Abstract
Ashes produced during on-farm burning of crop residues are mixed in soil before sowing next crop and studies suggest that they can enhance adsorption of pesticides in soil. Enhanced adsorption of pesticides in soil can reduce their availability for degradation and pest/weed control. Therefore, present study evaluated effect of sugarcane trash ash (STA) on degradation of atrazine (used to control annual grasses and many broad-leaved weeds) and fipronil (used for control of early shoot borer and termites) in three sugarcane growing soils and bioavailability of atrazine. Three sugarcane-growing soils (silty clay loam, sandy clay loam and loam) were used to study effect of 0.1 and 0.2% STA on atrazine and fipronil degradation in laboratory incubated soils. Field study for effect of STA on atrazine degradation was performed in silty clay loam soil. Effect of STA on atrazine bioavailability was assayed using its effect on mustard seed germination. Pesticides and their metabolites were quantified using high-performance liquid chromatography (HPLC)/ gas–liquid chromatography (GLC)/ liquid chromatography-mass spectroscopy (LC–MS/MS). Under laboratory condition 0.2% STA doubled atrazine half-life (t1/2) in sandy clay loam, increased t1/2 by 40% in silty clay loam, while no significant effect was observed in loam soil. The STA did not affect fipronil degradation in all three soils. Metabolites, hydroxyatrazine and deethylatrazine (atrazine) and sulfide, sulfone, amide and desulfinyl (fipronil) were detected in soil samples; but their spectrum and quantities varied with the soil type. The t1/2 of atrazine under field condition was 3.61, 2.73 and 2.86 days in control, 0.1% and 0.2% STA-mixed silty clay loam, respectively. The STA reduced atrazine availability suggesting that higher amount of herbicide will be needs for the desired effect in STA-mixed soils. This study has relevance in assessing fate and bioavailability of atrazine and fipronil in sugarcane soils where trash is burnt year after year and mixed in soils. The STA mixing might have cumulative effect due to regular burning of trash.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
On-farm burning of residue left after crop harvest by farmers is convenient, cost effective and less labor-intensive way to get rid of huge quantities of waste biomass (Jain et al. 2014; França et al. 2012). Pre-harvest and post-harvest burning of sugarcane or trash is commonly practice world wise in sugarcane-growing countries. Pre-harvest burning is done to make manual harvest easier and problem is more serious in Brazil that is the world’s largest producer of sugarcane. Post-harvest burning of trash is prevalent in India and sugarcane is third most important crop, after rice and wheat, whose residues are burnt in fields. State of Uttar Pradesh is the greatest contributor to burning of sugarcane trash followed by Karnataka (Jain et al. 2014). Post-harvest burning is done as trash resists biodegradation, is highly indigestible as fodder, voluminous in nature and difficult to transport. Although, mulching of chopped trash is advised to avoid burning in field as burning results in loss of soil organic matter, nutrients in the trash and causes environmental pollution.
The ashes produced are alkaline in nature and may change physico-chemical properties of soils. Reports suggest that ashes decrease soil bulk density and increase soil porosity and water holding capacity (Pandey and Singh 2010). Additionally, ashes, due to presence of alkaline metal oxide, can have agronomic benefits like regulation of pH of acidic soils and better soil nutrient status which resulted in better plant growth due to increased availability of nutrients (Trivedi et al. 2016; Basu et al. 2009). Further, ashes contain fair quantities of highly porous and have high surface area carbon residues; therefore, exhibited significant potential to adsorb pesticides (Yadav and Singh 2020; Kumar et al. 2019a, b; Deokar et al. 2016a, b, c). Thus, ash mixing in soils affected adsorption, leaching and degradation of pesticide applied for pest control (Kumar and Singh 2020a; Singh et al. 2013; Hillier et al. 2009, 2013; Sheng et al. 2005; Yang and Sheng 2003). This is attributed to reduce availability of pesticide in soil solution due to higher retention of pesticides in ash-mixed soils (Yadav and Singh 2021; Kumar et al. 2019a, b; Zhang et al. 2005). Zhang et al. (2005) reported that wheat residue chars enhanced initial degradation of benzonitrile and attributed it to higher cell density in the presence of the char nutrients; however, degradation slowed down in second phase due to adsorptive inhibition. Few reports suggested substantial reduction in diuron and MCPA degradation in presence of ash (Hiller et al. 2009; Yang et al. 2006). Loganathan et al. (2009) reported inhibition in atrazine degradation by Pseudomonas sp., where 14CO2 production was reduced by 11% in the char-mixed soil. Ash amendments resulted in lesser leaching of alachlor (Giori et al. 2014), sulfosulfuron and pretilachlor (Kumar and Singh 2020b). However, all these studies were performed at very high ash levels that are not realistic in field condition.
Atrazine and fipronil are commonly used pesticides in sugarcane cultivation in India. Atrazine is a selective, systemic, pre- and post-emergent herbicide used to control annual grasses and many broad-leaved weeds in sugarcane (Turner 2015). Fipronil is a broad-spectrum insecticide of phenylpyrazole group and is used for control of early shoot borer and termites in sugarcane. Burning sugarcane trash in fields generates a significant amount of sugarcane trash ash (STA) that is mixed in the soils. Recently, authors reported that STA (0.1 and 0.2%) enhanced adsorption of both pesticides in three sugarcane-growing soils; although effect varied with soil type and STA content (Yadav and Singh 2021). The Freundlich adsorption coefficient (Kf) values were increased by 135–395% for fipronil and 143–241% for atrazine. Thus, higher adsorption of pesticide in STA-mixed soils will reduce its concentration in soil solution that is available for degradation and biological action. Less availability of pesticide in soil solution will reduce it degradation by microbial community and will increase the persistence of pesticide. However, ashes are reported to increase soil pH and it has been reported to change soil microbial population and community composition (Bang-Andreasen et al. 2017); thus, can increase degradation of pesticides, especially atrazine, which is prone to microbial degradation (Govantes et al. 2009). Further, ashes can enhance surface catalyzed chemical degradation of pesticides, therefore, might result in faster degradation of pesticides in ash-mixed soils (Kumar et al. 2019a, b). However, no information is available on the effect of STA on degradation behaviour of atrazine and fipronil in Indian soils, where sugarcane residues are mainly burnt on fields. Therefore, the present study reports the effect of STA on degradation and bioavailability of atrazine and fipronil in three sugarcane soils of Northern India.
Materials and Methods
Chemicals
Analytical grade atrazine (98.9%) and fipronil (97.5%) were purchased from Sigma-Aldrich, India while metabolites, deethylatrazine, deisopropylatrazine and hydroxyatrazine were obtained from Fluka Analytics, Australia. Fipronil metabolites sulfone, sulfide, amide and desulfinyl were synthesized by methods earlier reported by Chatterjee and Gupta (2010).
Soils
Three soils from sugarcane growing regions of the state of Uttar Pradesh namely, the silty clay loam (Indian Institute of Sugarcane Research, Lucknow, 26.56° N and 80.52° E), the sandy clay loam (G.B. Pant University of Agriculture and Technology, Pantnagar, 29° N and 79° E) and the loam (Baraut, 29.10° N, 77.26° E) were used in the study. Soils were collected from the surface 0–15 cm, air-dried and stored at room temperature. Soils were characterized for physicochemical characteristics using standard analytical methods (Table 1).
Sugarcane Trash Ash (STA)
The sugarcane trash ash (STA) used in this study was reported earlier [10]. The STA was characterized for pH, organic carbon (OC), electrical conductivity (EC), specific surface area (SSA) and pore volume (Quantachrome NOVA 10.01, Quantachrome Instruments, Florida, USA). The properties of the STA were: pH 10.5, OC—8.21%, EC—7.01 mS m−1, surface area—27.23 m2 g−1, porosity—0.0596 cm3 g−1.
Degradation Studies
Laboratory studies were performed by taking soil (100 g) in sterilized flasks (250 mL), thoroughly mixed with STA (0, 0.1 and 0.2%) and sterile distilled water to attain 60% water holding capacity and incubated for 10 days at 27 ± 1 °C for stabilization. Treatments that were named as: (1) Control (2) STA (0.1%) (3) STA (0.2%). Samples, in triplicate, were treated with atrazine (2 µg g−1) or fipronil (1 µg g−1) in 0.1 mL acetone while untreated samples served as control. The soil samples were incubated at 27 ± 1 °C and water lost during incubation was supplemented every week (weight basis). The soil samples were withdrawn at regular intervals for atrazine/fipronil residues extraction and analysis by high-performance liquid chromatography (HPLC)/ gas–liquid chromatography (GLC)/ liquid chromatography-mass spectroscopy (LC–MS/MS).
Effect of STA on atrazine degradation was studied under field conditions in the silty clay loam soil at Indian Institute of Sugarcane Research, Lucknow. The experiment was conducted in July 2019, 1.5 × 1.0 m2 plot size, in triplicate using randomized block design. The STA was broadcasted in fields at 20 and 40 t ha−1 (equivalent to 0.1 and 0.2% STA). Plot without STA served as control. After STA application, plots were irrigated and 5–7 two budded setts (CoLK 09204) were planted. After 8-10 h, atrazine (Attari, 50% WP) was sprayed @ 2 kg ha−1 by dissolving required amount of formulation in 900 mL water. Plots were irrigated as and when required. Soil samples were drawn randomly from 0 to 15 cm depth using tube auger from 6 to 7 spots in each plot at different time intervals and analyzed for pesticide residues.
Bioavailability Studies
Effect of STA on atrazine availability was determined by observing its effect on mustard seedling. Mustard [Brassica juncea (L.) Coss] was selected as a test plant as it was sensitive to atrazine. The plastic pots (3.3 cm i.d. × 4.5 cm length) were filled with 50 g of silty clay loam soil mixed with 0, 0.1 and 0.2% STA. Atrazine concentration of 1, 1.5 and 2 µg g−1 were applied while untreated soil/soil + STA mixtures served as control. Treatments were replicated three times. Mustard seeds (5), soaked overnight in water, were sown in each pot, kept in laboratory under light condition at room temperature and watered as and when required. Ten days after sowing mustard seedling were uprooted and roots were washed with water to remove soil particles and root and shoot length measured.
Extraction and Analysis
Soil (5 g) was taken in 50 mL glass tube and distilled water (4 mL), 2-3 g anhydrous sodium sulphate and 10 mL ethyl acetate were added. The samples were shaken for 2 h using horizontal shaker. After shaking samples were centrifuged at 5000 rpm for 5 min and 1 mL of ethyl acetate fraction was transferred to a test tube and solvent evaporated. After evaporation, residue was re-dissolved in 1 mL of acetonitrile for atrazine/metabolite analysis by high-performance liquid chromatography (HPLC) or liquid chromatography-mass spectroscopy (LC–MS/MS) (Kumari et al. 2021) and 1 mL hexane for fipronil/metabolite analysis by gas chromatography (GC, Varian CP-3800) (Chatterjee and Gupta 2010).
Bacteria and Fungus Population Counts in Soil, Without and with STA
Population of bacteria and fungi, in terms of colony-forming unit (CFU), was studied in silty clay loam soil mixed with 0, 0.1 and 0.2% STA by serial dilution method. Soil samples: (1) Control (2) STA (0.1%) (3) STA (0.2%) were prepared as mentioned earlier 2.4 and CFU were counted on 10th day. After 10 days, soil sample (0.1 g) from each treatment was suspended in 0.9 mL sterilized phosphate buffer saline (PBS). Further, suspension was serially diluted using PBS to get dilutions up to 10–5 and 50 µL of these dilutions, in duplicate, were plated on tryptone soya agar medium (tryptone soya broth—0.6 g, agar—15 g, antifungal agent Delvo-cid—100 mg, water—1 L) for bacteria and potato dextrose agar medium (potato-dextrose broth—24 g, agar—15 g, water—1 L) for fungi. The plates were incubated in an incubator at 27 ± 1 °C and after 48–72 h of incubation, the colonies were counted and expressed as CFU per gram soil.
Results and Discussion
Degradation Studies
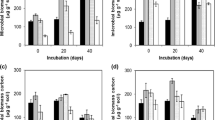
Degradation results in laboratory incubated soils suggested that atrazine was moderately persistent and except 0.2% STA-mixed silty clay loam, atrazine residues reached below detection limit on 30th days in all treatments. The STA (0.1%) slightly increased atrazine degradation in silty clay loam soil while increasing STA content (0.2%) slowed down degradation (Supplementary Table A.). At 20th days, 36.02, 20.34 and 54.03% of atrazine was recovered from control, STA (0.1%) and STA (0.2%) treatments. After 30 days, atrazine concentration reached below detectable limits except STA (0.2%) where 33.71% atrazine was recovered undegraded. The STA slowed down atrazine degradation in sandy clay loam and on 20th day 0.9, 11.13 and 14.01% atrazine was recovered undegraded from control, STA (0.1%) and STA (0.2%) treatments. However, atrazine degraded at faster rate in STA-mixed loam soil and on 10th day 17.11, 5.57 and 6.46% atrazine was recovered from the control, STA (0.1%) and STA (0.2%) treatments.
Compared to atrazine, fipronil was fairly persistent in all three sugarcane soils as insecticide was detected for 95 (silty clay loam) and 120 (sandy clay loam and loam) days (Supplementary Table B.). The STA did not show much impact on fipronil degradation. After 95 days, 30.77, 29.45 and 28.11% % fipronil was recovered from the control, STA (0.1%) and STA (0.2%) treatments in silty clay loam soil, respectively. Similarly, on 120th day 6.3, 6.2 and 7.6% (sandy clay loam) and 17.7, 12.2 and 12.4% (loam) fipronil was recovered from the respective treatments. Degradation of atrazine and fipronil in the laboratory incubated soils followed order: silty clay loam > sandy clay loam > loam.
Metabolites detected in soil samples are expressed in Supplementary Tables A-D. Atrazine can be degraded, both chemically and microbially, by (1) hydrolysis of chlorine group leading to formation of hydroxyatrazine and (2) dealkylation to deethylatrazine or deisopropylatrazine (Supplementary Figure A). Metabolite analysis suggested that hydroxyatrazine and deethylatrazine were detected as metabolites of atrazine (Supplementary Table C). No detection of deisopropylatrazine did not indicate that it was not formed during atrazine degradation. Probably, it might have formed, but degraded immediately; so, did not accumulate at detectable concentration. Both metabolites were detected on 10th day in silty clay loam soil. Concentration of deethylatrazine was more in the control than STA (0.1%) and STA (0.2%) treatments, while no effect of STA was observed on hydroxyatrazine concentration. Traces of only deethylatrazine were detected in sandy clay loam soil samples in 6-15th day samples and STA did not affect its concentration among different treatments. Both metabolites were detected on 6th day in loam soil, but, no difference in hydroxyatrazine concentration among the samples of different treatment was observed. However, concentration of deethylatrazine in STA (0.1%) treatment was more than in the control and STA (0.2%) treatments. Both metabolites were relatively less persistent in STA (0.2%) treatment. These results suggested that hydroxyatrazine was detected only in silty clay loam and loam soils having alkaline pH and STA further increased pH (> 8 in 0.2% STA). Earlier reports suggested that atrazine gets hydrolysed to hydroxyatrazine in alkaline soils (Nel and Reinhardt, 1984). Thus, hydrolysis and dealkylation predominated for atrazine degradation in silty clay loam and the loam soils, while dealkylation followed in sandy loam soil.
Fipronil get metabolized to different metabolites viz., sulfide is formed by reduction of > S = O moiety, sulfone is formed by oxidation of > S = O moiety, amide is formed by hydrolysis of cyanide group and desulfinyl is mainly formed by photolysis. Among them, only sulphide was detected on 20th day in silty clay loam soil and no effect of STA was observed on metabolite concentration (Supplementary Table D). All four metabolites were detected in sandy clay loam (Supplementary Table E); sulfide and sulfone on 10th day, amide on 30th day and desulfinyl traces (0.05 µg g−1) on 50th and 60th day. Among them, sulfide and sulfone were the major metabolites and their maximum concentrations were 0.28 and 0.46 µg g−1, respectively, in 70th day samples; thereafter, concentrations decreased. Sulfide, sulfone and amide were detected in loam soil (Supplementary Table F) and their concentrations were more in the control soil. Maximum amounts of sulfide in the control (0.414 µg g−1), STA (0.1%) (0.397 µg g−1) and STA (0.2% (0.238 µg g−1) treatments were observed on 70th, 70th and 60th day, respectively. Similarly, maximum concentration of sulfone in the control (0.215 µg g−1), STA (0.1%) (0.186 µg g−1) and STA (0.2% (0.151 µg g−1) treatments was observed on 70th, 70th and 40 day, respectively. Maximum amounts of amide (miner metabolite), were observed between 50th and 60th days. All metabolites formed degraded with incubation. Comparison of metabolites formed across all three soils suggested that concentrations of sulfone and sulfide metabolites were highest in loam, followed by sandy clay loam and silty clay loam. These results are in agreement with the rate of fipronil degradation in these soils. Further, highest recovery of sulfide metabolites from silty clay loam and loam, having high clay content suggested that reduction was the main mechanism of fipronil degradation in these soils. However, sulfone was the major metabolite in sandy loam soil suggesting oxidation was the main degradation mechanism operative in this soil.
Degradation data, without and with metabolites, fitted well to the first-order degradation equation as correlation coefficient (r) values were > 0.9 (Tables 2, Fig. 1). The Kobs values of atrazine alone in silty clay loam, sandy clay loam and loam were 0.042–0.059, 0.096–0.229 and 0.207–0.262 µg g−1 d−1, respectively. The half-life (t1/2) of atrazine degradation in the control, STA (0.1) and STA (0.2%) treatments were 11.77, 9.94 and 16.46 (silty clay loam), 3.03, 7.0 and 7.22 (sandy clay loam) and 3.18, 3.35 and 2.65 (loam) days, respectively. Thus, atrazine was maximum persistent in silty clay loam and least in loam soil. Degradation kinetics calculation including all metabolites detected during degradation, slightly decreased degradation rate. The Kobs of atrazine + metabolites in silty clay loam, sandy clay loam and loam were 0.033–0.0593, 0.096–0.207 and 0.113–0.253 µg g−1 d−1, respectively. This resulted in slightly increase in the t1/2, which in the control, STA (0.1) and STA (0.2%) were 14.59, 13.03 and 20.87 (silty clay loam), 3.35, 7.22 and 7.45 (sandy clay loam) and 5.42, 6.13 and 2.74 (loam) days, respectively. Thus, except silty clay loam (all three treatments) and control & 0.1% STA loam, t1/2 without and with metabolites did not vary significantly.
Degradation rate constants (µg g−1 d−1) for fipronil (Table 3, Fig. 1) were lowest in silty clay loam (0.013–0.014), followed by sandy clay loam (0.016–0.017) and loam soil (0.022–0.024). The t1/2 for fipronil in control, STA (0.1%) and STA (0.2%) treatments were: 53.31, 53.31, 49.5 d (silty clay loam); 43.31, 40.76, 40.76 d (sandy clay loam); 28.88, 28.88d, 31.50d (loam). Including metabolites in total residues recovered, decreased the rate of degradation and the Kobs of fipronil + metabolites in silty clay loam, sandy clay loam and loam soils were 0.0097–0.01, 0.006–0.0077 and 0.014–0.016 µg g−1 d−1, respectively. The t1/2 for fipronil residues in control, STA (0.1) and STA (0.2%) treatments were 60.30, 71.44 and 71.19 (silty clay loam), 115.52, 115.52 and 90.02 (sandy clay loam) and 49.51, 46.21 and 43.32 (loam) days, respectively. Thus, including metabolites in fipronil residues significantly increased the t1/2, suggesting that fipronil metabolites were more persistent than atrazine metabolites.
Effect of STA on atrazine degradation under field condition in silty clay loam soil (Supplementary Table G) indicated that on day 1, compared to the control (0.531 µg g−1), higher amounts of atrazine were recovered from STA (0.1%) (0.575 µg g−1) and STA (0.2%) (0.734 µg g−1) treatments. But, thereafter, degradation was faster in STA-mixed soils and on 10th day 11.24, 7.04 and 8.14% of initially applied atrazine was recovered from the control, STA (0.1%) and STA (0.2%) treatments, respectively, and residues reached below detection limits on 15th day in all three treatments. Hydroxyatrazine (minor) and deethylatrazine (major) were detected as atrazine metabolites in all three treatments. Maximum concentration of hydroxyatrazine was recovered on 1st day, while deethylatrazine concentration was the maximum between 3rd and 6th days, while both metabolites reached below detection limit on 15th day. Data fitted well to first-order kinetics equation (r > 0.93) (Table 2, Fig. 2) and t1/2 in the control, STA (0.1%) and STA (0.2%) were 3.61, 2.73 and 2.86 days, respectively. The respective t1/2 calculated including metabolites 3.77, 2.89 and 3.08 days. Thus, t1/2 calculated without and with metabolite were nearly same indicating that metabolites did not accumulate and degraded quickly. Faster atrazine degradation under field conditions can be attributed to photodegradation and volatilization. Further, microbial activity dynamics in laboratory incubated soils and field soils are entirely different and it might contribute to faster microbe-mediated degradation of pesticide.
This study suggested impact of STA was more on atrazine degradation than fipronil. Maximum impact of STA on atrazine degradation was observed in sandy loam, where 0.2% STA enhanced t1/2 value by > 2 folds. These results are not in line with the adsorption result (Yadav and Singh, 2021) which suggested that STA increased atrazine adsorption coefficient (Kf) by 113% (silty clay loam), 46% (sandy clay loam) and 43% (loam). Although, STA (0.2%) increased fipronil adsorption in silty clay loam (35%), loam (140%) and sandy clay loam (300%) soils, no significant effect was observed on fipronil degradation; indicating that degradation of fipronil was not limited by its availability in soil solution. Generally, pesticide adsorption and degradation are inversely related as adsorption decreases the amount of pesticide in soil solution (Yang et al., 2010). However, ashes, which contain oxide of some macro- and micro-nutrients, might increase microbial activity and pesticide degradation. Earlier, Zhang et al. (2005) observed faster benzonitrile degradation in wheat residue char mixed soil and attributed it to higher cell density in the presence of the char nutrients (stimulation by soluble phosphorus) than in their absence. Kumar et al. (2019a, b) reported that wheat straw ash enhanced pretilachlor degradation in soils, where 0.2% ash reduced t1/2 by nearly half.
Effect of STA on soil microbial population (culturable bacteria and fungi) in silty clay loam soil was studied by CFU counts. Results (Table 4) suggested that STA slightly increased CFU counts of bacterial and fungal population and CFU increased with increase in STA dose from 0.1 to 0.2%. It can be attributed to increased nutrient availability following STA mixing in soil (Bang-Andreasen et al. 2017).
Bioavailability Study
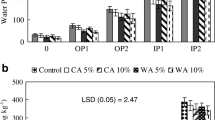
Effect of STA on bioactivity of atrazine was evaluated by observing its effect on mustard seed germination. Initial results to find out mustard seedling sensitivity to atrazine suggested that seedlings were sensitive to 1 μg mL−1 aqueous concentration (results not shown here) and atrazine affected both root and shoot lengths, but effect on root length was more evident. However, 1 and 1.5 μg g−1 atrazine concentrations (Table 5, Supplementary Figure C.) did not show any inhibition of mustard seedling’s root length in soil as part of atrazine get adsorbed to soil particles and was unavailable in soil solution. Atrazine concentration of 2 μg g−1 inhibited mustard seedling root length in the control soil, while did not show much inhibition of seedling growth in STA (0.1 and 0.2%) mixed treatments. This can be attributed to reduced availability of atrazine in STA-mixed soils due to higher adsorption of atrazine. These results suggested that compared to atrazine availability in the control soil, even 0.1% STA reduced availability of atrazine; therefore, no effect of atrazine on mustard seedling was observed. Therefore, probably higher amounts of atrazine will be required for weed control in STA-mixed soils.
Not many studies related to effect of plant biomass ashes on herbicide activity are available. Kumar and Singh (2020b) assayed the effect of rice straw ash (RSA) on bioavailability of sulfosulfuron by observing its effect on mustard seedlings and reported that even 0.1% RSA reduced herbicide’s availability.
Conclusion
Burning of crop residues for land clearing after crop harvest is a common agricultural practice in India. Crop residue ashes contain high surface area unburnt carbon content that increased retention of pesticides in soils and can affect fate of pesticides applied after burning. The present study evaluated the effect of sugarcane trash ash (STA) on atrazine and fipronil degradation behaviour in three sugarcane-growing soils. The STA affected atrazine degradation in laboratory incubated soils and effect was maximum in sandy clay loam soil, however, effect under field evaluation in silty clay loam soil was nonsignificant. No effect was observed on fipronil degradation suggesting fipronil degradation may not affected by its availability in soil solution. These results could not be explained by pesticide’s adsorption behavior in these soils which suggested enhanced retention of both pesticides in STA-mixed soils and should have affected subsequent degradation behaviour in soils. The STA (0.1%) reduced bioavailability of atrazine evaluated by its effect on mustard seedling, a sensitive plant. Present study has implications for assessing the fate of these pesticides in sugarcane fields, where trash is burnt year after year and STA is mixed in soil. However, the findings of laboratory studies cannot be directly extended to field situation as STA might have cumulative effect due to year after year burning of trash.
References
Bang-Andreasen, T., J.T. Nielsen, J. Voriskova, J. Heise, R. Rønn, R. Kjøller, H.C.B. Hansen, and C.S. Jacobsen. 2017. Wood ash induced pH changes strongly affect soil bacteria numbers and community composition. Frontiers in Microbiology 8: 1400. https://doi.org/10.3389/fmicb.2017.01400.
Basu, M., M. Pande, P.B.S. Bhadoria, and S.C. Mahapatra. 2009. Potential fly-ash utilization in agriculture: A global review. Progress in Natural Science 19: 1173–1186.
Chatterjee, N.S., and S. Gupta. 2010. Fipronil mobility and transformation in undisturbed soil columns. Bulletin of Environmental Contamination and Toxicology 85: 152–156.
Deokar, S.K., S.A. Mandavgane, and B.D. Kulkarni. 2016a. Adsorptive removal of 2,4-dichlorophenoxyacetic acid from aqueous solution using bagasse fly ash as adsorbent in batch and packed-bed techniques. Clean Technologies and Environmental Policy 18: 1971–1983.
Deokar, S.K., S.A. Mandavgane, and B.D. Kulkarni. 2016b. Agro-industrial waste: A low cost adsorbent for effective removal of 4-chloro-2-methylphenoxyacetic acid herbicide in batch and packed bed modes. Environmental Science and Pollution Research 23: 16164–16175.
Deokar, S.K., D. Singh, S. Modak, S.A. Mandavgane, and B.D. Kulkarni. 2016c. Adsorptive removal of diuron on biomass ashes: A comparative study using rice husk ash and bagasse fly ash as adsorbents. Desalination and Water Treatment 57: 22378–22391.
França, D.A., K.M. Longo, T.G. Soares Neto, J.C. Santos, S.R. Freitas, B.F.T. Rudorff, E.V. Cortez, E. Anselmo, and J.A. Carvalho Jr. 2012. Pre-harvest sugarcane burning: Determination of emission factors through laboratory measurements. Atmosphere 3: 164–180.
Giori, F.G., V.L. Tornisielo, and J.B. Regitano. 2014. The role of sugarcane residues in the sorption and leaching of herbicides in two tropical soils. Water Air & Soil Pollution 225: 1935. https://doi.org/10.1007/s11270-014-1935-8.
Govantes, F., O. Porrúa, V. García-González, and E. Santero. 2009. Atrazine biodegradation in the lab and in the field: Enzymatic activities and gene regulation. Microbiology and Biotechnology 2: 178–185.
Hiller, E., M. Bartal, J. Milicka, and S. Cernansky. 2009. Environmental fate of the herbicide mcpa in two soils as affected by the presence of wheat ash. Water Air & Soil Pollution 197: 395–402.
Hillier, J.L., T.H. Fletcher, M.S. Solum, and R.J. Pugmire. 2013. Characterization of macromolecular structure of pyrolysis products from a Colorado green river oil shale. Industrial & Engineering Chemistry Research 52: 15522–15532.
Jain, N., A. Bhatia, and H. Pathak. 2014. Emission of air pollutants from crop residue burning in India. Aerosol & Air Quality Research 14: 422–430.
Kumar, A., and N. Singh. 2020a. Effect of crop residue ashes on sorption behavior of herbicides used in the succeeding crop in Indian soils. Journal of Environmental Science & Health B55: 630–645.
Kumar, A., and N. Singh. 2020b. Crop residue ashes reduce leaching, persistence and bioavailability of sulfosulfuron and pretilachlor used in the succeeding crop. Soil Research 58: 551–560.
Kumar, A., A. Mandal, and N. Singh. 2019a. Rice and wheat straw ashes: Characterization and modeling of pretilachlor sorption kinetics and adsorption isotherm. Journal of Environmental Science & Health B54: 303–312.
Kumar, A., N. Singh, T. Banerjee, and S.B. Singh. 2019b. Chemical degradation of sulfosulfuron in aqueous suspension of rice and wheat straw ashes. Bulletin of Environmental Contamination and Toxicology 103: 484–489.
Kumari, U., T. Banerjee, and N. Singh. 2021. Evaluating ash and biochar mixed biomixtures for atrazine and fipronil degradation. Environmental Technology and Innovation 23: 101745. https://doi.org/10.1016/j.eti.2021.101745.
Loganathan, V.A., Y. Feng, G.D. Sheng, and T.P. Clement. 2009. Crop-residue-derived char influences sorption, desorption and bioavailability of atrazine in soils. Soil Science Society of America Journal 73: 967–974.
Nel, P.C., and C.F. Reinhardt. 1984. Factors affecting the activity of atrazine in plants and soil. South African Journal of Plant and Soil 1: 67–72.
Pandey, V.C., and N. Singh. 2010. Impact of fly ash incorporation in soil systems. Agriculture Ecosystem and Environment 136: 16–27.
Sheng, G., Y. Yang, M. Huang, and K. Yang. 2005. Influence of pH on pesticide sorption by soil containing wheat residue-derived char. Environmental Pollution 134: 457–463.
Singh, N., S.B. Singh, and T.K. Das. 2013. Effect of fly ash on persistence, mobility and bio-efficacy of metribuzin and metsulfuron-methyl in crop fields. Ecotoxicology and Environmental Safety 97: 36–241.
Trivedi, N.S., S.A. Mandavgane, and B.D. Kulkarni. 2016. Mustard plant ash: A source of micronutrient and an adsorbent for removal of 2, 4-dichlorophenoxyacetic acid. Environmental Science & Pollution Research 23: 20087–20099.
Turner, J.A. 2015. Pesticide manual. Worcestershire: British Crop Protection Council.
Yadav, S., and N. Singh. 2020. Sugarcane trash ash: A low cost adsorbent for atrazine and fipronil removal from water. Indian Journal of Chemical Technology 27: 319–325.
Yadav, S., and N. Singh. 2021. Increased sorption of atrazine and fipronil in the sugarcane trash ash–mixed soils of northern India. Journal of Soil Science and Plant Nutrition 21: 1263–1276.
Yang, Y., and G. Sheng. 2003. Enhanced pesticide sorption by soils containing particulate matter from crop residue burns. Environmental Science & Technology 37: 3635–3639.
Yang, Y., G. Sheng, and M. Huang. 2006. Bioavailability of diuron in soil containing wheat-straw-derived char. Science of Total Environment 354: 170–178.
Yang, X.B., G.G. Ying, P.A. Peng, L. Wang, J.L. Zhao, L.J. Zhang, and H.P. He. 2010. Influence of biochars on plant uptake and dissipation of two pesticides in an agricultural soil. Journal of Agricultural and Food Chemistry 58: 7915–7921.
Zhang, P., G. Sheng, Y. Feng, and D.M. Miller. 2005. Role of wheat-residue-derived char in the biodegradation of benzonitrile in soil: Nutritional stimulation versus adsorptive inhibition. Environmental Science & Technology 39: 5442–5448.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest is reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yadav, S., Singh, S.R., Bahadur, L. et al. Sugarcane Trash Ash Affects Degradation and Bioavailability of Pesticides in Soils. Sugar Tech 25, 77–85 (2023). https://doi.org/10.1007/s12355-022-01197-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-022-01197-1