initially applied MCPA (Fig. 3a). MCPA has been shown to degrade in soil by both physico-chemical and biological means although microbial breakdown is considered the primary mechanism, whereas photodecomposition and volatilization are of minor importance (Torstensson et al. 1975; Crespín et al. 2001).
4 Discussion
4.1 Sorption and Desorption
Each soil had a low affinity for MCPA as indicated by the low K d values and the corresponding K oc values (Table 2). MCPA as well as other acidic herbicides are predominantly present in an anionic form when soil pH is greater than 5. It is well-known that anionic forms of organic acids are generally weakly retained by most soils (Nicholls and Evans 1991; Dubus et al. 2001), and consequently considered as very mobile in soil profiles (Socías-Viciana et al. 1999; Sørensen et al. 2006).
Wheat ash effectively sorbed MCPA as compared with ash-free soils (Table 2; Fig. 2). Approximately 70 times higher K oc value for MCPA in wheat ash than that in ash-free soils indicates that the increase in MCPA sorption is not only due to the higher carbon content of wheat ash, but likely also the much greater surface affinity for MCPA as compared with typical soil organic matter. This result is consistent with previous studies, showing that ashes derived from the burning of vegetation are very effective sorbents of organic chemicals (Yang and Sheng 2003; James et al. 2005; Sheng et al. 2005). Interestingly, sorption of MCPA by wheat ash displayed the characteristics of the Langmuir sorption isotherm (Fig. 2), suggestive of the surface adsorption of MCPA by the ash. The isotherm of the Langmuir-type for MCPA sorption by wheat ash measured in this study was similar to those earlier reported for adsorption of 2,4-D and MCPA by activated carbons (Aksu and Kabasakal 2004; Nyazi et al. 2005). The similarity indicates that wheat ash may resemble activated carbon as a surface adsorbent (Chun et al. 2004; James et al. 2005).
Addition of 1% wheat ash to the soils caused a significant increase in MCPA sorption, as indicated by the sorption coefficients (Table 2). Assuming that the presence of wheat ash in soils did not change their intrinsic sorption affinities for MCPA, simple calculations show that 1% wheat ash contributed 85% and 91% to the overall sorption by ash-amended soils I and II, respectively, consistent with the results of earlier studies (Yang and Sheng 2003; Zhang et al. 2004; Yang et al. 2006). These results indicate the predominance of wheat ash over soil for MCPA sorption.
Desorption of MCPA from soils containing wheat ash was lower than that observed from soils without wheat ash (Table 2). Desorption of MCPA from pure wheat ash corresponding to the initial concentration 56.6 mg L−1 was not significantly different from that with the ash-amended soils. These results highlight the importance of wheat ash in affecting herbicide retention in soils. However, desorption of MCPA from pure wheat ash was lower at the concentration of 5.7 mg L−1 than that at the concentration of 56.6 mg.L−1. This could be explained by the limited number of sites available for high-energy binding, which are occupied first at lower solute concentrations, whereas at high solute concentrations, more molecules are retained by low-energy sites and therefore can more easily desorb (Celis and Koskinen 1999; Drori et al. 2005). It was expected that desorption of MCPA from ash-amended soils corresponding to the lower initial concentration would be similar to that from pure wheat ash, but such behavior was not observed (Table 2).
4.2 Degradation
A significantly faster degradation of MCPA in the soil I compared with the soil II might be explained by a higher organic carbon content in the soil I. Organic matter is an important source of nutrients, and the biological activity of soil microbial populations may increase with an increase in the organic carbon content (Anderson and Domsch 1989; Beulke and Malkomes 2001). An increase in soil microbial activity is likely to increase the rate of pesticide degradation (Bending et al. 2006; Rodríguez-Cruz et al. 2006). In this study, the degradation of MCPA over time was simply measured, and thus it could not provide mechanistic information about the degradation pathway. However, it has been observed that MCPA can be utilized by soil bacteria as a carbon source and converted to 4-chloro-2-methylphenol in the first step (Bollag et al. 1967; Crespín et al. 2001). The half-lives of MCPA for these two soils are similar to those reported in other studies (Torstensson et al. 1975; Thorstensen and Lode 2001).
Addition of wheat ash to the soils substantially altered and reduced the degradation rate and extent of MCPA (Fig. 3a,b; Table 3). Zhang et al. (2004) and Yang et al. (2006) have also observed reduced degradation of organic chemicals in ash-amended soil that was inoculated by the enriched bacteria suspension cell. The reduced degradation of MCPA in the ash-amended soils, as observed in this study, resembles previous results refering to the reduced degradation of MCPA and other acidic herbicides in soils treated with activated carbon (Guo et al. 1999, 2000; Jensen et al. 2004). Reduced degradation of organic chemicals in soil amended with activated carbon was mainly due to the high sorption by activated carbon that effectively decreased the solution-phase concentration of organic chemicals and inability of soil microbes to utilize sorbed organic chemicals directly (Guerin and Boyd 1997; Jensen et al. 2004). It is well-known that soil-sorbed chemicals are less available to microorganisms, and thus less degraded compared with organic chemicals in solution (Guo et al. 1999, 2000; Park et al. 2001). Therefore, it is likely that reduction in the degradation of MCPA in the presence of wheat ash was obviously due to high sorption of MCPA by the ash. As desorption is often a prerequisite for microbial degradation (Zhang et al. 2004), lower degradation of MCPA in the ash-amended soils might be also due to its lower desorption from these soils (Table 2).
5 Conclusions
This study was designated to investigate the effects of ashes originating from the burning of wheat residues (T. aestivum L.) on primary environmental fate processes occurring between soils and pesticides. Wheat ash was a highly effective sorbent for the weak acid herbicide MCPA. It increased significantly the sorption of MCPA when present in the soils. Moreover, the results have shown that sorption of MCPA by soils containing the wheat ash has a profound effect on herbicide desorption and degradation. Desorption of MCPA from the ash-amended soils was lower than that from the ash-free soils. In the absence of wheat ash, 40–78% of the sorbed MCPA were desorbed from the soils. However, the presence of wheat ash in soils caused a decrease in the MCPA desorption up to 20%, which was the same as that in wheat ash alone. It was found that MCPA was less degradable in soils containing the wheat ash. Taking into account the analogy with studies of activated carbons, reduced degradability of MCPA appeared to be a result of the enhanced sorption in soils in the presence of wheat ash. The field burning of crop residues appears to effectively immobilize the pesticides in agricultural soils, and thus reduce their leaching. However, the prolonged persistence of pesticides in soils containing the ashes gives rise to the attention regarding a problem of pesticide carryover. Although, the field burning of crop residues is still a common practice worldwide, it should not be recommended for reducing leaching of pesticides as it contributes to the greenhouse effect and negatively affects soil microbial populations.
References
Aksu, Z., & Kabasakal, E. (2004). Batch adsorption of 2,4-dichlorophenoxy-acetic acid (2,4-D) from aqueous solution by granular activated carbon. Separation and Purification Technology, 35, 223–240. doi:10.1016/S1383-5866(03)00144-8.
Anderson, T. H., & Domsch, K. H. (1989). Ratios of microbial biomass carbon to total organic carbon in arable soils. Soil Biology & Biochemistry, 21, 471–479. doi:10.1016/0038-0717(89)90117-X.
Bekbölet, M., Yenigün, O., & Yücel, I. (1999). Sorption studies of 2,4-D on selected soils. Water, Air, and Soil Pollution, 111, 75–88. doi:10.1023/A:1005089612111.
Bending, G. D., Lincoln, S. D., & Edmondson, R. N. (2006). Spatial variation in the degradation rate of the pesticides isoproturon, azoxystrobin and diflufenican in soil and its relationship with chemical and microbial properties. Environmental Pollution, 139, 279–287. doi:10.1016/j.envpol.2005.05.011.
Beulke, S., & Malkomes, H. P. (2001). Effects of the herbicides metazachlor and dinoterb on the soil microflora and the degradation and sorption of metazachlor under different environmental conditions. Biology and Fertility of Soils, 33, 467–471. doi:10.1007/s003740100354.
Bollag, J. M., Helling, C. S., & Alexander, M. (1967). Metabolism of 4-chloro-2-methylphenoxyacetic acid by soil bacteria. Applied Microbiology, 15, 1393–1398.
Celis, R., & Koskinen, W. C. (1999). Characterization of pesticide desorption from soil by the isotopic exchange technique. Soil Science Society of America Journal, 63, 1659–1666.
Celis, R., Real, M., Hermosín, M. C., & Cornejo, J. (2005). Sorption and leaching behaviour of polar aromatic acids in agricultural soils by batch and column leaching tests. European Journal of Soil Science, 56, 287–297. doi:10.1111/j.1365-2389.2004.00676.x.
Chun, Y., Sheng, G., Chiou, C. T., & Xing, B. (2004). Compositions and sorptive properties of crop residue-derived chars. Environmental Science & Technology, 38, 4649–4655. doi:10.1021/es035034w.
Cox, L., Cecchi, A., Celis, R., Hermosín, M. C., Koskinen, W. C., & Cornejo, J. (2001). Effect of exogenous carbon on movement of simazine and 2,4-D in soils. Soil Science Society of America Journal, 65, 1688–1695.
Crespín, M. A., Gallego, M., Valcárcel, M., & González, J. L. (2001). Study of the degradation of the herbicides 2,4-D and MCPA at different depths in contaminated agricultural soil. Environmental Science & Technology, 35, 4265–4270. doi:10.1021/es0107226.
Drori, Y., Aizenshtat, Z., & Chefetz, B. (2005). Sorption-desorption behavior of atrazine in soils irrigated with reclaimed wastewater. Soil Science Society of America Journal, 69, 1703–1710. doi:10.2136/sssaj2004.0345.
Dubus, I. G., Barriuso, E., & Calvet, R. (2001). Sorption of weak organic acids in soils: clofencet, 2,4-D and salicylic acid. Chemosphere, 45, 767–774. doi:10.1016/S0045-6535(01)00108-4.
Dyson, J. S., Beulke, S., Brown, C. D., & Lane, M. C. G. (2002). Adsorption and degradation of the weak acid mesotrione in soil and environmental fate implications. Journal of Environmental Quality, 31, 613–618.
Fiala, K., Kobza, J., Matúšková, L., Brečková, V., Makovníková, J., Barančíková, G., et al. (1999). Obligatory methods of soil analyses. Partial monitoring system-soil. Bratislava: Soil Science and Conservation Research Institute, (In Slovak).
Gaultier, J., Farenhorst, A., Cathcart, J., & Goddard, T. (2008). Degradation of [carboxyl-14C] 2,4-D and [ring-U-14C] 2,4-D in 114 agricultural soils as affected by soil organic carbon content. Soil Biology & Biochemistry, 40, 217–227. doi:10.1016/j.soilbio.2007.08.003.
Guerin, W. F., & Boyd, S. A. (1997). Bioavailability of naphthalene associated with natural and synthetic sorbents. Water Research, 31, 1504–1512. doi:10.1016/S0043-1354(96)00402-2.
Guo, L., Wagenet, R. J., & Jury, W. A. (1999). Adsorption effects on kinetics of aldicarb degradation: equilibrium model and application to incubation and transport experiments. Soil Science Society of America Journal, 63, 1637–1644.
Guo, L., Jury, W. A., Wagenet, R. J., & Flury, M. (2000). Dependence of pesticide degradation on sorption: nonequilibrium model and application to soil reactors. Journal of Contaminant Hydrology, 43, 45–62. doi:10.1016/S0169-7722(99)00097-2.
Haberhauer, G., Pfeiffer, L., & Gerzabek, M. H. (2000). Influence of molecular structure on sorption of phenoxyalkanoic herbicides on soil and its particle size fractions. Journal of Agricultural and Food Chemistry, 48, 3722–3727. doi:10.1021/jf9912856.
Hiller, E., Khun, M., Zemanová, L., Jurkovič, Ľ., & Bartaľ, M. (2006). Laboratory study of retention and release of weak acid herbicide MCPA by soils and sediments and leaching potential of MCPA. Plant, Soil and Environment, 52, 550–558.
James, G., Sabatini, D. A., Chiou, C. T., Rutherford, D., Scott, A. C., & Karapanagioti, H. K. (2005). Evaluating phenanthrene sorption on various wood chars. Water Research, 39, 549–558. doi:10.1016/j.watres.2004.10.015.
Jensen, P. H., Hansen, H. C. H. B., Rasmussen, J., & Jacobsen, O. S. (2004). Sorption-controlled degradation kinetics of MCPA in soil. Environmental Science & Technology, 38, 6662–6668. doi:10.1021/es0494095.
Nicholls, P. H., & Evans, A. A. (1991). Sorption of ionisable compounds by field soils. Part 1: Acids. Pesticide Science, 33, 319–330. doi:10.1002/ps.2780330306.
Nyazi, K., Baçaoui, A., Yaacoubi, A., Darmstadt, H., Adnot, A., & Roy, C. H. (2005). Influence of carbon black surface chemistry on the adsorption of model herbicides from aqueous solution. Carbon, 43, 2218–2221. doi:10.1016/j.carbon.2005.03.015.
Park, J. H., Feng, Y., Ji, P., Voice, T. C., & Boyd, S. A. (2003). Assessment of bioavailability of soil-sorbed atrazine. Applied and Environmental Microbiology, 69, 3288–3298. doi:10.1128/AEM.69.6.3288-3298.2003.
Park, J. H., Zhao, X., & Voice, T. C. (2001). Biodegradation of non-desorbable naphthalene in soils. Environmental Science & Technology, 35, 2734–2740. doi:10.1021/es0019326.
Rodríguez-Cruz, M. S., Jones, J. E., & Bending, G. D. (2006). Field-scale study of the variability in pesticide biodegradation with soil depth and its relationship with soil characteristics. Soil Biology & Biochemistry, 38, 2910–2918. doi:10.1016/j.soilbio.2006.04.051.
Sheng, G., Yang, Y., Huang, M., & Yang, K. (2005). Influence of pH on pesticide sorption by soil containing wheat residue-derived char. Environmental Pollution, 134, 457–463. doi:10.1016/j.envpol.2004.09.009.
Socías-Viciana, M. M., Fernández-Pérez, M., Villafranca-Sánchez, M., Gonzáles-Pradas, E., & Flores-Céspedes, F. (1999). Sorption and leaching of atrazine and MCPA in natural and peat-amended calcareous soils from Spain. Journal of Agricultural and Food Chemistry, 47, 1236–1241. doi:10.1021/jf980799m.
Sørensen, S. R., Schultz, A., Jacobsen, O. S., & Aamand, J. (2006). Sorption, desorption and mineralisation of the herbicides glyphosate and MCPA in samples from two Danish soil and subsurface profiles. Environmental Pollution, 141, 184–194. doi:10.1016/j.envpol.2005.07.023.
Thorstensen, C. H. W., & Lode, O. (2001). Laboratory degradation studies of bentazone, dichlorprop, MCPA, and propiconazole in Norwegian soils. Journal of Environmental Quality, 30, 947–953.
Torstensson, N. T. L., Stark, J., & Göranson, B. (1975). The effect of repeated applications of 2,4-D and MCPA on their breakdown in soil. Weed Research, 15, 159–164. doi:10.1111/j.1365-3180.1975.tb01116.x.
Yang, Y., & Sheng, G. (2003). Enhanced pesticide sorption by soils containing particulate matter from crop residue burns. Environmental Science & Technology, 37, 3635–3639. doi:10.1021/es034006a.
Yang, Y., Sheng, G., & Huang, M. (2006). Bioavailability of diuron in soil containing wheat-straw-derived char. The Science of the Total Environment, 354, 170–178. doi:10.1016/j.scitotenv.2005.01.026.
Zhang, P., Sheng, G., Wolf, D. C., & Feng, Y. (2004). Reduced biodegradation of benzonitrile in soil containing wheat-residue-derived ash. Journal of Environmental Quality, 33, 868–872.
Acknowledgements
This research was financially supported by grants VEGA No. 1/4036/07 and 1/3462/06. We thank also the Water Research Institute in Bratislava, Department of Groundwater, Slovakia, for its financial support from grant No. 6413 of Ministry of the Environment of the Slovak Republic and Ray Marshall for checking the English language of manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hiller, E., Bartaľ, M., Milička, J. et al. Environmental Fate of the Herbicide MCPA in Two Soils as Affected by the Presence of Wheat Ash. Water Air Soil Pollut 197, 395–402 (2009). https://doi.org/10.1007/s11270-008-9820-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-008-9820-y