Abstract
The present investigation intends to determine the physiochemical parameters and levels of toxic metals in surface soils, their possible sources, and their impacts on the tree carbon stock potential in a natural forest. Ninety soil samples were collected from three locations: Site-1: Vicinity to industries, Site-2: 150 m distance, and Site-3: 300 m distance from thirty industries in Bhawal Sal Forest. The toxic metal concentrations were quantified using an atomic absorption spectrophotometer (AAS). Multiple factor analysis (geo-accumulation index (Igeo), pollution index (PI), Pearson correlation, clustering, and regression) was done to measure the ecological risks and relationships of metals. The results revealed that the mean concentration of metal pollutants (Cu, Zn, Pb, and Cr) at Site-1 was 32.28, 7.99, 38.85, and 53.08 ppm, respectively. The geo-accumulation index (Igeo) values for Pb at Site-1 and Site-2 were 1.02±0.37 and 0.10±0.26, respectively, which means Igeo class 2 and 1, indicating moderately contaminated and unpolluted/moderately contaminated soils, respectively. A high value of pollution index (PI) for Pb (3.10±0.92) was recorded at Site-1. Correlation analysis found a strong positive link between metals: Cu-Zn (72%), Cu-Pb (83%), Cu-Cr (51%), Zn-Pb (71%), Zn-Cr (54%), and Pb-Cr (62%). Furthermore, negative correlations for tree biomass carbon stock of Cu (-87%), Zn (-78%), Pb (-84%), and Cr (-53%) were recorded, highlighting the negative effects of metals on tree diversity and biomass carbon production that require further monitoring. Nonetheless, policymakers and conservationists are obligated to take the necessary actions to reduce the impacts of industrial effluent discharge in protected forests.
Highlights
• Lead was the most significant contributor to the ecological risks.
• Cu, Zn, Pb, and Cr exhibited strong correlations, indicating similar origins.
• Toxic metals in surface soils had negative impacts on tree biomass carbon stocks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The industrialization has been a driving force behind human progress and development, yet it has caused significant environmental changes, especially in forest ecology. The rapid expansion of the industry is a major contributor to environmental pollution from a wide range of sources, particularly toxic metals (Jiang et al. 2017; Zouiten et al. 2016; Vignaroli et al. 2018). Human activities triggering toxic metal pollution in protected forests alter the physiochemical properties of the soil, threatening the integrity of forest ecosystems (Galal et al. 2021; Yang et al. 2022). With the advent of industrialization and urbanization, the abundance of toxic metals such as copper (Cu), zinc (Zn), chromium (Cr), nickel (Ni), iron (Fe), manganese (Mn), arsenic (As), mercury (Hg), lead (Pb), and cadmium (Cd) in the environment has skyrocketed over the past few decades, causing global concern (Dutta et al. 2021; Adimalla 2020; Shi et al. 2019; Ashraf et al. 2019; Bernardino et al. 2019). The toxicity, high degree of bioaccumulation, and pervasiveness of heavy metal contamination in soil have brought it to the forefront of research efforts on a worldwide scale (Zhang 2020). Their contamination causes disruptions in the natural biogeochemical cycle since they are not biodegradable (Kumar et al. 2019), pose ecological concerns, and have long biological half-lives (Kaur et al. 2020; Carvalho et al. 2020). In addition, they disrupt ecosystem functioning, human health, and nutrient cycling (Hiller et al. 2021; Huang et al. 2020). Particles of toxic metals discharged by urban megaprojects and industrial effluent gases infiltrate the atmosphere and are thus subsequently precipitating into urban forest ecosystems (Li and Liu 2017). Thus, ecologists and environmentalists are becoming more interested in deducing the nature of these connections. Their concern is a result of world researchers' emphasis on the environmental effects of toxic metals. The focus of environmental scientists throughout the globe on toxic metals is likely what piques their curiosity (Malunguja et al. 2022; Li et al. 2020; Kumar et al. 2020; Kothandaraman et al. 2020).
Bangladesh is one of the world's nations with the highest population density (Alam 2021). The soil near the industrial areas of Bangladesh's major cities was overabundant with toxic metals and metalloids (Islam et al. 2018; Zakir et al. 2017; Zakir and Hossain 2016), and those wastes containing toxic metals pose a serious threat to the environment, particularly to soils, sediments, and streams (Khanam et al. 2020), including protected forests. In addition to being one of the most contaminated countries, Bangladesh has forest areas covering approximately 2.53 million hectares, or 17.5% of its total land area (BFD 2020). Yet, the real area covered by forests does not reach 6% of the country's total land, and a person only has access to 0.022 hectares of forest (Uddin et al. 2020). The deciduous Sal forest in Bangladesh spans over 0.12 million hectares and accounts for 4.7% of the country's total forest area (Roshni et al. 2022). The Bhawal Sal Forest is one of the most endangered natural deciduous forests in Bangladesh. Due to invasion and illegal industrialization, the forest environment has suffered. The majority of industries in this forest are textiles (Alauddin et al. 2020), which release toxic metals like copper, manganese, lead, cadmium, mercury, zinc, and arsenic in their wastes and effluents. These metals have been identified as crucial markers among the different hazardous compounds generated by industrial operations because they can be efficiently and consistently studied in the majority of environmental matrices. The deposition rate of toxic metals in these areas are attributable for changes in the plant community, diversity, and biomass production potential (Sheng et al. 2021, Khalid et al. 2021, Danelli et al. 2021).
Toxic metals in forest soil have been proven to disrupt root physiological function and lower forest productivity (Kang et al. 2022). The accumulation of these heavy metals in the trees leads to their entry into food webs (Fajardo et al. 2020; Leclercq-Dransart et al. 2019; Kumar et al. 2019). For instance, Sheng et al. (2021) evaluated the impact of heavy metal pollution on moss taxa and the conservation of biodiversity in the Nancha Mn-mining region of China. Ng et al. (2020) discovered the extraordinary capacity of plant species to accumulate metals in soils. Khanam et al. (2020) reported that metal exposure decreases leaf growth and CO2 assimilation in plants. Moreover, the accumulation of toxic metals in forest soils can impact the ability of trees to sequester carbon, leading to potential implications for climate change mitigation.
Globally reported data on toxic metals accumulation in forest ecosystems owing to industrial activities, including those of Kang et al. (2022) revealed that Cd, Pb, and Cu in their respective enrichment regions were mostly from marble and cement manufacturing of forest soil in Western Guangdong Province, China. Malinova et al. (2022) measured heavy metal concentrations in forest soils in Bulgaria's Western Balkan Mountains. A Pb-Zn smelter's heavy metal contamination was examined by Hu et al. (2022). Bolou-Bi et al. (2021) examined Cd, Cr, Hg, Ni, Mn, and Zn in an urban forest in Abidjan. Raj et al. (2019) also found heavy metal contamination in forest soil from mining dust and waste ore. The nonferrous metallurgy smelter's air emissions damaged European North Russia's northern taiga pine forest, as reported by Lyanguzova et al. (2018). Li and Liu (2017) stated that industrial activities and transportation are the primary contributors to heavy metal contamination in urban forest ecosystem. Roztocze National Park's (Poland) heavy metal contamination was assessed by Mazurek et al. (2017).
Several studies on toxic metals and their sources in forest soil around the world along with human activities like roadways, traffic emissions, and development activities are by Malunguja et al. (2022) in India, Kupka et al. (2021) in central Poland, Mackowiak et al. (2021) in southern California, Vural et al. 2021) in Bingol Turkey, Wang et al. (2020) in China, Devi et al. (2019) in Kaziranga National Park in Indi, Alsbou and Al-Khashman (2018) in the Petra region, Jordan. On the other hand, numerous investigations have depicted industrial discharges as sources of soil toxic metals. For example, Bibi et al. (2023) reported industrial discharge sources of soil heavy metals in Vienna, Austria; Su et al. (2022) in South China. Holtra and Zamorska-Wojdyla (2020) observed high pollution surrounding the smelter. Petroleum, coal, nuclear, and high-tension wire industries emit toxic metals such as Cu, Zn, Cd, Ni, B, Se, and Cs as reported by Zhu et al. (2016), and Ahmed and Ahmaruzzaman (2016).
Notwithstanding the importance of data on metals, plant variety, and productivity in Bangladesh's protected forests, this topic is rarely discussed in the literature. The available reported data in the country focused on describing the levels of toxic metals in agricultural lands, water, and plants in industrial areas (Bhuiyan et al. 2021; Proshad et al. 2020; Yesmeen et al., 2018; Proshad et al. 2018, Zakir et al., 2017, Mottalib et al. 2016). The baseline data that characterize the levels of toxic metals in protected forests in terms of plant diversity, their potential for biomass production, and ecological hazards are still limited and rarely reported. Thus, quantifying the concentrations of toxic metals in forest ecosystems caused by industrialization is deemed a crucial endeavor. Consequently, to address this knowledge gap and facilitate efficient forest management, an ecological investigation was conducted in Bhawal Sal Forest, Gazipur District, Bangladesh. However, this study is unique as it measured the pollution level of toxic metals in forest soils, their probable sources, and their effects on tree diversity and biomass carbon stocks. We collected the forest soil samples from three sites of industrial activity and measured the concentration of heavy metals, i.e., Cu, Zn, Pb, and Cr, and recorded data on tree species. The multiple indicator analysis (geo-accumulation index, pollution index, Pearson correlation, clustering, and linear regression) was done to measure the ecological risks, possible sources, and their relationships. Furthermore, we also recorded the tree parameters and used allometric equations for estimating tree biomass carbon. This study aims to: (i) determine the physicochemical properties and levels of four toxic metals (Cu, Zn, Pb, and Cr) in the surface soil of forest using the ecological risk index (geo-accumulation index (Igeo) (Muller 1969) and pollution index (PI) (Dutta et al. 2021); and (ii) predict the effects of toxic pollutants on the soil nutrients and carbon stocking potential of trees.
However, we need consistent forest management monitoring to adjust to and lessen the effects of natural and anthropogenic shifts. We believe that this study will encourage scientists and policymakers to focus on forest ecosystems and work towards more environmentally responsible forest management practices. Decisions about forest conservation can only be made with accurate, up-to-date data. Thus, policymakers will find this study useful in safeguarding the forest zone from toxic metal pollution and in drafting rules and regulatory systems to protect the natural forest ecology and environment.
2 Materials and Methods
2.1 Description of the Study Area
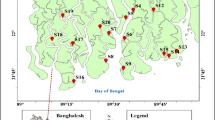
The research was conducted at Bhawal Sal Forest (BSF), Bangladesh, which is located between the latitudes 24°02′ to 24°11′ North and the longitudes 90°21′ to 90°28′ East (Alauddin et al. 2020). BSF has two administrative zones, the National Park Range (division) and the Bhawal Range, with seven forest beats (sub-divisions): Park Beat, Baupara Beat, Bankharia Beat, Rajendrapur West Beat, Baroipara Beat, Bhabanipur Beat, and Bishawakuribari Beat (B. K. Bari Beat) (Masum et al. 2016). Specifically, this study was conducted at three forest beats in the Bhawal Sal Forest; Park and Baupara beats within the National Park Range (Division) and the Bishawakuribari beat under the Bhawal range because of the existence of industries and land encroachment, which harm the soils and potential for carbon sequestration (Fig. 1). A total of 255.3 ha of forest land were taken by about 354 unauthorized industrial structures in the protected areas (Roshni et al. 2022). The "Chalas" (paddy fields) are split by "Baids" and surrounded by plains or low hills rising 3.0-4.5 m above them. According to Thornthwaite's classifications, this forest is considered to be humid (Rahman and Vacik 2010). The Bhawal-Madhupur and Barind tracts have acidic, well-drained brown terrace soils with a clayey to fine loamy texture. It is a part of Bangladesh's 28th AEZ and contains (0.72-2.45%) organic matter (Kabir and Ahmed 2005). The average rainfall is 1500 mm per year and optimum temperatures range from 11.5 °C to 38.5 °C. The yearly relative humidity (RH) is 85.2% with a rate of total evaporation of 1023.5 mm (Rahman and Vacik 2010).
2.2 Vegetation of the Study Area
In Bhawal Sal Forest, about 221 species of plants (including 24 species of climbers, 27 species of grasses, 3 species of palms, 105 species of herbs, 19 species of shrubs, and 43 species of trees) were recorded (Kabir and Ahmed 2005), with Sal (Shorea robusta) was the dominant species. Besides, other associated species such as Azuli (Dillenia pentagyna), Akashmoni (Acacia auriculiformis), Eucalyptus (Eucalyptus spp.), Teak (Tectona grandis), Mahogany (Swietenia spp.), Bot (Ficus benghalensis), Koroi (Albizia spp.), Jam (Syzygium cumini), Haritaki (Terminalia chebula), Bohera (Terminalia bellirica), Palash (Butea monosperma), Kumbhi (Careya arborea), Gandhi gajari (Miliusa velutina), Datai (Microcos paniculata) are also present in this forest. Other shrub species like Monkata (Randia dumetorum), Bhat (Clerodendrum squamatum), Bet (Calamus viminalis), Matkila (Glycosmis pentataphyla), and different climbers such as Payna lata (Cissus adnata), Samalata (Ichnocarpus frutescens), Swamalata (Cuscuta reflexa), Kumarilata (Smilax perfoliata) and Assamlata significantly exist in this forest area.
2.3 Design of the Study
The randomly selected thirty industries of this forest were considered as sampling points of this investigation; their spatial locations were taken through the Geographical Positioning System (GPS). Soil samples were obtained at three sites, i.e., Site-1: Vicinity to industries, Site-2: 150 m distance, Site-3: 300 m distance from each industry for determining the soil's physiochemical properties and toxic metals. The quadrate plot (10 m × 10 m) was used to estimate tree biomass stocking characteristics from each site. To estimate the tree carbon stocks, the girth at breast height (GBH) was recorded at 1.37 meters above the ground. The total height of all tree species was measured using the Sunnto Clinometer. The wood density of specific tree species was collected from a list of the global wood density database mentioned by Chave et al. (2009).
2.4 Collection of Soil Samples
The soil samples were collected from the surface layer (0–15 cm depth), followed by the Composite Soil Sampling technique as recommended by the USDA Soil Survey Team (1951). With the help of manual auger, soil samples from three sites (vicinity, 150 m and 300 m distance to industries), weighing a 0.5 kg of soil were collected. The total number of collected soil samples was 90, with 30 samples from each site. Five soil cores were extracted from various locations on each site, completely coated to create a composite sample, and packaged and labeled securely. The drainage pattern of effluent discharge was considered when selecting the site locations. To eliminate any damage, samples were kept in sealed poly bags with definite markings and tied carefully.
2.5 Preparation of Soil Samples for Analysis
The composite soil sample was brought to the Department of Agroforestry Laboratory, Bangladesh Agricultural University, to prepare for analysis. First, the soil was spread thinly on clean brown paper in the lab and air-dried for two weeks at 25 °C in a dust-free environment (Ngaba and Mgelwa 2020). Then, the soil samples were mashed using a wooden pestle and mortar to generate uniform fine particles, and sieved through a 2 mm stainless steel sieve to eliminate visible rubbish, stones, weed bits, roots, and other debris. Finally, soil samples were stored in zip-locked polybags with chemical analysis labels. Soil samples were analyzed chemically for heavy metals in the lab of Soil Resources Development Institute (SRDI), Dhaka, Bangladesh.
2.6 Soil Samples Analysis Methods
A glass electrode pH meter was used to measure soil pH in water where the soil and water ratio was 1:2.5 as outlined by Jackson (1969). The wet-oxidation technique described by Page et al. (1989) was used to calculate the organic carbon content of the soil. The quantity of organic matter in soil samples was estimated by dividing the organic carbon content by the Van Bemmelon factor, 1.73 (Piper 1950). Following the semi-micro Kjeldahl method, the total nitrogen concentration of the soil sample was ascertained (Jackson 1969). The soil sample available phosphorus was evaluated by extracting it with 0.5 M NaHCO3 solution at pH 8.5, following Kurtz (Bray and Kurtz 1945) method as reported by Tandon (1993). The content of exchangeable potassium in soil samples was determined with the help of a flame emission spectrophotometer as outlined by Page et al. (1989). In a digestion tube (100 mL), 1.0 g of powdered soil sample was digested with 10 mL of pure nitric acid (HNO3) and heated at 130 °C until the volume was reduced to roughly 1 mL to determine harmful metal concentration. After cooling, samples were combined with 5 mL of 1% HNO3 and filtered using Whatman No. 42 filter paper (Hseu 2004). An atomic absorption spectrophotometer (AAS) measured Cu, Zn, Pb, and Cr contents in aqueous extracts (Shimadzu AA7000 Japan).
2.7 Standards for Chemicals and Reagents
The chemicals and reagents employed in the laboratory analysis were of a known concentration and were spectrophotometrically pure to within 0.01 percent. Every step, from creating standards to diluting reagents to preparing samples, was performed with double-deionized water. Calibration curves were diluted from reference supplies. Periodic washings were provided, and blanks were routinely run, to guarantee the integrity of the analysis (Sharma et al. 2018). The average values from the three tests conducted on each sample were presented (Adhikari and Bhattacharyya 2015).
2.8 Geo-accumulation Index (Igeo)
The index of geo-accumulation (Igeo) is a quantitative indicator of trace metals. It uses (Muller 1969) the link between the element's concentration in soil/sediment (fraction 2 m) and the backdrop. The Eq. (1) is used to estimate the geo-accumulation index of the samples soil:
Here, Cn is the soil toxic metal concentration (mg kg-1) and Bn is the same element concentration (mg kg-1) in the geochemical background. The background value is the average earth's crust value of the metal given by Taylor (1964). There are seven Igeo classes: class 0 (0 ≥ Igeo) unpolluted; class 1 (0 ≤ Igeo ≤ 1) uncontaminated/moderately polluted; class 2 (1 ≤ Igeo ≤ 2) moderately polluted; class 3 (2 ≤ Igeo ≤ 3) moderately/strongly polluted; class 4 (3 ≤ Igeo ≤ 4) strongly polluted; class 5 (4 ≤ Igeo ≤ 5) strongly/extremely polluted; and class 6 is an open class and comprises all values of Igeo higher than 5, which indicates extremely polluted soil (Muller 1969).
2.9 Pollution Index (PI)
A soil sample's pollution index (PI) is calculated by dividing the concentration of toxic metals by the background concentration (Dutta et al. 2021). The pollution index (PI) is a common way to represent the amount by which a sample's metal concentration exceeds the reference (uncontaminated standard) To determine metal toxicity, the index compares the average levels of trace elements in a soil sample to a global or regional normal background value that accounts for land-use trends and human activities (Adhikari and Bhattacharyya 2015). The pollution index can be calculated using the Eq. (2):
where, Cn is the element's measured concentration in soil (mg kg-1), and Bn is the local natural background value (mg kg-1). The soil element concentration (Cn) is divided by the local natural background value (Bn) to derive the pollution index (PI). The metal pollution levels are ranked from low (if PI ≤ 1.0), medium (if 1.0 < PI ≤ 3.0), or high (if PI > 3.0) according to Dutta et al. (2021).
2.10 Quantification of Identified Tree Species Parameters
The density of the tree species was calculated following the Eq. (3) (Shukla and Chandel 2000):
where, a= Total number of individual species in all quadrates, b= Total number of quadrates of studied areaa (then it was converted into trees/ha).
The two equations Eq. (4) and Eq. (5) mentioned by Dallmeier et al. (1992) were used to determine relative frequency and relative density as follows:
where, Fi= Frequency of tree species
and
The Girth at Breast Height (GBH) of tree species was converted into Diameter at Breast Height (DBH) by dividing by the π=3.1416 value as stated by Islam et al. (2016). The basal area was calculated using the following Eq. (6) (Chowdhury et al. 2019):
2.11 Allometric Model for Estimating Tree Biomass Carbon Stocks
The aboveground (AGB) and belowground (BGB) biomass stocking potentials of trees were calculated using non-destructive methods (Chave et al. 2005; Hangarge et al. 2012). To estimate the carbon stocks in tree biomass, an allometric model was used which was developed for the tropical "moist forest" mentioned by (Chave et al. 2005). The Eq. (7) was used for estimating aboveground biomass:
where, ρ is the wood density (g/cm3), D is the DBH of the tree (cm) and H is the height of the tree (m).
The belowground biomass was calculated by multiplying the aboveground biomass (AGB) by 0.26 (root-to-shoot ratio) (Hangarge et al. 2012) as follows the Eq. (8):
The total biomass of the tree was then calculated (Sheikh et al. 2011) as follows the Eq. (9):
Typically, 50% of a plant's biomass is considered carbon (Pearson et al. 2005). After estimating the biomass using an allometric relationship, the biomass was multiplied by 0.5 because wood of tree species comprises 50% of the total biomass. Therefore, the total biomass carbon was calculated using the Eq. (10):
2.12 Statistical Analysis
Boxplots were generated to illustrate the descriptive statistics analysis and Tukey's test for statistically significant differences for every soil sample data. To test whether the values of soil nutrient elements, metals, and carbon stocks were substantially different, a one-way ANOVA was conducted. Correlation coefficient and dendrogram for cluster analysis using the Ward and Euclidean distance methods were utilized to identify interrelation and patterns of similarity between metals, soil physicochemical parameters, and tree biomass carbon stocks. To assess the effects of metal pollution on the biomass stocks of trees, predictor models were developed using stepwise multiple regression analysis. To separate the means of all components, the alpha level of Tukey's test for genuinely significant differences was adjusted to 0.001. The statistical analyses were done using R (version 3.6.3; R Core Team, Free software foundation, Europe) and Minitab (version 19; Minitab, LLC, Pennsylvania) statistical software.
3 Results and Discussion
3.1 Physiochemical Properties of Soil Samples
Within sampling sites, mean values of soil physiochemical parameters (pH, organic matter (OM), nitrogen (N), available phosphorus (P), and exchangeable potassium (K)) varied significantly (respectively: F=64.44, F=15.13, F=503.83, F=36.01, F=29.50; p < 0.000). In the present findings, the values of pH ranged from 4.8 to 6.8, respectively, which indicated that the soils were strongly acidic to slightly acidic. The highest mean and median pH values (6.06 and 6.1, respectively) were recorded at Site-1. The discharged items from industries such as wastes, effluents, chemicals, and salt could be responsible for pH fluctuations. But with increasing distance from industries, the pH value decreased, and the lowest average and median pH values (4.92 and 5, respectively) were recorded at Site-3 (Fig. 2). Similar finding was reported by Shivakumar et al. (2012) for the Indian city of Mysore. Soil acidity in the studied area may have been affected by heavy rain washing away exchangeable bases in the natural forest (Malunguja et al. 2022). High leaching and wet conditions accelerate base exchange and may escalate the release of hydrogen as a process of biochemical weathering, as reported by Dutta et al. (2021). As stated by Adhikari and Bhattacharyya (2015), toxic metals move more quickly through the soil and are better able to bind to soil particles in acidic soils. The organic matter (OM) values of soil samples were asymmetrically distributed over the three sites illustrated in the boxplot (Fig. 2). The OM content of all soil samples ranged from 1.7% to 6.1%, which indicated that the soil was high- to low-fertile. The results inferred that the greatest amount of organic matter (OM) (3.28%) was found to be at Site-1, whereas the lowest organic matter content (2.25%) was found at Site-3 (Fig. 2). The observed discrepancies between the studied sites could be attributed to large quantities of industrial waste and the breakdown of solid waste, poultry waste, and sewage. Hossen et al. (2017) revealed that polluted sediments have more organic matter than unpolluted ones due to the accumulation of enormous amounts of industrial waste, sewage sludge, and other organic material. The organic matter level of soil around dyeing businesses, textile industries, and glass industries in Bangladesh was highest, as reported by Tusher et al. (2017). The available N, P, and K concentrations vary significantly between the sites (Fig. 2). The total nitrogen content, exchangeable potassium (ppm), and available phosphorus (ppm) ranged between 0.02% and 0.9%, 0.1 and 0.7 meq/100 g, and 3 and 58 ppm, respectively. The levels of N and K were higher in samples collected from Site-3 than in samples collected from the other two sites in the Bhawal Sal forest. Because of the loss of natural vegetation due to tree falling, crushing, shearing off, and uprooting for industrial development, and the removal of the bulk of nutritive soil due to erosion, the value of total N and accessible K in the neighboring area to the industrial site was a little lower. Hasan et al. (2020) reported that the N content was the lowest (0.09%) of highly encroached forest sites in Madhupur Sal Forest, Bangladesh. In contrast to common belief, the highest P content (34.50 ppm) was obtained from Site-1 (Vicinity to Industries) than other sites (Fig. 2). The greatest P concentration is at Site-1, which is likely attributable to the disposal of garbage that includes P elements. Malunguja et al. (2022) found high P availability in protected forest soil samples along roadways in India.
Boxplot showing impacts of industrialization on soil chemical properties (a = pH, b = OM (%), c = Nitrogent (%), d = K (meq/100g), e = P (ppm)) and toxic metals accumulation (f = Cu (ppm), g = Zn (ppm), h = Pb (ppm), i = Cr (ppm)) at three sites (p < 0.001, significant level). The uppermost hanging bar depicts the high edge or maximum value, while the below-hanging bar represents the low edge or minimum value. The top and bottom box lines represent the third and first quartiles, respectively. The solid line within the box denotes the median value, while the dot represents the mean or average value of all samples
3.2 Toxic Metals Concentration of Soil Samples
The present investigation found a significant difference in levels of heavy metals between sampling sites (Site-1, Site-2 and Site-3) (Fig. 2). The recorded concentration of the studied heavy metals like Cu, Zn, Pb and Cr concentrations of all the soil samples ranged between 1.15 to 52 ppm, 2 to 10 ppm, 3.26 to 84.2 ppm, and 17.8 to 154.18 ppm, respectively (Fig. 2). As per sampling sites, toxic metals concentrations were found to be greater at Site-1 (Vicinity to industries) than other sites (Fig. 2). Cu had the highest concentrations of 32.28 ppm at Site-1, followed by Zn (7.99 ppm). Boxplot showed that Pb concentration of soil samples was both positively and negatively skewed distributed at Site-1 and Site-2 but was found negligible at Site-3. The maximum Pb content was found at Site-1 (38.85 ppm) while the minimum Pb content was found at Site-3 (4.81 ppm) which was at 300 m distance from industries. The highest average Cr concentration (53.08 ppm) was found at Site-1 and the lowest (20.99 ppm) was found at 300 m distance from industries, respectively. The sequence of heavy metal concentrations at Site-1 was in the order Cr > Pb > Cu > Zn.
Despite significant variances in toxic metal contents, the mean values were below the standards limit for earth crusts (Taylor 1964). The higher concentrations of metal pollutants in soil samples collected along Site-1 (Vicinity to industries) compared to other sites (Site-2 and Site-3) suggest that these metals could be from wastes, textile manufacturing effluent, dyeing wastewater, cement factory wastes, and vehicular emissions caused by rapid industrialization in natural forest areas. Due to a dearth of similar research in the region, it was difficult to make appropriate comparisons from the present study findings. For comparison and justification, certain metal contamination concentration studies from various countries are included (Table 1). The present study findings agree with other reported data in Bangladesh and the world at large. The present study is in accord with the findings of industrial areas of Gazipur, Bangladesh (Hossain et al. 2019). The mean concentrations of Pb, Cu, Zn, and Ni in forest soils in Western Guangdong Province, China, reported by Kang et al. (2022), were 0.02 mg·kg−1, 17.05 mg·kg−1, 10.20 mg ·kg−1, 18.14 mg·kg−1, and 7.14 mg·kg−1, respectively. Earlier research showed that car exhaust emissions and industrial output increased soil Cd, Pb, and Cu (Liu et al. 2018; Zhang et al. 2018). Pb and Cu in Yunan District were more likely to originate from cement production and Zn from processing and manufacturing, according to Cao et al. (2020). Tavakoli et al. (2019) found that lead and cadmium concentrations were substantially higher in mine area in Hyrcanian Forest, North Iran. Similarly, petroleum industries, coal-burning power plants, nuclear power stations, and high-tension cables emit B, Se, Cu, Zn, Cd, Ni, and Cs (Ahmed and Ahmaruzzaman 2016). Mining and refining are additional important contributors of heavy metal pollution (Srivastava et al. 2017). On the other hand, textile wastewater was the largest source of heavy metals revealed by Astuti et al. (2023). For instance, Proshad et al. (2018) stated that rapid industrialization were the causes of heavy metal toxicity in agricultural soil of Bangladesh. Islam et al. (2018) stated that untreated industrial effluents immediately contribute heavy metals and metalloids to water and soil of metropolitan areas in Bangladesh. According to Begum and Huq (2016), the presence of trace elements in the surrounding soil may be related to the textile industry's use of heavy metals (especially Cu, Cr, and Zn) in dying and printing steps. Many pertinent pieces of research demonstrate significant metal concentrations next to the industries (Table 1). Perhaps site-specificity explains the present study's striking heavy metal level discrepancies from those of earlier research. The diverse sources of metal pollutants may explain the differences in metal pollutant concentrations across all comparisons. These studies were done in vehicular emissions, urban, or agricultural areas. As this work focused on soil samples contaminated mostly by a single source of human activity (i.e., industrial activity), pinpointing the practical rationale for considerable enlargement from these findings proved challenging. The higher metal concentrations found at Site-1 of the present study support the idea that these metals are the product of industrial effluents. If not monitored and regulated, this concentration might rise to unmanageable levels and harm surrounding species' habitats in forest.
3.3 Assessment of Ecological Risks of Toxic Metals
3.3.1 Geo-accumulation Index (Igeo)
The geo-accumulation index (Igeo) for the metal contaminants exhibited different classes, as shown in Fig. 3. The mean Igeo values at Site-1 (vicinity to industries) for Cu, Zn, Pb, and Cr were -1.12, -3.74, 1.02, and -1.62, respectively. These values were relatively higher as compared to those recorded at Site-3, where Igeo values for Cu, Zn, Pb, and Cr, were -5.93, -5.11, -2.01, and -2.85, respectively (Fig. 3). In all examined soil samples, the Igeo values for Cu and Zn were zero, indicating that the soil quality at each site was uncontaminated. Nonetheless, all soil samples collected from Site-3 (300 m distance from industries) displayed uncontaminated levels (i.e., Igeo ≤ 0). The surface soils of Site-1 were determined to be moderately contaminated with Pb (1 < Igeo < 2), while Cr, Cu, and Zn had the lowest concentrations. At Site-2, it was determined that Pb (0 < Igeo < 1) in the surface soils was uncontaminated to moderately contaminated by lead metal pollutants. Therefore, Pb contributed the most contamination to the surface soils in Bhawal forest (Site-1), while Cu, Zn and Cr contributed the least. Pb has a significant impact on ecological and environmental risk, as well as plant diversity and biomass production. According to the research of Malunguja et al. (2022), all heavy metals at 200 m away from the highway towards the forest (control) posed no ecological risk, since the site represented a "practically uncontaminated category" (Igeo 0) for both Bhomoraguri and Balipara RF, India. Hossain et al. (2019) stated that Igeo values for Ni and Cd of industrially impacted soils demonstrated unpolluted/moderately polluted soil quality in the pre-monsoon and dry season. As stated by Zakir et al. (2015), the Igeo of soils in three distinct industrial areas of the Gazipur district showed moderate soil contamination by Pb, Zn, and Cd from multiple anthropogenic sources, preferably different industrial activities, supporting the current findings.
3.3.2 Pollution Index (PI)
According to the PI criteria, the studied forest soil samples revealed three levels of pollution (i.e., low, medium, and high levels). The present findings state that Pb (3.10±0.92) had the highest level of pollution, whereas Cu (0.72±0.20), Zn (0.11±0.03), and Cr (0.53±0.302) had the lowest levels (Table 2). At Site-2, all metals (Cu, Zn, Pb, and Cr) were classified as having low levels of pollution, with mean values of 0.38, 0.07, 0.64, and 0.31, respectively (Table 2). At Site-3 (300 m away from industries), all metals (Cu, Zn, Pb, and Cr) had minimal levels of contamination. The overall pollution in the adjacent industries was as follows: Pb > Cu > Cr > Zn. This demonstrates that the surface soils of the Bhawal Sal Forest next to industries were highly contaminated with Pb. The present findings are congruent with the data reported by Xu et al. (2021) for East China with comparable industries, indicating that the potential ecological risk index by Pb and Cd in the adjacent soil was at a medium (or high) level. The southwest region of Xinxing County was moderately and heavily contaminated (PI > 1), whereas the center districts of Yunan and Yuncheng were only slightly and moderately contaminated (Kang et al. 2022). The present findings support previous research (Tavakoli et al. 2019) where according to PI index for Pb (1.16) and Cd (1.77), soils were moderately polluted near a coal mine in Hyrcanian Forest, North Iran. The majority of metal pollution is said to originate from industrial effluents. The results indicate that industrial activities in the Bhawal Sal Forest provide a potential ecological risk to the soils on the forest floor. Effluent emissions are sources of large quantities of metal pollutants into forest soils, which may have a significant impact on biodiversity and productivity. The results could be very dangerous for the planet's ecosystem. Excessive concentrations may lead to pollution due to their cumulative effects over time causing serious environmental problems. Thus, it is important to track metal levels in soil to prevent excessive buildup.
3.4 Carbon Stock Potentials of Tree Species
A total of 30 mature tree species were recorded from 90 quadrate plots of three sites (i.e., 10 trees from Site-1, 19 tree species from Site-2 and 24 tree species from Site-3) to evaluate the impacts of the toxic metal pollutants (Cu, Zn, Pb and Cr) on tree biomass carbon stock and production potential in the forest where some species were common within the sites. Table 3 showed that at Site-1, the total stand density was 343.33 trees ha-1 while the total stand density at Site-3 was 946.65 trees ha-1. This indicates that tree density is a key factor contributing to tree biomass carbon production which means positive relationships between them. Random cutting down of trees for industrial developments leads to the change in the tree density and biomass carbon production at industrial-affected Site. At Site-1 (Vicinity to industries), a total biomass of 239.46 Mg ha-1 with a total carbon of 119.73 Mg ha-1 were recorded (Table 3). On the contrary, at Site-2 (150 m distance to industries), the total biomass was 482.15 Mg ha-1 and the total carbon was 241.08 Mg ha-1. At Site-3, the total biomass was 992.88 Mg ha-1 and the total carbon was 496.44 Mg ha-1 (Table 3). These values equalized 439.40 Mg ha-1, 884.75 Mg ha-1 and 1821.94 Mg ha-1 of CO2 sequestration by tree species. Acacia hybrid (31.54 Mg ha-1), Shorea robusta Gaetrn (28.73 Mg ha-1), Swietenia macrophylla King f. (19.33 Mg ha-1), Acacia auriculiformis A. Cunn ex Benth (15.87 Mg ha-1) were the dominant tree species that produced the most tree biomass and carbon stock at Site-1. The highest biomass stocks at Site-2 (150 m distance from industries) were found in: Shorea robusta Gaetrn (272.69 Mg ha-1), Swietenia macrophylla King f. (36.65 Mg ha-1), Artocarpus heterophyllus Lamk (36.46 Mg ha-1), Xylia xylocarpa (Roxb.) Taub (24.53 Mg ha-1) (Table 3). For Site-3 (300 m distance from industries), the top tree biomass carbon stock was found in Shorea robusta Gaetrn (567.69 Mg ha-1) and Haldina cordifolia (Roxb.) Ridsdale (110.42 Mg ha-1), respectively (Table 3). The dominant biomass stocks at Site-1 and Site-2 of Balipara RF were F. carica L (11.13 and 25.20 Mg ha−1), Mimusops elengi L (7.49 and 17.09 Mg ha−1), and Stereospermum chelonoides DC (5.19 and 9.99 Mg ha−1). The topmost biomass producer and carbon accumulator species were T. grandis L.f. and F. carica L., in Bhomoraguri and Balipara RF, India, respectively, as reported by Malunguja et al. (2022). The present study's findings provide a substantial chance for carbon sequestration and might lead to the right use of REDD+ carbon credits.
3.5 Correlation between Physicochemical Properties, Metal Pollutants and Tree Biomass Carbon Stocks
Table 4 and Fig. 4 provide the Pearson correlation coefficient matrix (r) and cluster analysis (CA) findings for toxic metals, physicochemical parameters and tree biomass carbon stock, respectively, to reveal the similarities and connections between these variables. Soil metals are shown to be associated with pH and OM as well as accessible soil nutrients (N, P, and K) at a 1% level of probability. To determine whether or not any of the soil-examined factors are associated, a correlation matrix was generated and provided the results in Table 5. Matrix analysis may also reveal the presence of carrier chemicals and the harmful metal chemical associations in the study area. The results reveal a positive correlation between pH and metal concentrations (Cu=0.67, Zn=0.66, Pb=0.61, Cr=0.56) (Table 4). However, OM was positively correlated to metal pollution, with 53% and 40% for Cu and Zn, respectively, and negatively correlated to Pb (-49%). Heavy metals had a negative correlation with nutrients with coefficient (r) values: for N -76%, -69%, -74%, and -54%; for K -55%, -42%, -54%, and -34%; for P 56%, 57%, 58%, and 38%; respectively (Table 4). It is shown that OM and nutrients such as N and K were negatively linked with metal pollutants in the present study. Cu had a highly significant positive correlation with Cu-Zn (72%), Cu-Pb (83%), and Cu-Cr (51%). Similar results were found for Zn with Zn-Pb (71%) and Zn-Cr (54%), and Pb with Pb-Cr (62%); all these suggest that these metals may have the same origins, ideally across diverse industrial activities. Given the strong positive correlation seen between the metals in this research, it is likely that these metals originated from the same place. A significant inverse relationship between tree biomass and the four metal pollutants was found and the correlation coefficient matrix is as follows: TBC-Cu (-87%), TBC-Zn (-78%), TBC-Pb (-84%), and TBC-Cr (-53%) (Table 4). Findings from the current investigation indicate a negative relationship between tree biomass carbon stocks and all metal pollutants from studied soils. This significant negative correlation between plant diversity and productivity suggests that metals have an effect on the diversity and productivity of trees. Variations in tree species biomass production can be attributed to plant diversity, which is positively correlated with tree production and stocking (Paletto et al. 2021).
On the contrary, tree biomass carbon stock had a positive relationship with tree stand characteristics viz. DBH (cm), height (m), basal area and density (trees/ha) at the three sites (Fig. 5). Similarly to the current work, Malunguja et al. (2022) found that TBC-Pb (-80%), TBC-Ni (-79%), TBC-Cr (-76%), and TBC-Cd (-71%) were negatively correlated with the four metal pollutants demonstrating to be highly adversely connected with tree biomass. Biswas and Hasan (2020) also stated that basal area m2/ha (r=0.99 and R2=0.99) and stand density/ha (r=0.93 and R2= 0.87) had a strong relationship with total above-ground carbon and the relationship was significant (p ˂ 0.05) which is quite similar to the present study. Other factors such as density, basal area, forest maturity, climate, terrain, and disturbance regime may have influenced differences in biomass production between tree species (Dibaba et al. 2019).
Cluster analysis (CA) was used to determine the existence of interconnected groups and to separate them into distinct clusters and sub-clusters. It was also used to back up the relationship observation results using the correlation coefficient (r). Figure 4 shows that the data can be roughly divided into three major groups, each of which exhibits its own distinct patterns. The remaining cluster includes pH, Cu, Pb, Zn, and Cr. The second cluster contains OM and available P, while the third has available K, tree biomass stock, and nitrogen values. The current study's cluster analysis (CA) indicated comparable grouping, which lends credence to the notion that the metal pollutants under examination have common sources of accumulation in surface soils, which may be considerably connected to industrialization. Kumar et al. (2019) and Shi et al. (2019) used Ward method and the Euclidean distance to quantify levels of similarity.
3.6 Quantifying the Potentials Impacts of Toxic Metal Pollution on Tree Biomass Carbon Stocks
In order to calculate the effects of toxic metal contaminants (Cu, Zn, Pb and Cr) on the carbon stock potentials for tree species at the three sites, a stepwise multiple regression analysis was performed (Table 5). Tree biomass stocks are influenced by metal concentrations in surface soils to varying degrees. Metals (Cu, Zn, Pb, and Cr) are shown to have a profoundly negative association with tree biomass stocks, as indicated by linear regression models:
Y = -10.07x+447.44 for Cu, R2=0.7691, p < 0.001; Y = -51.87x+564.65 for Zn, R2=0.615, p < 0.001; Y = -8.78x+464.93 for Pb, R2=0.719, p < 0.001; Y = -3.868x+413.79 for Cr, R2=0.290, p < 0.001 where, Y= Tree biomass stocks (dependent variable), x= Concentration of toxic metals (independent variable) (Table 5). Singh et al. (2020) reported that heavy metals tend to modify the physicochemical characteristics of soils, affecting plant production. Consequently, metal accumulations as Cu, Zn, Pb, and Cr in surface soils in industrial regions of Forest may have changed soil physical and chemical characteristics, affecting nutrient absorption. Due to excessive metal absorption, plant development and stocking potential are impaired.
3.7 Practical Implications of the Study
The present study provides the scientific data on the concentrations of four toxic metals (Cu, Zn, Pb, and Cr) and the ecological concerns in forest soils that can be used to improve environmental policies and regulations with the goal of limiting the emission of toxic metals. With the up-to-date data in hand, forest policymakers and government can implement appropriate mitigation strategies, such as phytoremediation, phyto-extraction, and phytoremediation technologies, customized for the particular plant kind and degree of contamination. Based on the findings of this study, Cr and Pb should be prioritized in future efforts to reduce the contribution of industrialization to soil heavy metal pollution.
3.8 Limitations of the Study
The researchers acknowledge the study's limitations while maintaining optimism that it provides a useful assessment of toxic metals contamination, its pollution levels, and effects on tree biomass carbon stock potentials in BSF that can drive subsequent policy considerations and forest management. First, the samples analyzed from thirty different categorized industries inside the natural forest could not reflect the full entire scenario of industrialization and its repercussions on the entire forest soil. Finally, the study was designed to determine the pollution levels of four toxic metals and their effects on tree biomass carbon, and did not identify the other toxic metals, possibly limiting the accuracy of the study. As a result, it is suggested that more research be conducted to learn more about the effects of toxic metals contamination on tree biomass carbon stock potentials in BSF.
4 Conclusions
The followings conclusions were drawn from the present investigation:
-
1.
Although the mean concentrations of all examined metals (Cu, Zn, and Cr) were within the limit set by the geochemical background concentration of the earth's crust, the soil samples from the vicinity of industries had the greatest levels of toxic metals than the other two sites in the forest area. The results show that industrial effluent discharges were a substantial contributor to hazardous metal pollution in the surface soils of natural forests. Hence, it is necessary to track the levels of these metals to ensure that their accumulation and toxicity do not impair the forest ecosystem. A database of heavy metal concentrations in forest soils is necessary because of the possible ecological risks they provide to forest-based ecosystems.
-
2.
The ecological risk assessment results showed that the geo-accumulation (Igeo) and pollution index (PI) were found to be greater in all industrially contaminated areas (Site-1), with the highest contribution of Pb. Strong positive correlations between metals in the study revealed that they have a similar source. The observed significant negative relationship indicates that metals are contributing factors to tree biomass carbon. Furthermore, a comprehensive investigation of the mechanism and distribution of metals in soils and tree species in protected forests is required. As a result, ecologists and policymakers ought to give protective efforts the highest priority to lessen the harshness of this impact on the environment.
Data Availability
The raw data of this study will be available from the corresponding author upon request.
Abbreviations
- Cu :
-
Copper
- Zn :
-
Zinc
- Pb :
-
Lead
- Cr :
-
Chromium
- NaHCO 3 :
-
Sodium Bicarbonate
- DBH :
-
Diameter at breast height
- TB :
-
Tree biomass
- TBC :
-
Tree biomass carbon
References
Adhikari G, Bhattacharyya KG (2015) Ecotoxicological risk assessment of trace metals in humid subtropical soil. Ecotoxicology 24(9):1858–1868. https://doi.org/10.1007/s10646-015-1522-9
Adimalla N (2020) Heavy metals pollution assessment and its associated human health risk evaluation of urban soils from Indian cities: a review. Environ Geochem Health 42(1):173–190. https://doi.org/10.1007/s10653-019-00324-4
Ahmed MJK, Ahmaruzzaman M (2016) A review on potential usage of industrial waste materials for binding heavy metal ions from aqueous solutions. J Water Process Eng 10:39–47. https://doi.org/10.1016/j.jwpe.2016.01.014
Alam MZ (2021) MS Is Population Density a Risk Factor for Communicable Diseases Like COVID-19? A Case of Bangladesh. Asia Pac J Public Health 33(8):949–950. https://doi.org/10.1177/1010539521998858
Alauddin M, Hossain M, Islam M, Islam S (2020) Management Strategies for Sustainable Forest Biodiversity Conservation in Protected Areas of Bangladesh: A Study of Bhawal National Park, Gazipur. Grassroots J Natl Resour 3(3):56–72. https://doi.org/10.33002/nr2581.6853.03035
Alsbou EME, Al-Khashman OA (2018) Heavy metal concentrations in roadside soil and street dust from Petra region, Jordan. Environ Monit Assess 190(1):48. https://doi.org/10.1007/s10661-017-6409-1
Ashraf S, Ali Q, Zahir ZA, Ashraf S, Asghar HN (2019) Phytoremediation: environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotox Environ Safe 174:714–727. https://doi.org/10.1016/j.ecoenv.2019.02.068
Astuti D, Awang N, Othman MSB, Kamaludin NFB, Meng CK, Mutalazimah M (2023) Analysis of Heavy Metals Concentration in Textile Wastewater in Batik Industry Center. J. Penelit. Pendidik . IPA 9(3), 1176–1181. https://doi.org/10.29303/jppipa.v9i3.3085
Begum M, Huq SI (2016) Heavy metal contents in soils affected by industrial activities in a southern district of Bangladesh. Bangladesh J Sci Res 29:11–17
Bernardino CAR, Mahler CF, Santelli RE, Freire AS, Braz BF, Novo LAB (2019) Metal accumulation in roadside soils of Rio de Janeiro, Brazil: impact of traffic volume, road age, and urbanization level. Environ Monit Assess 191(3):156. https://doi.org/10.1007/s10661-019-7265-y
BFD (Bangladesh Forest Department): http://www.bforest.gov.bd/ (2020). Accessed 23 Mar 2020
Bhuiyan MA, Chandra-Karmaker S, Bodrud-Doza M, Rakib MA, Saha BB (2021) Enrichment, sources and ecological risk mapping of heavy metals in agricultural soils of Dhaka district employing SOM, PMF, and GIS methods. Chemosphere 263:128339. 10.1016/j
Bibi D, Tőzsér D, Sipos B, Tóthmérész B, Simon E (2023) Heavy Metal Pollution of Soil in Vienna, Austria. Water Air Soil Pollut 234(4):232. https://doi.org/10.1007/s11270-023-06244-5
Biswas M, Hasan M (2020) Measurement of above-ground carbon stocks of roadside agroforestry plantation at Sadar Upazila of Mymensingh district in Bangladesh. J Bangladesh Agric Univ 18:252–259. https://doi.org/10.5455/JBAU.911985455/JBAU.91198
Bolou-Bi BE, Ettien DJB, Guety TP, Pitta MA Balland-Bolou-Bi C (2021) Heavy Metal Fluxes in Tropical Urban Forest Soil in Abidjan District (Côte d’Ivoire). J Agri Chem Environ 10, 169-183. https://doi.org/10.4236/jacen.2021.102011
Bray RH, Kurtz LT (1945) Determination of total, organic, and available forms of phosphorus in soils. Soil Science 59:39–45. https://doi.org/10.1097/00010694-194501000-00006
Cao X, Ma R, Zhang QS, Wang WB, Liao QX, Sun SC, Zhang PX, Liu XL (2020) The factors influencing sludge incineration residue (SIR)-based magnesium potassium phosphate cement and the solidification/stabilization characteristics and mechanisms of heavy metals. Chemosphere 261:127789. https://doi.org/10.1016/j.chemosphere.2020.127789
Carvalho MEA, Castro PRC, Azevedo RA (2020) Hormesis in plants under Cd exposure: From toxic to beneficial element? J Hazard Mater 384:121434. https://doi.org/10.1016/j.jhazmat.2019.121434
Chave J, Andalo C, Brown S, Cairns MA, Chambers JQ, Eamus D, Fölster H, Fromard F, Higuchi N, Kira T (2005) Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145:87–99. https://doi.org/10.1007/s00442-005-0100-x
Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE (2009) Towards a worldwide wood economics spectrum. Ecol Lett 12:351–366
Chen H, Teng Y, Lu S, Wang Y, Wu J, Wang J (2016) Source apportionment and health risk assessment of trace metals in surface soils of Beijing metropolitan, China. Chemosphere 144:1002–1011. https://doi.org/10.1016/j.chemosphere.2015.09081
Chowdhury M, Islam K, Hafiz N, Islam K (2019) Diversity of trees in a community managed forest: the case of Komolchori VCF, Khagrachari, Bangladesh. Geol Ecol Landsc 3:95–103. https://doi.org/10.1080/24749508.2018.1508980
Dallmeier F, Kabel M, Rice RA (1992) Methods for long-term biodiversity inventory plots in protected tropical forests. In: Dallmeier F (ed) Ling-term Monitoring of Biological Diversity in Tropical Forest Areas: Methods for Establishment and Inventory of Permanent Plots. UNESCO, Paris, pp 11–46
Danelli T, Sepulcri A, Masetti G, Colombo F, Sangiorgio S, Cassani E, Anelli S, Adani F, Pilu R (2021) Arundo donax L. biomass production in a polluted area: effects of two harvest timings on heavy metals uptake. Appl Sci (Switzerland) 11(3):1–16. https://doi.org/10.3390/app11031147
Devi U, Taki K, Shukla T, Sarma KP, Hoque RR, Kumar M (2019) Microzonation, ecological risk and attributes of metals in highway road dust traversing through the Kaziranga National Park, Northeast India: implication for confining metal pollution in the national forest. Environ Geochem Health 41(3):1387–1403. https://doi.org/10.1007/s10653-018-0219-4
Dibaba A, Soromessa T, Workineh B (2019) Carbon stock of the various carbon pools in Gerba-Dima moist Afromontane forest, South-Western Ethiopia. Carbon Balance Manag 14(1):1–10. https://doi.org/10.1186/s13021-019-0116-x
Dutta N, Dutta S, Bhupenchandra I, Karmakar RM, Das KN, Singh LK, Bordoloi A Sarmah T (2021) Assessment of heavy metal status and identification of source in soils under intensive vegetable growing areas of Brahmaputra valley, North East India. Environ Monit Assess 193(6): 1–18. https://doi.org/10.1007/s10661-021-09168-x
Fajardo C, Sánchez-Fortún S, Costa G, Nande M, Botías P, García-Cantalejo J, Mengs G, Martín M (2020) Evaluation of nanoremediation strategy in a Pb, Zn and cd contaminated soil. Sci Total Environ 706:136041. https://doi.org/10.1016/j.scitotenv
Galal TM, Essa B, Al-Yasi H (2021) Heavy metals uptake and its impact on the growth dynamics of the riparian shrub Ricinus communis L. along with Egyptian heterogenic habitats. Environ Sci Pollut Res 28(1):37158–37171. https://doi.org/10.1007/s11356-021-13383-x
Hangarge LM, Kulkarni DK, Gaikwad VB, Mahajan DM, Chaudhari N (2012) Carbon Sequestration potential of tree species in Somjaichi Rai (Sacred grove) at Nandghur village, in Bhor region of Pune District, Maharashtra State, India. Ann Biol Res 3:3426–3429
Hasan M, Islam M, Roshni N, Hemel S (2020) Effects of forest encroachment on tree stock parameters and soil nutrient status in the Madhupur Sal (Shorea robusta CF Gaertn) forest of Bangladesh. J Agric Food Environ 1:35–40
Hiller E, Pilkova Z, Filova L, Jurkovič Ľ, Mihaljevič M, Lacina P (2021) Concentrations of selected trace elements in surface soils near crossroads in the city of Bratislava (the Slovak Republic). Environ Sci Pollut Res 28(5):5455–5471. https://doi.org/10.1007/s11356-020-10822-z
Holtra A, Zamorska-Wojdyla D (2020) The pollution indices of trace elements in soils and plants close to the copper and zinc smelting works in Poland’s Lower Silesia. Environ Sci Pollut Res 27:16086–16099
Hossain MB, Islam MN, Alam MS, Hossen MZ (2019) Industrialisation scenario at Sreepur of Gazipur, Bangladesh and physico-chemical properties of wastewater discharged from industries. Asian J Environ Ecol 9:1–14
Hossen MZ, Islam MM, Hossain MS (2017) Heavy metal contents in sediments of an urban industrialized area—a case study of Tongi Canal, Bangladesh. Asian J Water Environ 14:59–68. https://doi.org/10.3233/AJW-170007
Hseu ZY (2004) Evaluating heavy metal contents in nine composts using four digestion methods. Bioresour Technol 95:53–59. https://doi.org/10.1016/j.chemosphere.2015.09081
Hu Y, Wu D, Liu J, Yan C, Sun J (2022) Contamination and health risk assessment of heavy metals form a typical Pb-Zn smelter in Northwest China. Bangladesh J Bot 51(4):995–1015. https://doi.org/10.3329/bjb.v51i40.63844
Huang J, Wang C, Qi L, Zhang X, Tang G, Li L, Guo J, Jia Y, Dou X, Lu M (2020) Phosphorus is more effective than nitrogen in restoring plant communities of heavy metals polluted soils. Environ Pollut 266:115259. https://doi.org/10.1016/j.envpol.2020.115259
Islam M, Tusher T, Kabir M, Hassan M, Khan M (2016) Carbon storage and sequestration potentiality of tree species in Madhupur Sal Forest of Bangladesh. Bangladesh J Environ Sci 30:33–39
Islam MM, Karim MR, Zheng X, Li X (2018) Heavy Metal and Metalloid Pollution of Soil, Water and Foods in Bangladesh: A Critical Review. Int J Environ Res Public Health 15(12):2825. https://doi.org/10.3390/ijerph15122825
Jackson ML (1969) Soil chemical analysis: advanced course. University of Wisconsin-Madison, College of Agriculture, Libraries, Parallel Press, USA, p 930
Jarva J, Tarvainen T, Lintinen P, Reinikainen J (2009) Chemical characterization of metalcontaminated soil in two study areas in Finland. Water Air Soil Pollut 198:373–391. https://doi.org/10.1007/s11270-008-9852-3
Jiang Y, Chao S, Liu J, Yang Y, Chen Y, Zhang A, Cao H (2017) Source apportionment and health risk assessment of heavy metals in soil for a township in Jiangsu Province, China. Chemosphere 168:1658–1668. https://doi.org/10.1016/j.chemosphere.2016.11.088
Kabir DS, Ahmed AZ (2005) Wildlife biodiversity in Bhawal National Park: Management techniques and drawbacks of wildlife management and nature conservation. Our Nature 3:83–90
Kang J, Ding X, Ma H, Dai Z, Li X, Huang J (2022) Characteristics and Risk of Forest Soil Heavy Metal Pollution in Western Guangdong Province, China. Forests 13:884. https://doi.org/10.3390/f13060884
Kaur M, Kumar A, Mehra R, Kaur I (2020) Quantitative assessment of exposure of heavy metals in groundwater and soil on human health in Reasi district, Jammu and Kashmir. Environ Geochem Health 42(1):77–94. https://doi.org/10.1007/s10653-019-00294-
Khalid N, Rizvi ZF, Yousaf N, Khan SM, Noman A, Aqeel M, Latif K, Rafique A (2021) Rising metals concentration in the environment: a response to effluents of leather Industries in Sialkot. Bull Environ Contam Toxicol 106(3):493–500. https://doi.org/10.1007/s00128-021-03111-z
Khanam R, Kumar A, Nayak AK, Shahid M, Tripathi R, Vijayakumar S, Bhaduri D, Kumar U, Mohanty S, Panneerselvam P (2020) Metal (loid)s (As, Hg, Se, Pb and Cd) in paddy soil: Bioavailability and potential risk to human health. Sci Total Environ 699:134330
Kothandaraman S, Dar JA, Sundarapandian S, Dayanandan S, Khan ML (2020) Ecosystem-level carbon storage and its links to diversity, structural and environmental drivers in tropical forests of Western Ghats, India. Sci Rep 10(1):1–15. https://doi.org/10.1038/s41598-020-70313-6
Kumar A, Cabral-Pinto M, Kumar A, Kumar M, Dinis PA (2020) Estimation of risk to the eco-environment and human health of using heavy metals in the Uttarakhand Himalaya, India. Appl Sci (Switzerland) 10(20):1–18. https://doi.org/10.3390/app10207078
Kumar V, Sharma A, Kaur P, Sidhu GP, Bali AS, Bhardwaj R, Thukral AK, Cerda A (2019) Pollution assessment of heavy metals in soils of India and ecological risk assessment: A state-of-the-art. Chemosphere 216:449–462. https://doi.org/10.1016/j.chemosphere.2018.10.066
Kupka D, Kania M, Pietrzykowski M, Lukasik A, Gruba P (2021) Multiple Factors Influence the Accumulation of Heavy Metals (Cu, Pb, Ni, Zn) in Forest Soils in the Vicinity of Roadways. Water Air Soil Pollut 232:194. https://doi.org/10.1007/s11270-021-05147-7
Leclercq-Dransart J, Demuynck S, Waterlot C, Bidar G, Sahmer K, Pernin C, Deram A, Lepretre A (2019) Douay F (2019) Distribution of Metals and Cell Wall Compounds in Leaf Parts of Three Tree Species Suitable for the Phytomanagement of Heavy MetalContaminated Soils. Water Air Soil Pollut 230:237. https://doi.org/10.1007/s11270-019-4290-y
Li C, Zhang Z, Wang Z, Wu Q (2020) Effects of environmental factors and heavy metals on the vertical distribution. Plant Biol 22(5):822–831. https://doi.org/10.1111/plb.13129
Li Z, Liu Z (2017) Hazards, Sources and Control Measures of Heavy Metal Pollution of Forest Soil: Taking Jin-Jing-Ji Region of China as an Example. Nat Environ Pollut Technol 16(4):1141–1147
Liu Y, Huang H, Sun T, Yuan Y, Pan Y, Xie Y, Fan Z, Wang X (2018) Comprehensive risk assessment and source apportionment of heavy metal contamination in the surface sediment of the Yangtze River Anqing section, China. Environ Earth Sci 77:493. https://doi.org/10.1007/s12665-018-7621-1
Lyanguzova I, Yarmishko V, Gorshkov V, Stavrova N, Bakkal I (2018) Impact of Heavy Metals on Forest Ecosystems of the European North of Russia. Heavy Metals. https://doi.org/10.5772/intechopen.73323
Mackowiak TJ, Mischenko IC, Butler MJ, Richardson JB (2021) Trace metals and metalloids in peri-urban soil and foliage across geologic materials, ecosystems, and development intensities in Southern California. J Soils Sediments 21(1-2):1–17. https://doi.org/10.1007/s11368-021-02893-3
Malik R, Jadood W, Husain S (2010) Metal contamination of surface soils of industrial city Sialkot, Pakistan: a multivariate and GIS approach. Environ Geochem Health 32:179–191. https://doi.org/10.1007/s10653-009-9274-1
Malinova L, Petrova K, Pavlov P (2022) Assessment of heavy metal concentrations in soils of Western Balkan Mountains. Bulg J Agric Sci 28(1):129–136
Malunguja GK, Thakur B, Devi A (2022) Heavy Metal Contamination of Forest Soils by Vehicular Emissions: Ecological Risks and Effects on Tree Productivity. Environ Process 9:11. https://doi.org/10.1007/s40710-022-00567-x
Masum KM, Islam MN, Saha N, Hasan MZ, Mansor A (2016) Assessment of land grabbing from protected forest areas of Bhawal National Park in Bangladesh. Landsc Res 41:330–343
Mazurek R, Kowalska J, Gąsiorek M, Zadrożny P, Józefowska A, Zaleski T, Kępka W, Tymczuk M, Orłowska K (2017) Assessment of heavy metals contamination in surface layers of Roztocze National Park forest soils (SE Poland) by indices of pollution. Chemosphere 168:839–850. https://doi.org/10.1016/j.chemosphere.2016.10.126
Mottalib MA, Somoal SH, Aftab M, Shaikh A, Islam MS (2016) Heavy metal concentrations in contaminated soil and vegetables of tannery area in Dhaka, Bangladesh. Int J Curr Res 8:30369–30373
Muller G (1969) Index of geoaccumulation in sediments of the Rhine river. Geojourna. 2:108–118
Nezat CA, Hatch SA, Uecker T (2017) Heavy metal content in urban residential and park soils: A case study in Spokane, Washington, USA. Appl Geochem 78:186–193. https://doi.org/10.1016/j.appgeochem.2016.12.018
Ng CC, Boyce AN, Abas MR, Mahmood NZ, Han F (2020) Evaluation of vetiver grass uptake efficiency in single and mixed heavy metal contaminated soil. Environ Process 7(1):207–226. https://doi.org/10.1007/s40710-019-00418-2
Ngaba MJY, Mgelwa AS (2020) Ecological risk assessment of heavy metal contamination of six forest soils in China. Int J Innov Appl Sci 30:1–10
Page AL, Miller RH, Keeny DR (1989) Methods of soil analysis. American Society of Agronomy, Madison, Wisconsin, USA
Paletto A, Agnelli AE, Meo IM (2021) Carbon stock in deadwood: the mountain birch (Betula pubescens subsp. czerepanovii) forests in the Khibiny Mountains (Russia). J Sustain For 40(4):385–400. https://doi.org/10.1080/10549811.2020.1767144
Pearson TRH, Brown S, Ravindranath NH (2005) Integrating carbon benefits estimates into GEF. Projects UNDP GEF;1-56
Piper CS (1950) Soil and Plant Analysis. The University of Adelaide Press, Adelaide
Proshad P, Islam MS, Kormoker T (2018) Assessment of heavy metals with ecological risk of soils in the industrial vicinity of Tangail district, Bangladesh. Int J Adv Geosci 6:108–116
Proshad R, Kormoker T, Islam MS, Chandra K (2020) Potential health risk of heavy metals via consumption of rice and vegetables grown in the industrial areas of Bangladesh. Human Eco Risk Assess 26(4):921–994
Rahman MM, Vacik H (2010) Vegetation analysis and tree population structure of Sal (Shorea robusta CF Gaertn) forests: A case study from the Madhupur and Bhawal National Park in Bangladesh. National Parks: Vegetation, Wildlife and Threats 193-206
Raj D, Kumar A, Maiti SK (2019) Evaluation of toxic metal (loid)s concentration in soils around an open-cast coal mine (Eastern India). Environ Earth Sci 78:645. https://doi.org/10.1007/s12665-019-8657-6
Roshni NA, Hasan MK, Akter R, Prodhan AKM, Sagar A (2022) Impacts of Industrialization on Plant Species Composition, Diversity, and Tree Population Structure in Tropical Moist Deciduous Forest in Bangladesh. Int. J. For. 1-14. https://doi.org/10.1155/2022/3959617
Sharma S, Nagpal AK, Kaur I (2018) Heavy metal contamination in soil, food crops and associated health risks for residents of Ropar wetland, Punjab, India and its environs. Food Chem 255:15–22. https://doi.org/10.1016/j.foodchem.2018.10.126
Sheikh MA, Kumar M, Bussman RW, Todaria NP (2011) Forest carbon stocks and fluxes in physiographic zones of India. Carbon Balanc Manag 6:15. https://doi.org/10.1186/1750-0680-6-15
Sheng X, Zhaohui Z, Zhihui W (2021) Effects of heavy metals on moss diversity and analysis of moss indicator species in Nancha manganese mining area, southwestern China. Glob Ecol Conserv 28:01665. https://doi.org/10.1016/j.gecco.2021.e01665
Shi C, Ding H, Zan Q, Li R (2019) Spatial variation and ecological risk assessment of heavy metals in mangrove sediments across China. Mar Pollut Bull 143:115–124. https://doi.org/10.1016/j.marpolbul.2019.04.043
Shivakumar D, Srikantaswamy S, Kiran B, Sreenivasa S (2012) Study of impacts of industries on soil characteristics of Mysore city, India. Int J Geol Earth Environ Sci 2:25–33
Shukla R, Chandel P (2000) Plant Ecology and Soil Science. Company Limited, New Delhi
Singh H, Yadav M, Kumar N, Kumar A, Kumar M (2020) Assessing adaptation and mitigation potential of roadside trees under the influence of vehicular emissions: A case study of Grevillea robusta and Mangifera indica planted in an urban city of India. PLoS One 15:e0227380
Srivastava V, Sarkar A, Singh S, Singh P, de Araujo ASF, Singh RP (2017) Agroecological Responses of Heavy Metal Pollution with Special Emphasis on Soil Health and Plant Performances. Front Environ Sci 5:64. https://doi.org/10.3389/fenvs.2017.00064
Su C, Meng J, Zhou Y, Bi R, Chen Z, Diao J, Wang T (2022) Heavy metals in soils from intense industrial areas in south China: spatial distribution, source apportionment, and risk assessment. Front Environ Sci 10:23. https://doi.org/10.3389/fenvs.2022.820536
Tandon HLS (1993) Methods of analysis of soils, plants, waters, and fertilisers. Fertiliser Development and Consultation Organisation. 63:1–204
Tavakoli M, Hojjati S, Kooch Y (2019) Lead and cadmium spatial pattern and risk assessment around coal mine in Hyrcanian Forest, North Iran. Int J Environ Ecol Eng 13:205–208
Taylor SR (1964) Abundances of chemical elements in the continental crust: a new table. Geochim Cosmochim Acta 28:1273–1285
Tusher T, Piash A, Latif M, Kabir M, Rana M (2017) Soil Quality and Heavy Metal Concentrations in Agricultural Lands around Dyeing, Glass and Textile Industries in Tangail District of Bangladesh. J Environ Sci Nat Resour 6:207–212
Uddin M, Chowdhury FI, Hossain MK (2020) Assessment of tree species diversity, composition and structure of Medha Kachhapia National Park, Cox’s Bazar, Bangladesh. Asian J For 4(1):15–21
Vignaroli C, Pasquaroli S, Citterio B, Di Cesare A, Mangiaterra G, Fattorini D (2018) Antibiotic and heavy metal resistance in enterococci from coastal marine sediment. Environ Pollut 237:406–413. https://doi.org/10.1016/j.envpol.2018.02.073
Vural H, Akbana A, Meral A (2021) The effect of heavy metal pollution on urban ecosystem and the evaluation of different land classifications; in Bingöl city/Turkey. Manag Environ Qual Intl J. https://doi.org/10.1108/MEQ-09-2020-0193
Wang Y, Duan X, Wang L (2020) Spatial distribution and source analysis of heavy metals in soils influenced by industrial enterprises distribution. Case study in Jiangsu province. Sci Total Environ 710:134953. https://doi.org/10.1016/j.scitoteny
Xu X, Zhang X, Peng Y, Li R, Liu C, Luo X, Zhao Y (2021) Spatial distribution and source apportionment of agricultural soil heavy metals in a rapidly developing area in East China. Bull Environ Contam Toxicol 106:33–39. https://doi.org/10.1007/s00128-020-03079-2
Yang Z, Zhang R, Li H, Zhao X, Liu X (2022) Heavy Metal Pollution and Soil Quality Assessment under Different Land Uses in the Red Soil Region, Southern China. Int J Environ Res Public Health 19:4125. https://doi.org/10.3390/ijerph19074125
Yesmeen R, Zakir HM, Alam MS, Mallick S (2018) Heavy metal and major ionic contamination level in effluents, surface and groundwater of an urban industrialised city: A Case Study of Rangpur City, Bangladesh. 5(1): 1-16. https://doi.org/10.9734/AJOCS/2018/45061
Zakir H, Sumi SA, Sharmin S, Mohiuddin KM, Kaysar S (2015) Heavy metal contamination in surface soils of some industrial areas of Gazipur, Bangladesh. J Chem, Biol Phys Sci 5(2):2191–2206
Zakir HM, Hossain MS (2016) Metallic pollution level in soils of Mymensingh town, Bangladesh: an impact of urbanization and industrialization. J Ind Saf Eng 3(3):17–25
Zakir HM, Hossain MA, Alam MS (2017) Metallic pollution in top soils of an urban industrialized city: a case study of Chittagong city, Bangladesh. J Chem Biol Phys Sci 7(4):835–850. https://doi.org/10.24214/jcbps.D.7.4.83550
Zhang C (2020) Heavy mineral assemblages and provenance analysis of eolian sand in the Alashan Desert, northwestern China. Msc Thesis. Lanzhou: Lanzhou University. (in Chinese)
Zhang X, Wei S, Sun Q, Wadood SA, Guo B (2018) Source identification and spatial distribution of arsenic and heavy metals in agricultural soil around Hunan industrial estate by positive matrix factorization model, Principle components analysis and geo statistical analysis. Ecotoxicol Environ Saf 159:354–362
Zhu C, Tian H, Cheng K, Liu K, Wang K, Hua S et al (2016) Potentials of whole process control of heavy metals emissions from coal-fired power plants in China. J Clean Prod 114:343–351. https://doi.org/10.1016/j.jclepro.2015.05.008
Zouiten A, Beltifa A, Van Loco J, Ben Mansour H, Reyns T (2016) Ecotoxicological potential of antibiotic pollution–industrial wastewater: bioavailability, biomarkers, and occurrence in Mytilus galloprovincialis. Environ Sci Pollut Res 23:15343–15350. https://doi.org/10.1007/s11356-016-6713-2
Acknowledgments
Authors are indebted to the National Science and Technology (NST) fellowship provided by the Ministry of Science and Technology, Government of the People's Republic of Bangladesh, and to the authorities of the Bhawal Sal Forest in Bangladesh for their assistance and support throughout the data collection.
Funding
We would like to express our gratitude to the Ministry of Science and Technology, Government of the People’s Republic of Bangladesh who gave a fellowship award named National Science and Technology (NST) fellowship for (54000 BDT) conducting this research work.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Nasima Akther Roshni, Mohammad Kamrul Hasan, Md. Abdul Wadud, Ahmed Khairul Hasan, Rojina Akter, Rafat Nur Abdullah Khan and Syed Aflatun Kabir Hemel. The first draft of the manuscript was written by Nasima Akther Roshni and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Non-applicable.
Competing Interests
The authors state that to the best of their knowledge, they have no financial or personal interests that may be construed as having influenced the work disclosed in this paper.
Consent for Publication
Non-applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Roshni, N.A., Hasan, M.K., Wadud, M.A. et al. Toxic Metal Levels in Forest Soils Caused by Industrialization and Impacts on Tree Biomass Carbon Stock. Environ. Process. 10, 45 (2023). https://doi.org/10.1007/s40710-023-00656-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40710-023-00656-5