Abstract
In this work, several physicochemical properties of sub-tropical soil (up to 20 cm depth) like water holding capacity, organic carbon content, cation exchange capacity, texture, pH, and electrical conductivity were determined along with the trace metals, Co, Cr, Cu, Mn, Ni, Pb and Zn, in order to evaluate inter-relations among the trace metals and the soil properties. The contribution of the trace metals to ecotoxicological risk was assessed using various tools. Cr, Cu, Mn and Zn contents were found to be lower than the world average, but Co, Ni, and Pb had higher contents. The trace metal concentrations were utilized to obtain the pollution index and the potential ecotoxicological aspects. The trace metals were shown to have come from similar origin and their retention in the soil was contributed by properties like organic carbon, cation exchange capacity, clay content and water holding capacity of the soil. The pollution index showed that the trace metals had the sequence of Pb (considerably polluted) > Co, Ni (moderately polluted) > Cr, Cu, Mn and Zn (unpolluted). The composite ecological risk index was the highest in agricultural land with irrigation and fertilizer use, and was the lowest in the forest land.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil trace metals are sensitive indicators of environmental pollution levels (Pekey et al. 2004) as the metals have a role in determining chemical behavior, mineralogy, and fertility of the soil. Independent of the entry route, the trace metals in soil undergo biochemical, geochemical and chemical processes (chemical weathering, adsorption and ion exchange). There is growing concern throughout the world about the trace metals in soil, their toxicity levels, their entry into the food chain and the associated risks (Alfaro et al. 2015).
The soil gets naturally enriched with trace metals from the pedogenic biogeochemical cycles. The soil trace metal levels are mainly controlled by the chemical composition of the parent rock materials, but there are also contributions from peripheral anthropogenic activities such as dumping of industrial and municipal wastes, mining for minerals, use of fertilizers, insecticides, and other chemicals in agriculture. The presence of an iron-manganese layer is a common subtropical soil feature that acts as the source of various soil trace metals (Huang et al. 2008). Geochemical processes lead to the weathering of minerals and development of soil. The consequent feature of mineral weathering, release and translocation of the trace metals to soil varies to different extents based on the chemical behaviour of the trace metals (Galán et al. 2008). The pathways of the trace metals to the soil signify the relative distribution of the trace metals in different soil horizons and the related consequences to various segments of the environment. The trace metals themselves are sensitive to the changes in the environmental conditions and this factor determines their chemical speciation in the soil as well as their chemical behaviour in the ecosystem (N’gussan et al. 2009).
For the ecological risk assessment, the quantification of the available trace metals is considered to be a necessary condition. It is known that various soil physic-chemical properties can influence the mobility of the trace metals leading to their enrichment or depletion in a particular location. If there is biomagnification of the trace metals in soil, they become much more detrimental to the ecology—the overall scenario being determined by the reactivity, magnification potential, human exposure and ecotoxicological aspects of the trace metals (Baize 2010).
Subtropical soils experiences three kinds of climate a year ranging from the hot dry to cold dry through the hot wet season with varied extent of humidity. The changes in climate conditions perturb the soil–water system and prevent the soil pore water from attaining equilibrium conditions when concentrations of the metal ions in solid soil phase and the pore water become well-defined. The climatological and anthropogenic activities, associated with the dry-wet cycles, induce stress in the form of shock to the soil microorganisms to release their body fluids that alter the trace metal equilibrium in soil. The body fluids of microorganisms accumulate trace metals by the process of passive diffusion and may mistakenly uptake the unwanted trace metal ions along with the nutrient metal ions (Driscoll and Akeiverfeldt 1994; Carrillo-Gonzãlez et al. 2006).
Soil trace metals are sensitive indicators of environmental pollution levels (Pekey et al. 2004) as the metals have a role in determining chemical behavior, mineralogy, and fertility of the soil. Independent of the entry route, the trace metals in soil undergo biochemical, geochemical and chemical processes (chemical weathering, adsorption and ion exchange). Chemical reactions in presence of available soil water includes hydration, hydrolysis and complexion processes. During these processes, the trace metals either get adhered to the soil particles or released to the environment. As a consequence, the mobility of the trace metals in the soil is affected. Hydration, complexation and intrusion of the metals into a fixed organic or inorganic constituent through exchange processes, reduce the mobility of the metals (Huang, 2008). On the other hand, soil microbes through their enzyme catalytic activities influence the dissolution, precipitation, adsorption and exchange properties of the metals and determine their composition ion the soil (Driscoll and Akeiverfeldt 1994). Plants utilize some of the trace metals as micro-nutrients and reduce their availability in soil. Higher uptake of trace metals by plants may create a situation of micronutrient deficiency and metabolic stress in the soil leading to constrains also in the input of organic matter through decomposition and transformation (Liang et al. 2009).
Adsorption of trace metals by the soil particles is affected by the soil pH (Green et al. 2003), cation exchange capacity (CEC) (Ziper et al. 1988) and soil organic matter (SOM) (Kashem and Singh, 2001). Organic matter in soil actively retains the metallic elements, usually by complexation reactions.
Higher water holding capacity (WHC) results from a larger content of pore water in the soil that provides a medium for transporting the trace metals through the pores. Soil pH influences the trace metal mobility and it is known that acidic soils favor and alkaline soils lower their mobility across the soil matrix. Lack of oxygen in the soil can also trigger mobility of the metal ions (Gang et al. 2010). The lower the mobility of the metal ions, the higher is the chance to be retained by the soil maximizing their stay at a particular location and contributing to their role as soil contaminants. In agricultural soil, the extent of removal of nutrients from the soil cannot be counterbalanced by the post-harvest decomposition of the straws, leaving a negative balance (Whitbread et al. 2003). The trace metals may undergo redox reactions on clay mineral surface facilitating the involvement of these metals in the biological processes in soil.
Subtropical soils experiences three kinds of climate a year ranging from the hot dry to cold dry through the hot wet season with varied extent of humidity. The changes in climate conditions perturb the soil–water system and prevent the soil pore water from attaining equilibrium conditions when concentrations of the metal ions in solid soil phase and the pore water become well-defined. The climatological and anthropogenic activities, associated with the dry-wet cycles, induce stress in the form of shock to the soil microorganisms to release their body fluids that alter the trace metal equilibrium in soil. The body fluids of microorganisms accumulate trace metals by the process of passive diffusion and may mistakenly uptake the unwanted trace metal ions along with the nutrient metal ions (Driscoll and Akeiverfeldt 1994; Carrillo-Gonzãlez et al. 2006).
Soil trace metal analysis has a key role in arriving at a comparison with the natural background levels and in assessing the extent of weathering processes governing the association and dissociation of trace metals to soil (Wilson et al. 2008; Bini et al. 2011). The present work incorporates monitoring of Cr, Co, Cu, Mn, Ni, Pb, and Zn in tropical agricultural soil to establish their ecological hazard potential. The potent source of the trace metals in soil constitutes both the free and bound states. The bound metals might become bioavailable under appropriate unfavorable soil conditions. This work aims to determine the regional averages of the trace metals in the soil and to establish their correlation with soil physicochemical parameters.
Materials and methods
Soil sampling site description
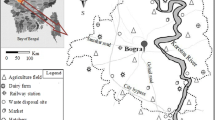
Soil samples were collected from seven sites belonging to the tropical rainforest climate region subjected to humid subtropical climate in the state of Assam, India. The average rainfall in the area is 1530 mm, with temperatures of 25.5–32.9 °C (summer) and 19.8–24.8 °C (winter), the annual average being 9.0 –30.4 °C. The sampling locations are shown in Fig. 1 and the sample identities and brief description of the sites are given in Table 1. To incorporate the effects of various land use patterns on the abundance of soil trace metals, the sampling sites were selected from virgin forest land (S1), wetland (S2), paddy cultivated land affected by floods every season (S3), agricultural land having irrigation and fertilizer use facilities (S4) and without such facilities (S5), land experiencing urban (S6) and rural (S7) residential impacts.
Soil sampling and processing
Three samples were collected from 1 m2 area, from A horizon, up to 20 cm depth (average conventional tillage depth) using a 5 cm corer. The collected soil samples were homogenized to form a composite sample, representing the specific soil type for a particular sampling location. The samples were air dried, passed through 2 mm sieve after which they were stored for laboratory analysis. The samples were collected annually in three seasons viz. hot dry summer (April to June), wet hot summer (July to September) and cold dry winter (November to February) to take into account the impact of change in climate as well as the land use patterns over the rate of soil organic matter (SOM) decomposition and stabilization. The results described here are the seasonal averages` for a span of two years from April 2012 to November 2013.
Analysis of soil samples
The soil samples were analyzed as per standard methods, i.e. soil composition with respect to sand, clay and silt percentage by hydrometer method, soil texture using ISSS triangle (Sarkar and Halder 2010), water holding capacity (WHC) by perforated base cylinder method (Wilke 2005). Electrical conductivity (EC) (1:5 soil: water suspension, Elico CM 180) (Jackson 1958), pH (1:5 soil: water suspension, Elico LI 120), soil organic carbon (SOC) was determined by modified Walkley–Black method in which the organic carbon was oxidized by a mixture of K2Cr2O7 and concentrated H2SO4 and nullifying the interferences of iron, chlorides, etc. (Sarkar and Halder 2010). The cation exchange capacity (CEC) was measured by ammonium acetate extraction method (Hasse 1994). To determine the total amounts of trace metals present in the soil samples, approximately 1 g of soil was digested with tri-acid mixture (concentrated hydrochloric, nitric and sulphuric acids in 1:2:4 proportions), the volume was made up to 100 ml and the trace metals were analysed in a Atomic Absorption Spectrophotometer (PerkinElmer AAnalyst 200) using air acetylene flame. The determined concentrations were converted to mg/kg using the formula: Trace metal (mg/kg) = (AAS reading in mg l−1 × volume of extract)/oven dry weight of soil.
All the chemicals used were of analytical grade and were procured from E. Merck. These were used without further purification. All the solutions were made in double distilled water. The analysis of each sample was done in triplicate and the mean of the three values is reported.
Calculations
Various indices have been computed and interpreted on the basis of world average trace metal concentrations and available literature (Hakanson 1980; Bhattacharya et al. 2006; Krupadam et al. 2006; Gong et al. 2008; Ogunkunle and Fatoba 2013; Jiang et al. 2014). The impact of environmental indicators is directly reflected through the single pollution index (Ogunkunle and Fatoba, 2013).This index most often indicates the trace metal pollution incorporating the land use practices or anthropogenic impacts on soil. It tries to correlate the average trace metal concentrations of a particular soil with the world reference values.
The ecological risk index incorporates the total weighted values of trace metal concentrations of the soil in order to evaluate the total potential ecological risk. As this index includes the toxicity of different trace metals and their bioavailability in the soil, and nullifies the regional background levels (Horst, 1997), it serves as a good indicator of the risk factor to ecology. This index is used generally to assess the soil quality with respect to the trace metals.
The pollution Index (PI) is obtained from
where Ci is the mean trace metal concentration obtained by averaging values measured for six seasons for a particular sampling location and Cref is the world average value (Co 11.3, Cr 59.6, Cu 38.9, Mn 488.0, Ni 29.0, Pb 27.0 and Zn 70.0; all values in mg/kg) for the particular trace metal (Kabata-Pendias 2011; Jiang et al. 2014). The Potential Ecological Risk Index for a single trace metal (Er) and the overall Potential Ecological Risk Index (PERI) are calculated using the expressions:
where Tr is toxicity response coefficient with a value of 5 for Co, Cu, Pb and Ni; 2 for Cr and 1 for Zn (Hakanson 1980; Krupadam et al. 2006). The basis of interpretation of the indices is given in Table 2.
Results
Physico-chemical characteristics of the soil
The average values for a few physicochemical parameters (water holding capacity WHC, clay, sand and silt percentages, pH, electrical conductivity EC, soil organic carbon SOC and cation exchange capacity CEC) for the three seasons (hot-dry, hot-wet and cold-dry) are given in Tables 3 and 4. It is found that clay, silt and sand composition of the soil follows the sequence:
-
Clay: S2 > S4 > S3 > S5 > S7 > S1 > S6
-
Silt: S4 > S5 > S6 > S2 > S3 > S1 > S7
-
Sand: S1 > S7 > S3 > S6 > S5 > S2 > S4
and the soil texture is of three types: sandy loam (S1, S6), clayey (S2, S4; clay > 40 %), and sandy-clay-loam (S3, S5, S7). The water holding capacity, WHC, of the soil is in the order of S4 > S2 > S5 > S3 > S6 > S7 > S1 and it is observed generally that the clayey soil has the highest and the sandy-loam soil the least WHC.
Soil texture is an important factor for the retention and release of trace metals. Fine grained soils compared to their coarse counterparts are able to provide large surface area and net negative surface charge to contribute to the electrostatic sorption of the trace metals (Tack 2010). Heavy soils are characterized by higher soil aggregation and retention capacity of soil organic carbon. The soil aggregates acts as a barrier for solute transportation. Water and solutes are absorbed by soil aggregates through the action of capillary forces. These forces increase with the fineness of the soil texture, higher soil organic carbon and dry phase of the soil (Bachmann et al. 2003).
Metals are characterized by their well-known property of low ionization potential which make them prone to exist in the cationic form and to get associated with the soil available anions. Higher clay content and aggregation exert greater capillary force due to distribution of the solute metal ions in the vertical direction and this contributes to the localization of the trace metals in soil.
The pH does not follow any particular order, and the values are in the sequence of S6 > S5 > S4 > S7 > S1 ≈ S2 ≈ S3, the mean values varying from 6.6 to 8.0, mostly in the alkaline range. The soil electrical conductivity (EC) is from 125.8 to 196.2 µS/cm with large deviations from season to season. The mean EC is in the order of S5 > S4 > S2 > S3 > S6 > S1 > S7. The soil organic carbon, SOC, is highest in S2 and lowest in S1 (sequence S2 > S4 > S3 > S5 > S7 > S6 > S1) within the range of mean values from 2.41 to 6.88 % indicating wide variability among the sites. The cation exchange capacity has also a wide variance from 3.91 (S1) to 12.22 (S6) cmol/kg (order S6 > S4 > S2 > S3 > S5 > S7 > S1).
Trace metals
The average seasonal concentrations of 7 trace metals (Co, Cr, Cu, Mn, Ni, Pb, Zn) are given in Table 5. The mean trace metal concentrations are in the sequences of Co: S4 > S6 > S2 > S3 > S5 > S7 > S1; Cr: S4 > S1 > S5 > S3 > S2 > S7 > S6; Cu: S4 > S2 > S6 > S2 > S3 > S7 > S1; Mn: S6 > S7 > S4 > S5 > S3 > S2 > S1; Ni: S4 > S2 > S6 > S5 > S3 > S1 > S7; Pb: S4 > S2 > S6 > S5 > S3 > S7 > S1 and Zn: S2 > S5 > S4 > S3 > S6 > S7 > S1. No two sequences are similar, but the soil from the site S4, is found to be richest in the trace metals (except Mn and Zn) while the site S1 has the least concentration of the trace metals (except Cr and Ni). The mean concentrations of the trace metals are in the ranges of Co: 26.75–51.22 mg/kg, Cr: 5.10–52.94 mg/kg, Cu: 10.02–47.24 mg/kg, Mn: 129.55–445.81, 48.17–99.51 mg/kg, Pb: 100.9–145.3 mg/kg and Zn: 37.80–59.41 mg/kg. The soil thus has considerable contents of the trace metals.
Comparative study of the observed values of the soil trace metals with the world average (Kabata-Pendias 2011) values (Table 6) indicates that Cu, Cr, Mn and Zn are present below the world average while Co, Ni, and Pb have much higher concentrations in the present study sites.
Correlation of trace metals with physicochemical properties
In order to correlate the trace metal concentrations to the major physicochemical characteristics of the soil, a linear regression analysis was done and the correlation coefficients are given in Table 7. A few significant correlations are found. The trace metal, Co, showed good positive correlation with CEC (77 %) and WHC (65 %), and also to some extent with silt (43 %) and clay (32 %) while fairly good negative correlation exists with sand (57 %), but it is very poorly related to SOC, pH and Electrical Conductivity, EC. The sequence at which Co occurs, i.e., S4 > S6 > S2 > S3 > S5 > S7 > S1, is very similar to the sequence of combined (WHC + CEC) except that in the latter, S2 precedes S6.
Cr does not show strong correlation with any of the physicochemical properties taken for consideration, but its weak positive correlation to clay (25 %) and water holding capacity (26 %) as well as negative correlation to sand (33 %), cannot be ignored.
Cu is correlated with most physicochemical parameters except pH. It has strong or moderately strong positive correlation with WHC (91 %), clay (64 %), CEC (59 %), EC (48 %) and SOC (42 %) while it is negatively correlated with sand (88 %). In contrast, Mn is strongly and positively correlated to pH (77 %) and it has also weak, positive correlation with silt (31 %) and CEC (33 %). The soil containing the highest amount of Mn in this work was found to be alkaline (pH ~ 8.0) supporting the fact that Mn retention is favored by higher pH lowering the mobility of the trace metal.
The trace metal, Ni, has also strong or moderately strong correlation with several of the physicochemical properties of the soil, for example, with water holding capacity (74 %), silt (65 %), CEC (60 %), EC (47 %) and clay (34 %), electrical conductance (EC) (~ 47 %) while it has strong negative correlation with sand (70 %). It is observed that Ni-occurrence sequence (S4 > S2 > S6 > S5 > S3 > S1 > S7) is similar to the sequence in which WHC, silt and CEC together appear for the soil samples with a minor exception in the combined value for S1 and S7, i.e. towards the end of the sequence.
The toxic trace metal, Pb, is not dependent on the soil pH as observed from the correlation analysis, but is shows strong positive correlation with WHC (92 %) and moderately strong positive correlation with clay (58 %), CEC (57 %), SOC (47 %), EC (46 %) and silt (36 %). Pb has a very strong, negative correlation with sand (84 %) in the soil. It has again been found that the sequence of Pb-contents (S4 > S2 > S6 > S5 > S3 > S7 > S1) agree very closely with that of the sum of the two highly correlated properties of WHC and CEC with exceptions in S3 and S5.
Zn has very poor or no correlation with silt content of the soil, pH and CEC, but it shows moderately strong positive correlation with EC (67 %), SOC (58 %), clay (51 %) and WHC (54 %), and similar negative correlation with sand (58 %). The observed sequence of Zn contents (S2 > S5 > S4 > S3 > S6 > S7 > S1) is identical to that of the combined SOC, clay and WHC with two exceptions in S5 and S6.
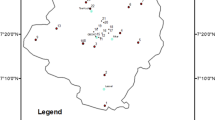
The spatial variation of the average contents of the trace metals, Co, Cr, Cu, Ni, Pb and Zn, for all the seasons is shown in Fig. 2. Since Mn is much more abundant than the other trace metals, it is excluded from this figure. On the basis of the average concentrations of the trace metals, the relative abundance of the trace metals is in the order of Mn > Pb > Cu > Zn (excepting S4) > Co in all the sites. Differences occur mainly with respect to Cr and Ni. Kabata-Pendias (2011) has shown top soil mean contents in mineral soil to be in the sequence of Mn > Cr ≈ Zn > Pb > Cu > Ni > Co, which is different from the one obtained in this work. The observed sequence indicates a relative enrichment of Pb in the soil of the study area and depletion of Cr, Zn, Ni and Co.
Correlation among the soil trace metals
The correlation matrix for the trace metals (Table 8) shows very high positive correlation between Co and Cu (R2 = 0.87), Co and Ni (0.93), Co and Pb (0.91), Cu and Ni (0.92), Cu and Pb (0.93), and Ni and Pb (0.93). The presence of these metals in the soil is thus interrelated strongly suggesting a common origin of the metals. Relatively weak correlation is seen between Cu and Zn (0.61), Pb and Zn (0.61), Cr and Cu (0.58), Cr and Ni (0.58), and Ni and Zn (0.49). The correlation among the trace metals indicates that their enrichment in the soil has originated from a few common sources as observed in other similar work (e.g., Hu et al. 2013).
Contamination and risk indices
The pollution indices, PI (Ci/Cref) computed for each metal separately for each site (Table 9) suggest that the concentrations of Cr, Cu, Mn and Zn represent the uncontaminated state of the soil (PI < 1.0) with respect to these trace metals. However, the soil is moderately polluted with respect to Co and Ni (1 ≤ PI < 3) and considerably polluted with Pb (3 ≤ PI < 6). The situation is more or less similar in all the sites.
Ecological risks due to individual trace metal and the overall risk are summarized in Table 10. The data show a low level of ecological risk due to individual trace metals, but the cumulative potential ecological risk index (PERI) is considerable to put the sites under the medium potential ecological risk category.
Discussion
Higher concentrations of Pb, Cu, and Zn are of ecological concern. These metals might have originated from two industrial sources, a pulp and paper industry (close to S1, S6 and S7) and a cement industry (close to S2, S3, S4 and S5). The probability of deposition from air also cannot be ruled out. The region has been experiencing rapid development works in recent times involving blasting, crushing and transportation of granite rocks among others, and these activities might have contributed to the trace metal contents of the soil in the area. Natural weathering processes are also likely to release some of the trace elements present in the rock. Reports of industrial contributions to trace metal composition of soil are abundant (e.g. Ogunkunle and Fatoba 2013).
In the present study, it is observed that physicochemical properties of the soil such as WHC, SOC, CEC and clay content are correlated to the soil trace metal concentration. In general, higher retention capacity for the trace elements lowers their mobility resulting in local enrichment of the trace elements. It has been suggested that amorphous or particulate soil organic matter increases the effective surface area of the soil particles which helps in retaining more of the trace metals on their surface (Adrino 2001). Further, high molecular weight organic compounds in the SOM can provide sufficient space to entrap the trace metals (Schmitt et al. 2002). Significant correlation of SOM to Co, Mn, Zn and Cu has been known for a long time (Haynes and Swift 1984). It is also known that trace metals, particularly Cu and Pb, are capable of forming insoluble complexes with the organic matter making them less mobile (Battaillard et al. 2003). All these factors lead to accumulation of the trace metals in the soil.
The trace metals can also be retained on the soil particles by a cation exchange mechanism (Martinez and Mottor 2000) when the CEC is considerable. Irrespective of the nature of the soil clay, higher clay content supports higher retention of soil trace elements (Murray et al. 2004). Again solubility of trace metals in soil is a pH regulated phenomenon (Henry 2000) and it has been observed that high soil pH contributes to greater retention of the metals (Basta et al. 1993; Ghosh and Singh 2005). This work has indicated a higher correlation between water holding capacity and trace metal contents which might have resulted from interactions among several soil physicochemical characteristics. For example, CEC is regulated by soil pH and both these properties are influenced by the presence of soil water in the form of WHC providing a medium to support soil chemical processes contributing to higher retention of the trace metals.
This work assesses the enrichment of trace metals without taking into consideration their intrusion into the food chain and consequent biomagnifications that are governed by antagonistic and synergistic interactions of other elements present in the soil. Soil microorganisms with their complex structures can also act as excellent biosorbents for the trace metals retaining them through cell surface adsorption/desorption, intercellular accumulation and metabolism. The presence of the trace metals in the present study should be considered in the context of all these mutually interactive forces.
Conclusion
The trace metals, Co, Cr, Cu, Mn, Ni, Pb, and Zn, were found to follow the same order in which the cumulative (WHC + CEC) existed. Similarly, close correspondence was observed between the sequence in which Ni occurred in the sampling sites and the cumulative (WHC + silt + CEC) and also for Zn and the cumulative (WHC + SOC + clay). The other trace metals, Cu, Cr and Mn, the sequences of their occurrence do not show such relationship with the bulk physicochemical properties. Regressional analysis show significant correlation between Cu and WHC, Clay, CEC, EC and SOC, Mn is related to pH only and Cr does not show any correlation with soil physicochemical properties.
Correlation studies exploring soil trace metal origin suggests a common origin of soil trace metals. Individual pollution indices for Zn, Cu, Cr and Mn suggest uncontaminated state, Ni, Co and Pb values suggest contamination to considerable extent. Though there is no individual potential ecological risk for all the trace metals, when the cumulative potential ecological risk indices (PERI) are evaluated, it is seen that all the sites fall under the medium potential ecological risk category.
References
Adrino DC (2001) Trace elements in terrestrial environments: biogeochemistry, bioavailability and risks of metals, 2nd edn. Springer, New York, p 840
Alfaro MR, Montero A, Ugarte OM, do Nascimento CWA, Accioly AM, Biondi CM, de Silva YJAB (2015) Background concentrations and reference values for heavy metals in soils of Cuba. Environ Monit Assess 187:4198. doi:10.1007/s10661-014-4198-3
Bachmann J, Woche SK, Goebel M-O, Kirkham MB, Horton R (2003) Extended methodology for determining wetting properties of porous media. Water Resour Res 39:1353
Baize D (2010) Concentrations of trace elements in soils: the three keys. In: 19th World Congress of Soil Science, Soil Solutions for a Changing World 1–6 August 2010, Brisbane, Australia. Published on DVD. pp 1–4
Basta NJ, Pantone DJ, Tabataba M (1993) Path analysis of heavy metal adsorption by soil. Agronomy Journal 85:1054–1057
Battaillard P, Cambier P, Picot C (2003) Short-term transformations of lead and cadmium compounds in soil after contamination. Eur J Soil Sci 54:365–376
Bhattacharya A, Routh J, Jacks G (2006) Environmental assessment of abandoned mine tailings in Adak, Västerbotten District (Northern Sweden). Appl Geochem 21:1760–1780
Bini C, Sartori G, Wahsha M, Fontana S (2011) Background levels of trace elements and soil geochemistry at regional level in N E Italy. J Geochem Explor 109:125–133
Carrillo-Gonzãlez R, Ŝimůnek J, Sauvé S, Adrino D (2006) Mechanisms and pathways of trace element mobility in soil. Advances in Agronomy, vol 91. Elsevier, New York, pp 111–178
Driscoll C, Akeiverfeldt JKO (1994) Trace metals speciation and cycling, biogeochemistry of small catchments. In: Moldanand B, Cemy J (eds) A tool for environmental research. Wiley, Chichester, pp 299–322
Galán E, Fernández-Caliani JC, González I, Aparicio P, Romero A (2008) Influence of geological setting on geochemical baselines of trace elements in soils. Application to soils of South-West Spain. J Geochem Explor 98:89–106
Gang W, Hubiao K, Xiaoyang Z, Hongbo S, Liye C, Chengjiang R (2010) A critical review on the bio-removal of hazardous heavy metals from contaminated soils: issues, progress, eco-environmental concerns and opportunities. J Hazard Mater 174:1–8
Ghosh M, Singh SP (2005) An overview on phytoremediation of heavy metals and utilization of its byproducts. Appl Biol Environ Res 3(1):1–18
Gong Q, Deng J, Xiang Y, Wang Q, Yang L (2008) Calculating pollution indices by heavy metals in ecological geochemistry assessment and a case study in parks of Beijing. J China Univ Geosci 19(3):230–241
Green CH, Heil DM, Cardon GE, Butters GL, Kelly EF (2003) Solubilization of manganese and trace metals in soils affected by acid mine runoff. J Environ Qual 32(4):1323–1334
Hakanson L (1980) An ecological risk index for aquatic pollution control: a sedimentological Approach. Water Res 14:975–1001
Hasse PR (1994) A textbook of soil analysis. Indian reprint. CBS Publishers and Distributors Pvt. Ltd, New Delhi. ISBN 978-81-239-1833-4
Haynes RJ, Swift RS (1984) Amounts and form of micronutrient cations in a group of loessal grassland soils of New Zealand. Geoderma 33(1):53–62
Henry JR (2000) An overview of phytoremediation of lead and mercury. NNEMS Report, Washington DC
Horst MF (1997) Heavy metal distribution in sediments and ecological risk assessment: the role of diagenetic processes in reducing metal toxicity in bottom sediments. Environ Pollut 97(3):317–325
Hu Y, Liu X, Bai J, Shih K, Zeng EY, Chen H (2013) Assessing heavy metal pollution in the surface soils of a region that had undergone three decades of intense industrialization and urbanization. Environ Sci Pollut Res 20:6150–6159
Huang PM (2008) Impacts of physicochemical-biological interactions of metals and metalloid transformatons in soils: an overview. In: Violante A, Huang PM, Gadd GM (eds) Biophysico-chemical processes of heavy metals and metalloids in soil environments. Wiley, New York, pp 3–52
Huang L, Hong J, Tan WF, Hu HQ, Liu F, Wang MK (2008) Characteristics of micromorphology and element distribution of iron-manganese cutans in typical soils of subtropical China. Geoderma 146:40–47
Jackson ML (1958) Soil Chemical Analysis. Prentice-Hall Inc, Englewood Cliffs
Jiang X, Lu WX, Zhao HQ, Yang QC, Yang ZP (2014) Potential ecological risk assessment and prediction of soil heavy-metal pollution around coal gangue dump. Nat Hazards Earth Syst Sci 14:1599–1610
Kabata-Pendias A (2011) Trace elements in soils and plants, 4th edn. CRC Press, Boca Raton
Kashem MA, Singh BR (2001) Metal availability in contaminated soils: I. Effects of flooding and organic matter on changes in Eh, pH and solubility of Cd, Ni and Zn. Nutr Cycl Agroecosyst 61(3):247–255
Krupadam RJ, Smita P, Wate SR (2006) Geochemical fractionation of heavy metals in sediments of Tapi estuary. Geochem J 40:513–522
Liang HM, Chiou JM, Yeh KC (2009) Model evaluation of phytoextraction potential of heavy metal hyperaccumulators and non-hyperaccumulators. Environ Pollut 157:1945–1952
Martinez CE, Mottor HL (2000) Solubility of lead, zinc and copper added to mineral soils. Environ Pollut 107:153–158
Murray KS, Rogers DT, Kaufman MM (2004) Heavy metals in an urban watershed in Southeastern Michigan. J Environ Qual 33:163–172
N’guessan YM, Probst YL, Bur T, Probst A (2009) Trace elements in stream bed sediments from agricultural catchments (Gascogne region, S-W France): where do they come from? Sci Total Environ 407(8):2939–2952
Ogunkunle CO, Fatoba PO (2013) Pollutant loads and the ecological risk assessment of soil heavy metals round mega cement factory in Southwest Nigeria. Pol J Environ Stud 22(2):487–493
Pekey H, Karakas D, Ayberk S, Tolun L, Backoglu M (2004) Ecological risk assessment using trace elements from surface sediments of Izmit Bay, Turkey. Mar Pollut Bull 48:946
Sarkar D, Halder A (2010) Physical and chemical methods in soil analysis, 2nd edn. New Age International Publishers, New Delhi. ISBN 978-81-224-2725-7
Schmitt D, Taylor HE, Aiken GR, Roth DA, Frimmel FH (2002) Influence of natural organic matter on the adsorption of metal ions onto clay minerals. Environ Sci Technol 36:2932–2938
Tack FMG (2010) Trace elements: general soil chemistry, principles and processes (chapter 2). In: Hooda PS (ed) Trace elements in soils. Wiley, Chichester, pp 9–37
Whitbread A, Blair G, Konboon Y, Lefroy R, Naklang K (2003) Managing crop residues, fertilisers and leaf litters to improve soil C, nutrient balances, and grain yield of rice and wheat cropping systems in Thailand and Australia. Agric Ecosyst Environ 100(2–3):251–263
Wilke BM (2005) Water holding capacity. In: Margesim R, Schinner F (eds) Manual for soil analysis-monitoring and soil bioremediation, vol 5. Springer, Berlin, pp 47–52
Wilson A, Burt R, Indorante SJ, Jenkins AB, Chiaretti JV, Ulmer MG, Scheyer JM (2008) Geochemistry in modern soil survey program. Environ Monit Assess 139:151–171
Ziper C, Komarneni S, Baker DE (1988) Specific cadmium sorption in relation to the crystal of clay minerals. Soil Sci Soc Am J 52:49–53
Acknowledgments
This work was carried out under the Faculty Improvement Programme of University Grants Commission, New Delhi, India for one of the authors (G.A). The authors are grateful to the reviewers for suggesting a few important points for improving the quality of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adhikari, G., Bhattacharyya, K.G. Ecotoxicological risk assessment of trace metals in humid subtropical soil. Ecotoxicology 24, 1858–1868 (2015). https://doi.org/10.1007/s10646-015-1522-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-015-1522-9