Abstract
Fipronil, {5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-4-[(trifluoromethyl)sulfinyl]-1H-pyrazole-3-carbonitrile} is commonly applied to soil to protect structures against termite infestations. The fate and bioavailability of fipronil in soil is dependent upon the variability of sorption processes and will differ from soil to soil. Adsorption of fipronil to three Nebraska soils with varying organic matter (OM) content was determined. At the concentrations tested (0.025, 0.1, 0.25, 0.5, 1.0, 1.5, and 2.0 mg L−1), adsorption curves showed constant partitioning of fipronil to the soil matrices (r 2 = 0.998 − 0.999). Calculated organic carbon partitioning coefficients (K oc) ranged from 244 to 628 with an average K oc of 396. Reported K d and K f values increased with increasing organic matter content. Desorption hysteresis was observed as fipronil has a propensity to stay in the adsorbed state. After five soil washes with 0.003 M CaCl2, ~30% of adsorbed fipronil residues were desorbed. Reported K oc values for fipronil suggests that it has intermediate mobility in the field collected soils utilized in this study.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
A common method for controlling subterranean termites involves the creation of a subsoil chemical barrier around and under a structural foundation (Kamble 2006). Fipronil [Termidor® Soluble Concentrate (SC)] is one of the most commonly used liquid termiticides to control subterranean termites. Fipronil, a broad-spectrum phenyl pyrazole insecticide, interferes with the passage of chloride ions through gamma-aminobutyric acid (GABA) regulated chloride ionophore thereby disrupting inhibitory post synaptic neuron activity, resulting in uncontrolled central nervous system function, and eventually causing death (Cole et al. 1993; Tingle et al. 2003). Fipronil is slightly water soluble with solubility ranges from 1.9 to 2.4 mg L−1 at 20°C (Hainzl and Casida 1996; Tingle et al. 2003). Fipronil degrades to its major metabolites by reduction to sulfide, oxidation to sulfone, hydrolysis to amide, and photolysis to des-sulfinyl (Hainzl and Casida 1996). The des-sulfinyl photodegradate is extremely stable and actually more toxic than the parent compound (USEPA 1998). Fipronil, as an aqueous solution, is stable to hydrolysis in mildly acidic to neutral water under dark conditions and at a constant ambient temperature (USEPA 1996).
The propensity of an organic pesticide to bind, or sorb to soil solids is dependant on a number of factors that include: the physical and chemical characteristics of the pesticide; nature of the soil solution; and composition of the soil (Comfort et al. 1994). Generally, pesticides will continuously sorb and desorb as it leaches downward through the soil profile. The characteristics of the chemical (i.e., solubility, size, hydrophobicity, presence of charged or uncharged functional groups, etc.) and the soil (i.e., particle type, level of organic matter, pH, CEC, etc.) are key factors into the likelihood of sorption and to the degree that it occurs. According to Comfort et al. (1994), adsorption strength is inversely related to the solubility of the pesticide and directly related to its partitioning coefficient (K d ). Pesticides are generally adsorbed by the organic matter (OM) fraction in soils which acts like a non-polar “oil-like” film that coats the surface of soil particles (Comfort et al. 1994). Non-polar pesticides, such as fipronil, seek to avoid the polar soil solution and become sequestered in organic matter.
A common method for determining pesticide adsorption is by conducting laboratory experiments that combines a known amount of soil with a pesticide solution. A number of mathematical expressions have been developed to describe the adsorption of a pesticide to soil, referred to as isotherms (Watts 1998). The Langmuir and Freundlich equilibrium-based adsorption models are two types of isotherms that are commonly used. The shape of the curves expressed in these models can be described by four general isotherm types (S, L, H, and C) (Sparks 2003). Each of the different isotherms can lend insight into the nature of the pesticide/soil interaction.
A number of studies on soil sorption of fipronil have been published (Bobé et al. 1997; Ying and Kookana 2001; Kamble and Saran 2005; Doran et al. 2006; Mukherjee and Kalpana 2006; Masutti and Mermut 2007). However, results vary as different soils were used and testing methods differ. Soils from different geographic locations have distinct physical and chemical properties thus altering the behavior and fate of fipronil. Therefore, data on the behavior of fipronil in different soils are important. To better understand the specific behavior of fipronil in soils from our geographic region we established the following objectives: (1) determine fipronil adsorption in soils with varying characteristics; and (2) determine desorption of fipronil after repeated soil washings.
Materials and Methods
BASF Corp. (Research Triangle Park, NC) provided 14C-fipronil {5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-4-[(trifluoromethyl)sulfinyl]-1H-pyrazole-3-carbonitrile} in toluene (CAS No. 120068-37-3). The specific activity was 77.03 μCi per mg with a purity of 99.4% (Batch No. 849-0101).
Three soils were collected from Cumming and Lancaster Counties in Nebraska targeted for having various characteristics. Soil samples were analyzed by the Soil and Plant Analytical Laboratory at the University of Nebraska. The three soils designated SL (sandy loam), SCL-1 (silty clay loam), and SCL-2 (silty clay loam) (classification, USDA 1993). Soils were passed through a 2-mm sieve and autoclaved (1.25 h @ 120°C and 1 atm) prior to use.
Fipronil working solutions were prepared with the target concentrations of 0.025, 0.1, 0.25, 0.5, 1.0, 1.5, and 2.0 mg L−1. CaCl2 (0.003 M) was spiked with 14C-fipronil in ≥ 99.9% MeOH (Sigma–Aldrich, St. Louis, MO) to achieve the desired target concentration. Methanol accounted for 0.8% of the aqueous solution for all concentrations tested. Approximately 5 g soil (dry wt. equivalent) was measured into individual 50 mL Nalgene® 50 mL Teflon® FEP centrifuge tubes. Fipronil working solution was added in the ratio of 1 part soil to 1.8 parts fipronil solution. Tubes were agitated for 15 h in an incubator shaker (New Brunswick Sci. Co. Inc., Edison, NJ) at 250 rpm. The temperature was held constant at 22.0 ± 1.0°C. Control tubes containing no soil were treated with the same fipronil working solutions and experimental conditions. All soil by fipronil stock concentrations were replicated 3 times.
After shaking, tubes were centrifuged in an Eppendorf 5804 Centrifuge (Eppendorf, Westbury, NY) for 10 min at 5,000 rpm. The clear supernatant phase was tested for remaining 14C-fipronil concentrations. A 0.5 mL sample was extracted from each tube and combined with 6 mL UltimaGold™ Scintillation Cocktail (Perkin Elmer, Inc., Waltham, MA) and directly analyzed by liquid scintillation counting (LSC) in a 1209 Rackbeta liquid scintillation counter (LKB Wallac Inc., Gaithersburg, MD).
Desorption of fipronil was evaluated using the SCL-2 soil type. After adsorption analysis the supernatant was removed and replaced with 0.003 CaCl2. Samples in tubes were again shaken using incubator shaker at 250 rpm for 12 h. After shaking the analysis of samples was conducted similarly to sorption procedures. These procedures were repeated 5 times over 60 h.
At the conclusion of the desorption soil washes, remaining fipronil residues in the soils were extracted using methanol. After removing the supernatant 10 mL of MeOH were added to each tube containing soil and shaken for 12 h. Similar methods of analysis were used to determine the amount of extracted fipronil present in methanol. The mass balance for the desorption experiment are reported in Table 3. For the purposes of this study all detectable residues were assumed to be parent fipronil molecules.
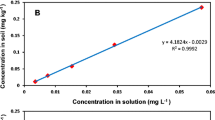
The adsorption isotherms were constructed by plotting the equilibrium concentration of the supernatant phase (C eq in mg L−1) against the concentration adsorbed to the soil (C ads mg Kg−1). Both freundlich and linear curves were fitted to the data. The Freundlich isotherm is quantified by (Watts 1998):
where C ads = concentration of fipronil sorbed on the soil (mg kg−1), C eq = equilibrium concentration of fipronil in solution (mg L−1), K f = Freundlich sorption coefficient, 1/n = slope.
In curves where the slope = 1, the soil distribution coefficient (Kd) is expressed as follows:
The most common sorbent in soils is organic matter (OM) (Watts 1998). As a result K d values which are specific for a particular soil type can be normalized by conversion to the organic carbon partitioning coefficient (K oc). First the percent OM is converted to percent organic carbon (OC) using the Van Bemmelen factor of 1.724. Conversions are as follows:
Desorption curves were plotted similarly to adsorption data and differences reported as % fipronil recovery. Regression analysis for adsorption curves was completed using SigmaPlot® (Systat Software Inc. 2004, Chicago, IL).
Results and Discussion
Soils used in this study are representative of those found in Nebraska. Properties for each soil are reported in Table 1. We used fipronil dilutions at or below the solubility limit of fipronil in water. Solutions above the solubility limit would give inaccurate sorption results as soil bound and precipitated residues may not be distinguished. Kamble and Saran (2005) published fipronil isotherm data using fipronil concentrations (600, 950, 1,250 mg L−1) which are much higher than its solubility; however, fipronil working solutions were prepared from formulated product which can greatly increase solubility of the compound. Bobé et al. (1997) also used fipronil concentrations higher than solubility but this issue was remedied by increasing the organic solvent concentration in the working solutions.
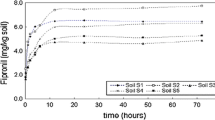
Sorption isotherms are presented in Fig. 1. Soil partition coefficient (K d ) and Freundlich coefficients (K f ) are presented in Table 2. Freundlich curve n values are all near 1 meaning that they are exhibiting a C type isotherm, or constant partitioning. Giles et al. (1960) defines the C type curve as constant partitioning of the solute molecule between the solvent (soil solution) and substrate (soil matrix). The linearity shows that sorption sites in the soil matrices have not yet become limiting factors. According to Giles et al. (1960) the C type curve will exhibit this behavior until sorption sites have been exhausted causing an abrupt shift in the curve resulting in a horizontal plateau. Reported n values ranged from slightly below 1.0 (0.94 ± 0.01) to 1.0 (1.02 ± 0.03). Values of n < 1.0 are represented by L type isotherms. L type curves reflect decreasing sorption site availability with an increase in solute concentration. This sorption pattern could be explained by high affinity for the solute molecules at low concentrations with decreasing affinity as concentration increase (Sparks 2003). Our reported K f and K d values from Freundlich and linear curves are similar supporting the C type isotherm.
Bobé et al. (1997) reported the factors influencing fipronil sorption in soils. In that study, S type isotherms were reported using two soils with low organic matter content (0.1%–0.3%); however, a C type isotherm was reported using a soil with higher organic matter (6.5%). Temperature and methanol cosolvent amount influenced fipronil adsorption in isotherms with increasing K f coeffiecients reported at increasing temperatures and decreasing coefficients with increasing methanol content (Bobé et al. 1997). Mukherjee and Kalpana (2006) reported fipronil adsorption as an S type curve. The initial slope of this curve is indicative of increasing adsorption with increased solute molecule concentration (Giles et al. 1960). Ying and Kookana (2001) reported S or C type isotherms for fipronil in soils from South Australia. Kamble and Saran (2005) reported fipronil sorption as an L type curve at termiticide concentrations in soils collected around urban structures in Nebraska. Doran et al. (2006) also reported L type isotherms with the possibility of a C type in one soil.
In the current study, increasing trends of Kd and Kf values in soils with increasing organic matter are reported (Table 2). SCL-1 and SCL-2 soils had nearly similar pH, sand, and clay content (Table 1) leaving OM as the main difference. Ying and Kookana (2001) reported that sorption of fipronil was better correlated with soil organic carbon than with soil clay content. Fipronil K oc values were calculated from reported K d coefficients (Table 2). The K oc values ranged from 244 to 628 with an average K oc of 396 putting it into the class of having intermediate mobility (Comfort et al. 1994). A wide range of fipronil K oc values have been reported in the literature. Tingle et al. (2003) reports a fipronil K oc average of 727 (range, 427–1248). K oc coefficients of 1,568 and 3,725 were reported for fipronil applied as a formulated product; whereas, the K oc for technical grade fipronil was 292 and 320 in two different soils (Doran et al. 2006). Other reported K oc coefficients include a range of 3,787–11,935 (Kamble and Saran 2005) and 542–1,176 (Ying and Kookana 2001).
Fipronil desorption curves are reported in Fig. 2. At all initial fipronil concentrations desorption was less than adsorption. The lesser slope of desorption curves indicates a non-singularity, or hysteresis effect (Bowman and Sans 1985). This hysteresis effect may indicate a partial irreversibility of fipronil from its adsorbed state. The difficulty of fipronil to desorb from the soil matrix is likely due to the hydrophobic interaction between soil and organic matter (Mukherjee and Kalpana 2006). Fipronil desorption hysteresis has also been reported in the literature (Mukherjee and Kalpana 2006; Masutti and Mermut 2007). After 5 cycles of soil washing with 0.003 CaCl2 an average of 30.24 ± 0.56% fipronil desorbed from the soil. Fipronil desorption decreased at each successive cycle with 7.63 ± 0.23% desorbing during the first soil wash and 4.63 ± 0.09% during the final wash. Masutti and Mermut (2007) reported 12%–19% fipronil desorption from various soils. Data presented in Fig. 3 depicts the cumulative recovery of fipronil at each concentration of adsorbed fipronil for each desorption cycle. The trend indicates that slightly higher desorption (% fipronil recovery) was observed at the lower adsorbed fipronil starting concentrations.
The mass balance of recoverable fipronil residues is reported in Table 3. Approximately 70% of fipronil remained bound to soil after 5 successive washes with 0.003 M CaCl2 solution. Overall recovery of fipronil residues ranged from 96.24% to 102.6%. Recovery of 14C-fipronil from control tubes ranged from 91.2% to 102.4%.
To accurately describe adsorption to soil we used concentrations at or below fipronil solubility. Cosolvents can be used to model soil sorption at higher working solution concentrations. However, the use of cosolvents generally results in lower K d and K f coefficients (Bobé et al. 1997; Ying and Kookana 2001). In the current study, it was necessary to use 14C-fipronil in methanol to spike working solutions. Low amounts of methanol (<1.0%) were applied to lessen its impact on sorption coefficients.
The use of fipronil at termiticide rates may cause precipitation of the compound due to low solubility. Resulting in a higher “apparent” K oc value which is likely what was observed by Kamble and Saran (2005). However, when applying liquid soil termiticides at label rates this phenomenon may occur. Additionally, precipitated compounds could potentially remain more bioavailable to soil insects as they may remain unbound, at least initially, to soil or the organic matter fraction.
References
Bobé A, Coste CM, Cooper JF (1997) Factors influencing the adsorption of fipronil in soils. J Agric Food Chem 45:4861–4865
Bowman BT, Sans WW (1985) Partitioning behavior of insecticides in soil-water systems: II. Desorption hysteresis effects. J Environ Qual 14:270–273
Cole LM, Nicholson RA, Casida JE (1993) Action of phenyl pyrazole insecticide at the GABA-gated chloride channel. Pestic Biochem Physiol 46:1–47
Comfort SD, Shea PJ, Roeth FW (1994) Understanding pesticides and water quality in Nebraska. Nebraska Cooperative Extension EC 94-135. University of Nebraska, Lincoln, NE 68583, p 16
Doran G, Eberbach P, Helliwell S (2006) The sorption and degradation of the rice pesticides fipronil and thiobencarb on two Australian rice soils. Aust J Soil Res 44:599–610
Giles CH, MacEwan TH, Nakhwa SN, Smith D (1960) Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J Chem Soc 3:3973–3993
Hainzl D, Casida JE (1996) Fipronil insecticide: novel photochemical desulfinylation with retention of neurotoxicity. Proc Natl Acad Sci USA 93:12764–12767
Kamble ST (2006) Termites. Nebraska Cooperative Extension, NebGuide 1062-A, University of Nebraska, Lincoln, NE 68583, p 4
Kamble ST, Saran RK (2005) Effect of concentration on the adsorption of three termiticides in soil. Bull Environ Contam Toxicol 75:1077–1085
Masutti CSM, Mermut AR (2007) Sorption of fipronil and its sulfide derivative by soils and goethite. Geoderma 140:1–7
Mukherjee I, Kalpana (2006) Sorption of fipronil in tropical soils. Bull Environ Contam Toxicol 76:334–340
Sparks DL (2003) Environmental soil chemistry, 2nd edn. Academic Press, San Diego
Systat Software Inc (2004) SigmaPlot® 9.0 users guide. Systat Software Inc, Point Richmond
Tingle CCD, Rother JA, Dewhurst CF, Lauer S, King WJ (2003) Fipronil: environmental fate, ecotoxicology, and human health concerns. Rev Environ Contam Toxicol 176:1–66
USDA (1993) Soil survey manual. USDA handbook. No. 18. USDA, Washington
USEPA (1996) Fipronil pesticide fact sheet. EPA 737-F-96-005. USEPA, Washington DC, USA, p 7
USEPA (1998) Fipronil for use on rice (Regent®, Icon®) and pets (Frontline®). HED Risk Assessment. Office of Prevention, Pesticides and Toxic Substances, USEPA, Washington DC, p 90
Watts RJ (1998) Hazardous wastes: sources, pathways, receptors. John Wiley, New York
Ying GG, Kookana RS (2001) Sorption of fipronil and its metabolites on soils from South Australia. J Environ Sci Health B36:545–558
Acknowledgments
The authors thank BASF Corp. for providing the 14C-fipronil used in this study. We are grateful to Dr. Steven D. Comfort for his assistance in conducting this research. This article is published as contribution no. 1300, Department of Entomology-Institute of Agriculture and Natural Resources, University of Nebraska, Lincoln.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spomer, N.A., Kamble, S.T. Sorption and Desorption of Fipronil in Midwestern Soils. Bull Environ Contam Toxicol 84, 264–268 (2010). https://doi.org/10.1007/s00128-009-9915-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-009-9915-1