Abstract
Laboratory study on adsorption–desorption of flubendiamide was conducted in two soil types, varying in their physical and chemical properties, by batch equilibrium method. After 4 h of equilibrium time, adsorption of flubendiamide on soil matrix exhibited moderately low rate of accumulation with 4.52 ± 0.21 % in red soil and low rate with 3.55 ± 0.21 % in black soil. After amending soils with organic manure, adsorption percentage increased to 6.42 ± 0.21 % in red soil and (4.18 ± 0.21 %) in black soil indicating that amendment significantly increased sorption. Variation in sorption affinities of the soils as indicated by distribution coefficient (K d) for sorption was in the range of 2.98–4.32, 4.91–6.64, 1.04–1.45 and 1.92–2.81 ml/g for red soil, organic manure-treated red soil, black soil and organic manure-treated black soil, respectively. Desorption was slightly slower than adsorption indicating a hysteresis effect having hysteresis coefficient ranges between 0.023 and 0.149 in two test soils. The adsorption data for the insecticide fitted well the Freundlich equation. Results revealed that adsorption–desorption was influenced by soil types and showed that the maximum sorption and minimum desorption of the insecticide was observed in soils with higher organic carbon and clay content. It can be inferred that crystal lattice of the clay soil plays a significant role in flubendiamide adsorption and desorption. Adsorption was lower at acidic pH and gradually increased towards alkaline pH. As this insecticide is poorly sorbed in the two Indian soil types, there may be a possibility of their leaching to lower soil profiles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Adsorption and desorption are important processes that affect the mobility and fate of contaminants in soil. The behaviour of pollutants in soil depends on their sorption to soil components. Although, organic matter and minerals present in soil play an important role in sorption process, differences exist in their relative behaviour (Bailey and white 1964; Bailey et al. 1968; Helling et al. 1971; Das et al. 2012). Two of the major factors known to influence sorption are (i) soil properties like the nature of soil colloids including clay, organic matter and metal oxides, soil reaction, soil acidity, soil temperature, electrical potential of soil surface and (ii) molecular characteristics of pesticide like chemical characteristics, shape and configuration of pesticide molecule, ionization constant, water solubility, charge distribution on pesticide molecule, molecular charge, polarity and polarizability (Murphy et al. 1992; Das and Mukherjee 2014). Spark and Swift (2002) studied the influence of soil constituents, physico-chemical factors of soil and the chemical structure of pesticides on their adsorption behaviour in soils. Thus, it is fundamentally important to explore the factors that modulate the behaviour of pesticides in soil (Zhang et al. 2012).
Increasing awareness of the potential impact of persistent crop protection agents have led to the development of eco-friendly new molecules to ensure minimum risk to man and environment. Flubendiamide N 2-[1,1-dimethyl-2(methylsulfonyl)ethyl]-3-iodo-N 1-[2-methyl-4-[1,2,2,2-tetrafluoro-1-(trifluoromethyl)ethyl]phenyl]-1,2 benzenedicarboxamide (Fig. 1) was introduced in India by Bayer Crop Science. It represents a novel class of insecticides with extremely high activity against a broad spectrum of lepidopteron insect–pest like fruit and shoot borer including resistant strain (Das and Mukherjee 2011). It belongs to the chemical class of benzenedicarboxamides or phthalic acid diamides. In contrast to most other commercially available pesticides which act on nervous system, flubendiamide disrupts the calcium balance in the muscles of the insects by acting on the ryanodine receptor, affecting the muscle contraction (Ebbinghaus-Kintscher et al. 2007). The ground water ubiquity score (GUS) is an indicator of pesticide environmental impact. It is a function of field half-life (DT 50) and organic carbon sorption constant (K oc). GUS of flubendiamide is 0.559, indicating that it has very low potential to leach down to groundwater (Gustafson 2002). Information on flubendiamide leaching behaviour is mainly restricted to the registration/regulatory documents mentioning that flubendiamide satisfies most of the leaching criteria of Cohen et al. (1984).
Investigation of sorption phenomena of pesticides in soils is of great importance from environmental point of view (Khoury et al. 2003). Any soil having different physico-chemical properties affect the sorption, transport and degradation of the soil-applied pesticides. The organic matter and clay fractions of the soil are the major constituents that affect the sorption–desorption behaviour of the pesticides (Wauchope et al. 2002; Das and Mukherjee 2012b; Das et al. 2014). In general, pesticide adsorption is exothermic, with more pesticide being adsorbed at lower temperatures. However, for a specific pesticide, adsorption may increase, decrease or remain unchanged at low soil temperatures (Hamaker and Thompson 1992; Masaki et al. 2006). In this study, the two soils have major differences in pH, clay content, acid oxalate Fe content and Olsen P which may play a significant role in the sorption process. Thus, it is found that there is no published information available regarding the sorption of flubendiamide in soil mainly under Indian subtropical condition. Therefore, there is a need to generate more information on the sorption behaviour of flubendiamide pesticide in red soil and black soil. Therefore, this experiment was designed to evaluate the adsorption and desorption of flubendiamide in two different Indian red soil and black soil varying in physico-chemical properties.
Materials and methods
Soil sample collection and analysis
Soil samples used for this experiment namely red and black soil were collected from experimental farm of CRIDA, Hyderabad (11.01° N, 76.93° E) and experimental farm of TNAU, Coimbatore (17.34° N, 78.50° E) from a depth of 0–15 cm (plough layer) with no history of pesticide application. Samples were collected in polythene sheet, dried in shade, sieved through a 2-mm sieve and stored at hot air oven. Selected physico-chemical properties were analysed with standard methods. Organic carbon was measured using the Walkley and Black method (Black et al. 1965), clay, sand and silt content was measured by employing the Bouyoucos hygrometer (Jackson 1967). Soil pH (1:2.5 H2O), cation exchange capacity [by 1 M NH4OAc (pH 7.0) extraction], Olsen P (by 0.5 M NaHCO3 extraction) were measured by the method of Black and Walkley (Black et al. 1965). Acid oxalate-extractable Fe and aluminium (Al), exchangeable potassium (K) were determined for the initial soil samples by the standard methods of Blakemore et al. (1987). The properties of the collected soils are given in Table 1.
Chemicals
High-purity (98.5 %) flubendiamide was purchased from M/s Bayer Crop Science Limited, New Delhi, India. Other chemical reagents and solvents used were of HPLC grade procured from Ranchem, India. Glassware used was supplied by Borosil, India, and were cleaned with distilled water and dried at 110 °C for 5 h prior to use. The properties of the pesticide are given in Table 2 (USEPA 2008).
Adsorption and desorption study
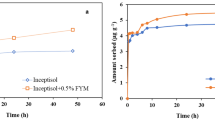
Adsorption study of flubendiamide was carried out using batch equilibration technique in two soil types, namely red soil and black soil and with organic manure-treated soils at concentrations of 0.5, 1.0, 2.0 and 5.0 μg/ml in laboratory condition at room temperature (27 ± 1 °C) in triplicate. The two soil types were treated with 1.0 % organic manure separately. The physicochemical characteristics of the organic manure were a pH of 6.7 and an organic carbon content of 24.6 %. The total carbon, nitrogen and hydrogen contents of manure were determined by elemental analysis and were 24.9, 10.2 and 3.1 %, respectively. Weighed 100 mg of flubendiamide (98.5 % purity) into a 100-ml standard flask and volume was made up using 0.01 M CaCl2. From this stock solution, different concentrations of the test solution were prepared in 0.01 M CaCl2 solution and stored at 4 °C. The CaCl2 (0.01 M) was used as the background electrolyte to keep the ionic strength of the soil solution constant and to facilitate flocculation. For determination of equilibration time, 10 g of soil was taken in a series of centrifuge tubes and 25 ml of known concentration of flubendiamide (0.001, 0.005, 0.01, 0.05, 0.1, 0.5, 1.0 μg/ml) was added and kept shaking for different time intervals (2, 4, 8, 12, 18 and 24 h). The equilibration time was found to be 4.0 h and was used in adsorption studies (Fig. 2). A 10-g (oven dry basis) portion of red soil and black soil each were transferred to 50-ml centrifuge tubes, and 25 ml of the flubendiamide solution (1 μg/ml) was introduced into it. A blank with 25 ml of 0.01 M CaCl2 was used for comparison. The contents were kept shaking for 4 h, and at the end of the equilibrium period, the contents were centrifuged at 2000 rpm for 10 min. The supernatant solution was withdrawn without disturbing the soil and quantified for flubendiamide content as described in the method (see partitioning). The amount of flubendiamide adsorbed was calculated from the difference in the concentration of flubendiamide in initial aqueous solution before equilibrium and supernatant after equilibration. Desorption studies were carried out at three different initial concentrations (0.5, 2.0 and 5.0 μg/ml) of flubendiamide. Three desorption cycles were carried out in triplicate for each concentration. After adsorption, the supernatant was decanted and 25 ml 0.01 M CaCl2 was added to the centrifuge tube. The retained solution in the soil pellet was less than 0.3 ml and was not taken into account in calculation. The contents were thoroughly mixed and care was taken that no soil remained sticking to the tube. These tubes were kept shaking for 4.0 h and centrifuged to obtain a clear supernatant. The supernatant was analysed for flubendiamide content. The amount of insecticide desorbed was calculated by subtracting the amount of insecticide in the entrapped solution after the adsorption experiment from the solution concentration measured after the desorption experiment. Adsorption desorption study were also carried out for control sample (no soil). This information is vital to investigate any losses of the chemical during the test. If found to be significant, they should be accounted in adsorption desorption calculation.
Partitioning and cleanup
The water samples (10 ml) were transferred into a clean 250-ml separatory funnel and extracted with 50 ml of dichloromethane. The contents were shaken vigorously to partition the entire test chemical into dichloromethane. The funnel was kept undisturbed for the layers to separate and the organic layer was collected in an evaporation flask. The aqueous layer was again partitioned with 30 ml of dichloromethane, shaken and the funnel kept undisturbed and collected the organic layer. Combined the collected organic layers and concentrated to near dryness (1–2 ml) in a vacuum rotary evaporator at 40 °C. A glass column was packed with 10 g of silica (30–50 mesh) and washed with 10 ml portions of 5 % methanol in dichloromethane followed by 10-ml portions of acetonitrile. After the column had been conditioned, the sample was redissolved in 1-ml acetone and diluted with 10-ml acetonitrile. The entire sample was then transferred into the column; the sample was eluted with 40 ml of 2 % methanol in acetonitrile. The collected eluate was evaporated to dryness by rotary evaporator. The residue was dissolved in 2 ml of acetonitrile, filtered and analysed by high performance liquid chromatography (HPLC).
HPLC analysis method
Flubendiamide residues were quantified by HPLC system (Merck-Hitachi), consisting of L-7100 (computer operated dual pump), a L-7400 (UV detector) and a L-7200 (Auto sampler), HPLC column (30 cm)-Lichrospher, RP-18. A mixture of acetonitrile–water (70:30, v/v) was used as the mobile phase, with a flow rate of 1.0 ml/min. The injection volume was 10 μl and the wavelength was set at 260 nm ((λ max) determined by using spectrophotometer. At this setting with 260-nm wavelength, the retention time for flubendiamide was 10.1 min. At the limit of detection 0.001 μg/g, the signal to noise ratio maintained was 3:1.
Recovery and detection limits
Different known concentrations of flubendiamide (1.0, 0.2, 0.1, 0.0, 0.005 and 0.001 μg/g) were prepared in acetonitrile by diluting the stock solution. Then 10 μl of standard solution of flubendiamide was injected and measured the peak area. Validation of the method was performed in terms of recovery studies before the analysis of unknown sample. Recovery study was conducted for adsorption study with 10 ml of 0.01 M CaCl2. Using pipette, 1 ml of standard solution of flubendiamide of known concentration was added in 10 g of soil sample uniformly on the surface of the matrix, mixed well before extraction and kept for 4 h. The extraction was performed as described in the methodology. The recovery of flubendiamide is shown in Table 3. The recovery ranged from 82 to 88 % in different substrates; the quantification limit is 0.05 μg/g and the signal to noise ratio is 3:1. No substrate interference was observed at this quantification level as evidenced by the control sample.
Calculation and statistical analysis
For calculating distribution coefficient (K d) the following expression was used: C s = K d C e , where (C s) is concentration of analyte adsorbed onto soil and C e is the equilibrium concentration in solution. Soil organic carbon (OC) and distribution coefficient (K d) is related by the following equation: K OC = 100 K d/% OC.
The sorption isotherm parameters were calculated using the linear form of Freundlich equation; log C s = log K F + 1/n log C e, K F is Freundlich sorption coefficients, and n is a linearity factor.
Desorption isotherms were fitted to the linear form of the Freundlich equation log C s = log K Fdes + 1/n des log C e, where C s is the amount of pesticides still adsorbed (μg/g), C e is the equilibrium concentration of pesticides in solution after desorption (μg/ml), and K Fdes and n des are two characteristic constants of soil. Hysteresis coefficient (H 1) was calculated as the ratio of slope of sorption curve and slope of desorption curve.
Using SAS 9.3, a two-way analysis of variance (ANOVA) was performed by taking the initial concentration of flubendiamide as factor one (with levels 0.5, 1.0, 2.0 and 5.0 μg/ml) and soil types as factor two (viz., red soil, organic manure treated red soil, black soil and organic manure-treated black soil); significant effects were observed. Critical difference (CD) was calculated for comparing initial concentration effects, soil type effects and their interaction.
Results and discussion
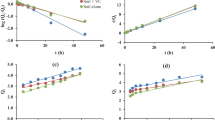
The limit of quantification of flubendiamide in soil was 0.05 μg/g and the limit of detection was 0.01 μg/g (10 μl injection). Recovery percent of flubendiamide in red soil and black soil was 82.0 ± 2.1 and 88.0 ± 2.6 at 5.0 μg fortification levels, respectively (Fig. 1). Capacity of sorption increased with time and within 4.0 h maximum level of adsorption reached in both soils, i.e. 5.60 % was obtained and change in adsorbed amount was negligible as reported by Kumar and Mukherjee 2013. At higher concentration, sorption percentage is lower (Table 4) and is indicated by the 1/n values, which are less than 1.0 (Table 6), indicating that sorption is nonlinear as observed by “L” type isotherm with respect to concentration. This result has been reported for metsulfuron-methyl (Ismail and Ooi 2012). 1/n is the slope and log K F is the intercept of the straight line resulting from the plot of log C s versus log C e as shown in (Figs. 3 and 4). The slope of the isotherm steadily decreases with rise in solute concentration because of vacant sites become less accessible with the progressive covering of the surface (Rhodes et al. 1970). Nonlinear isotherm indicates that a number of available sites for the sorption become a limiting factor, which may be due to the fact that flubendiamide molecules occupy most of the sorption sites at the higher concentrations.
The highest flubendiamide sorption was observed in red soil having K d value that varied between 2.98 and 4.32 and 1.02–1.45 ml/g for red soil and black soil, respectively, and for desorption process, it varied from 32.16 to 59.28 and 20.26–39.23 ml/g for red soil and black soil, respectively (Table 6). The data indicated that clayey soil adsorbed a greater amount of flubendiamide per unit organic carbon; therefore, a higher K oc value was found in soil with high clay content. The soil organic carbon partition coefficient (K oc) value for adsorption process varied between 620.83 and 900.00 and 329.03–467.74 ml/g for red soil and black soil, respectively. On amendment with 1.0 % organic amendment, the K oc value for adsorption process increased for both selected soils and ranged 791.93–1070.96 and 468.29–685.36 ml/g for red and black soil, respectively. The Freundlich coefficient K F for adsorption process were 5.79 and 1.79 ml/g for black soil and red soil, respectively. The K d values for desorption were consistently higher than the corresponding values for adsorption which might have occurred due to hysteresis effect during desorption process that involved various forces that caused higher retention of flubendiamide molecules after desorption than that of adsorption at unit equilibrium concentration. When desorption isotherm has a lesser slope than its respective adsorption isotherm, the hysteresis effects manifest themselves, indicating a partial irreversibility. Occurrence of hysteresis suggests that adsorption of pesticides occurs with a limited degree of reversibility depending upon both soil type and properties of flubendiamide. This also indicates that adsorption process was reversible. The flubendiamide K d values were generally smaller than the K F of adsorption process in both two soil types because of nonlinearity in sorption, as reported in triazine herbicides by Gannon et al. 2013.
Adsorption–desorption studies with 1 % organic manure-treated soil revealed that Freundlich coefficient K F for adsorption process also increased in the two tested soils; 8.80 ml/g (organic manure-treated red soil) and 3.90 ml/g (organic manure-treated black soil) as compared to without amended soil. Data revealed that adsorption of flubendiamide (Table 4) was higher in organic manure-treated soil than without organic manure-treated soil. Similar results were reported by Fernandes et al. (2006). The dynamics of pesticides in soil was greatly affected by the varying physical and chemical properties of the soil, especially the organic matter and clay content as reported by Li et al. (2003), Gajbhiye and Gupta (2001) and Beyer et al. (1996). The increase in percentage of adsorption of flubendiamide in organic manure-treated soils may be related to the adsorption of the flubendiamide to the soil by increasing the available sites for sorption. Zhang et al. (2012) also reported that soil organic matter played critical roles in absorption of organic chemicals in soil ecosystem.
After each desorption step, quantification of amount of flubendiamide desorbed was performed and expressed as a percentage of the amount adsorbed. Based on three consecutive desorption steps, the initial flubendiamide concentration of 0.5, 2.0 and 5.0 μg/ml, which corresponded to a final desorption percentage was 5.11 ± 0.25, 6.05 ± 0.25 and 8.16 ± 0.25 of the initially sorbed flubendiamide amount, respectively, from red soil and 4.65 ± 0.25, 6.84 ± 0.25 and 8.37 ± 0.25 of the initially sorbed flubendiamide amount, respectively, from black soil (Table 5). In organic manure-treated tested soils percent desorption were decreased for both red and black soil at all corresponding concentrations. At the time of three desorption cycle, the initial flubendiamide concentration of 0.5, 2.0 and 5.0 μg/ml corresponding to the total desorption percentage was 4.27 ± 0.25, 5.76 ± 0.25 and 7.45 ± 0.25 of the initially sorbed flubendiamide amount, respectively, from organic manure-treated red soil and 5.11 ± 0.25, 5.86 ± 0.25 and 6.94 ± 0.25 of the initially sorbed flubendiamide amount, from organic manure-treated black soil. For red, black, red with organic manure-treated and black with organic manure-treated soil, average percentage of flubendiamide desorbed for three initial flubendiamide concentrations was 5.86 ± 0.15, 6.62 ± 0.015, 5.82 ± 0.15, and 5.97 ± 0.15, respectively. The Freundlich coefficient values for the desorption process K Fdes were 12.3, 9.29, 5.29 and 7.40 ml/g, for red, organic manure-treated red, black and organic manure-treated black soil, respectively. A nonlinear curve is formed by the value of n des in desorption isotherm. This was used as an index of hysteresis. Scientifically, lower H 1 values indicate increase in difficulty of sorbed analyte to desorb from the matrix (Cox et al. 1997; Drori et al. 2005; Ismail and Kalithasan 2004). The combined effect of increased clay and organic carbon content is responsible for the increased irreversibility of flubendiamide sorption. H 1 values (Table 6) in two tested soil types indicated this and values were 0.035 (red soil) and 0.149 (black soil) and in organic manure-treated soil 0.023 (red soil) and 0.132 (black soil), respectively. This indicates that in organic manure-treated black soil, desorption of flubendiamide was more easy as compared to that in other tested soils. Lower organic carbon content and less clay content in black soil may be the reason for this.
Based on Tukey’s HSD test, statistical analysis showed that all the effects were significant for initial concentration; for soil types and for concentration × soil types interaction. Tukey’s HSD also revealed that maximum adsorption of flubendiamide was observed on organic manure-treated red soil (6.42° ± 0.21) followed by red soil (4.52p ± 0.21), organic manure-treated black soil (4.18M ± 0.21) and black soil (3.55N ± 0.21). According to Turkey’s HSD test, in both 1.0 % organic manure-treated red and black soil adsorption capacity increased significantly. Further, because of organic manure amendment, adsorption capacity of soils has increased due to an increase in binding site. Maximum flubendiamide adsorption was observed on all tested soils with 0.5 μg/g initial concentration (5.53M ± 0.23), and there was a significant difference from the rest of the initial concentrations. Keeping into consideration the interaction between initial concentration and soil type, it was observed that maximum adsorption of flubendiamide occurred at 0.5 μg/ml with treated red soil (7.42T ± 0.34) and is significantly different from the rest of the combinations.
Based on Tukey’s HSD test, statistical analysis of desorption study revealed that all the effects were significant for initial concentration; for soil types and for concentration × soil types interaction. Tukey’s HSD tests also showed that there was minimum desorption of flubendiamide observed on organic manure treated red soil (5.82N ± 0.15), followed by red soil (5.86M ± 0.15), organic manure-treated black soil (5.97P ± 0.15) and, finally, maximum percent desorption found in black soil (6.62O ± 0.15). This may be attributed to more clay content in red soil resulting in more surface area, and it would further be increased by amendment with organic manure. The percent desorption was decreased significantly by organic manure amendment in both selected soils. This test also revealed that there was a highest percent desorption with initial concentration 5.0 μg/ml (8.23M ± 0.08) and minimum percent desorption was observed with initial concentration 0.5 μg/ml (4.29O ± 0.08). Deng et al. (2010) also reported similar results in the case of atrazine desorption.
As adsorption was higher in red soil than black soil, observation was recorded to reveal the effect of pH on adsorption of flubendiamide in red soil (Fig. 5). Adsorption was very minimal at pH 2. With increase in pH from 2 to 6, there was an increase in adsorption of flubendiamide in red soil. From pH 6 to 8, there was a slight reduction in adsorption capacity for the insecticide. But after pH 8 to 10 again adsorption of this pesticide increased. This type of adsorption pattern is rarely seen in pesticide which may be due to their peculiar structure. Thus, pH has greater influence on adsorption process of flubendiamide in red soil. The free energy change during adsorption as influenced by organic matter was calculated using the equation ΔG om = −RT lnK om, where ΔG om is the free energy change (cal/mol), R is gas constant (2.0 cal/mol), K om = K × 100/organic matter (%) and T = Kelvin temperature (298°K). Calculated ΔG om value is −2.45 Kcal/mol for red soil and −2.76 Kcal/mol for black soil (Table 7). The study has shown that the ΔG was higher for black soil than red soil. The negative value of ΔG om suggested that adsorption of flubendiamide on soil is spontaneous and exothermic. Further, relatively low value of ΔG om revealed a more or less physical nature of adsorption on the soil organic matter surface (Jana and Das 1997). In a study conducted by Singh et al. (2001), adsorption–desorption of metalachlor, isoproturon and terbuthylazine in two different soil gave ΔG om values ranging from −2.60 to −3.34. Low values of ΔG om were explained on the basis of entropy generation forces like hydrogen bonding, van der Waals or hydrophobic interactions involved in the adsorption.
Conclusion
Adsorption–desorption studies of flubendiamide indicate that in these two Indian soils this insecticide is weakly sorbed. The adsorption–desorption of flubendiamide is affected by soil properties, adsorption being greater in soils with higher soil organic carbon and clay content. Freundlich sorption coefficient (K d) values were positively correlated with soil organic matter and clay content. Sorption was much stronger at lower concentrations, as the molecule attaches itself to the binding site of the soil particles. In soils having high organic matter content (red soil) or treated with 1.0 % organic manure, a higher amount of flubendiamide residue is retained in soil as compared to soils with low organic matter content (black soil). There was a positive correlation of values of Freundlich sorption coefficient (K d) with organic carbon and clay content. Flubendiamide is predominantly applied to rice, cotton, etc., grown in Indian soils characterized by high organic matter and clay content. Lower desorption of flubendiamide in a more sorptive system indicates that hysteresis is attributed to irreversible binding of the molecules to soil surfaces. As this insecticide is poorly sorbed in the two Indian soil types, there may be a possibility of their leaching to lower soil profiles. Hence, increasing the OM and clay content in the soil can be an alternative solution to prevent the mobility of flubendiamide.
References
Bailey, G. W., & White, J. L. (1964). Review of adsorption and desorption of organic pesticides by soil colloids with implications concerning pesticide bioactivity. Journal of Agriculture & Food Chemistry, 12, 324–332.
Bailey, G. W., White, J. L., & Rothberg, T. (1968). Adsorption of organic herbicides by montmorillonite: role of pH and chemical characters of the adsorbate. Soil Science Society of America, Proceedings, 32, 222–234.
Beyer, L., Ahlsdorf, B., Sorge, C., Schulton, H. R., & Blume, H. P. (1996). Soil organic matter composition and pesticide bonding in sandy soils in relation to groundwater protection in north-west German lower plain. Biology and Fertility of Soils, 23, 266–272.
Black, C. A., Evans, D. D., White, J. L., Ensminger, L. E., & Clark, F. E. (Eds.) (1965). Methods of soil analysis, 2nd edn. In Agronomy monograph 9. Madison: Agronomy Society of America and Soil Science Society of America.
Blakemore, L.C., Searle, P.L., & Daly, B.K. (1987). Methods for chemical analysis of soils. NZ Soil Bureau Scientific Report, series 80. New Zealand: NZ DSIR, 103 p.
Cohen, S.Z., Creeger, S.M., Carsel, R.F., & Enfield, C.G. (1984). Potential pesticide contamination of groundwater from agricultural uses. In R.F. Krueger, & J.N. Seiber (Eds.), Treatment and disposal of pesticide wastes, ACS Symposium Series No. 259 (pp. 297–325). Washington, DC: American Chemical Society.
Cox, L., Koskinen, W. C., & Yen, P. Y. (1997). Sorption–desorption of imidacloprid and its metabolites in soils. Journal of Agricultural and Food Chemistry, 45, 1468–1472.
Das, S. K., & Mukherjee, I. (2011). Effect of light and pH on persistence of flubendiamide. Bulletin of Environmental Contamination and Toxicology, 87, 292–296.
Das, S. K., & Mukherjee, I. (2012). Effect of moisture and organic manure on persistence of flubendiamide in soil. Bulletin of Environmental Contamination and Toxicology, 88, 515–520.
Das, S. K., & Mukherjee, I. (2014). Influence of microbial community on degradation of flubendiamide in two Indian soils. Environmental Monitoring and Assessment, 186, 3213–3219.
Das, S. K., Mukherjee, I., & Das, S. K. (2012). Dissipation of flubendiamide in/on Okra [Abelmoschus esculenta (L.) Moench] Fruits. Bulletin of Environmental Contamination and Toxicology, 88, 381–384.
Das, S. K., Avasthe, R. K., Singh, R., & Babu, S. (2014). Biochar as carbon negative in carbon credit under changing climate. Current Science, 107, 1090–1091.
Deng, J., Jiang, X., Hu, W., & Hu, L. (2010). Quantifying hysteresis of atrazine desorption from a sandy loam soil. Journal of Environmental Sciences, 22, 1923–1929.
Drori, Y., Aizenshtat, Z., & Chefetz, B. (2005). Sorption–desorption behavior of atrazine in soils irrigated with reclaimed wastewater. Soil Science Society of America Journal, 69, 1703–1710.
Ebbinghaus-Kintscher, U., Lummen, P., Raming, K., Masaki, T., & Yasokawa, N. (2007). Flubendiamide, the first insecticide with a novel mode of action on insect ryanodine receptors. Pf lanzenschutz-Nachrichten Bayer, 60, 117–140.
Fernandes, M. C., Cox, L., Hermosin, M. C., & Cornejo, J. (2006). Organic amendments affecting sorption, leaching and dissipation of fungicides in soils. Pest Management Science, 62, 1207–1215.
Gajbhiye, V. T., & Gupta, S. (2001). Adsorption-desorption behavior of flufenacet in five different soils of India. Pest Management Science, 57, 633–639.
Gannon, T. W., Hixson, A. C., Weber, J. B., Shi, W., Yelverton, F. H., & Rufty, T. W. (2013). Sorption of simazine and S-metolachlor to soils from a chronosequence of turf grass systems. Weed Science, 61, 508–514.
Gustafson, D. I. (2002). Groundwater ubiquity score: a simple method for assessing pesticide leachability. Environmental Toxicology and Chemistry, 8, 339–357.
Hamaker, J. W., & Thompson, J. M. (1992). Adsorption. In C. A. I. Goring & J. W. Hamaker (Eds.), Organic chemical in the soil environment (pp. 49–144). New York: Marcel Dekker Inc.
Helling, C. S., Kearney, P. C., & Alexander, M. (1971). Behavior of pesticides in soils. Advances in Agronomy, 23, 147–240.
Ismail, B. S., & Kalithasan, K. (2004). Adsorption, desorption and mobility of permethrin in Malaysian soils. Journal of Environmental Science and Health. Part. B, 39, 419–429.
Ismail, B. S., & Ooi, K. E. (2012). Sorption, desorption and mobility of metsulfuron-methyl in soils of the oil palm agro ecosystem in Malaysia. Journal of Environmental Biology, 33, 573–577.
Jackson, M. L. (1967). Soil chemical analysis. New Delhi: Prentice Hall.
Jana, T. K., & Das, B. (1997). Sorption of carbaryl (1-Naphthyl N-methyl carbamate) by soil. Bulletin of Environmental Contamination and Toxicology, 59, 65–71.
Khoury, R., Geanchan, A., Corte, C. M., Cooper, J. F., & Bobe, A. (2003). Retention and degradation of metribuzin in sandy loam and clay soils of Lebanon. Weed Research, 43, 252–259.
Kumar, N., & Mukherjee, I. (2013). Effect of soil physicochemical properties on adsorption of tricyclazole. International Journal of Agriculture and Food Science Technology, 4, 391–396.
Li, H., Sheng, G., Teppan, B. J., Johnston, C. T., & Boyd, S. A. (2003). Sorption and desorption of pesticides by clay minerals and humic acid-clay complexes. Soil Science Society America Journal, 67, 122–131.
Masaki, T., Yasokawa, N., Tohnishi, M., Motoba, K., & Hirooka, T. (2006). Flubendiamide: a novel Ca+2 channel modulator, reveals evidence for functional cooperation between Ca+2 pumps and Ca+2 release. Molecular Pharmacology, 69, 1733–1739.
Murphy, E. M., Zachara, J. M., Smith, S. C., & Phillips, J. L. (1992). The sorption of humic acids to mineral surfaces and their role in contaminant binding. Science of Total Environment, 117, 413–423.
Rhodes, R. C., Belasco, I. J., & Pease, H. L. (1970). Determination of mobility and sorption of agrochemicals on soils. Journal of Agricultural and Food Chemistry, 18, 524–527.
Singh, N., Kloeppel, H., & Klein, W. (2001). Sorption behavior of metolachlor, isoproturon and terbuthylazine in soils. Journal of Environmental Science and Health. Part. B, 36, 397–407.
Spark, K. M., & Swift, R. S. (2002). Effect of soil composition and dissolved organic matter on pesticide sorption. Science of Total Environment, 298, 147–161.
USEPA. (2008). Pesticide fact sheet, flubendiamide. Office of prevention, pesticides and toxic substances. http://www.epa.gov/opprd001/factsheet/flubendiamide.pdf. Accessed on 1st July 2010.
Wauchope, R. D., Yeh, S., Linders, J. B., Kloskowski, R., Tanaka, K., Rubin, B., Katayama, A., Kordel, W., Gerstl, Z., Lane, M., & Unsworth, J. B. (2002). Pesticide soil sorption parameters: theory, measurement, uses, limitations and reliability. Pest Management Science, 58(5), 419–445.
Zhang, J., Yang, L., Wei, L., Du, X., Zhou, L., Jiang, L., Ding, Q., & Yang, H. (2012). Environmental impact of two organic amendments on sorption and mobility of propachlor in soils. Journal of Soils Sediments, 12, 1380–1388.
Acknowledgments
The authors thank the head of the Division of Agricultural Chemicals, IARI, New Delhi, India, for providing the facilities to carry out this work (contribution no. 1066).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Das, S.K., Mukherjee, I. & Kumar, A. Effect of soil type and organic manure on adsorption–desorption of flubendiamide. Environ Monit Assess 187, 403 (2015). https://doi.org/10.1007/s10661-015-4623-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4623-2