Abstract
Heavy metals are well known for their toxicity and become significant environmental pollution with a continually rising technology and public outcry to ensure the safest and healthiest environment. The present study aims to investigate the uptake capability of heavy metals and its impact on the growth dynamics of Ricinus communis L. (castor bean), along various habitats in Qalyubia Province, Egypt. Three composite plants and soil samples were collected from four different habitats: urban (residential area), canal banks, field edges, and drain banks. The samples were analyzed for nutrients and heavy metals. At the same time, forty quadrats (5 × 5 m) were selected to represent the micro-variations of castor bean in the selected habitats to determine its growth criteria and normalized vegetation index (NDVI). The lowest size index, volume, and number of leaves of castor bean were recorded along canal banks and they were characterized by high soil heavy metal concentration, especially Zn, Cu, and Ni, while the highest values were recorded along field edges with lower heavy metal concentration. Moreover, the NDVI indicated that castor bean from most studied habitats, except field edges, was healthy population. This study revealed that the leaves collected from all habitats were considered to be toxic with Cu. The bioconcentration factor (BF) of the investigated heavy metals was greater than 1. The BF order for heavy metals uptake by castor bean leaves was Fe > Ni > Mn > Cu > Zn. Consequently, the species selected in the present study can be used as a biomonitor of these heavy metal polluted soils. Moreover, it could be used as a phytoremediator, taken into consideration its use in all medicinal purposes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental factors such as soil conditions, interactions with other species, and dispersal are key factors in determining a plant species abundance and distribution within a particular climatic zone (Farahat et al. 2015). The plant size variations and density reflect the status of a plant population, which may be caused by age variations, resource heterogeneity, and competition through variances in growth rates or directly (Galal 2011). The temporal difference in numerous abiotic and biotic factors determines the growth and size structure of plant population that affect the individuals in the population (Bullock et al. 1996). Studies on population size and density are important for testing hypotheses relevant to its management (Sagarin et al. 2006). Woody plants provide sources of fuel for native inhabitants; have potential industrial, medicinal, and phytoremediation values; and play an essential role in soil shelter and stabilization against movement of water or wind (Galal 2011; Galal et al. 2016a).
The castor bean plant is a perennial shrub of the spurge family Euphorbiaceae (Worbs et al. 2011). Weiss (2000) referred that the castor bean is native in both Ethiopia and tropical Africa regions and was used by the Romans, Greeks, and Ancient Egyptians. It became naturalized as a weed almost all over the world, in both tropical and/or subtropical climates (Manpreet et al. 2012). Castor bean plant is successful at invading non-native habitats with frequent and/or intense disturbance regimes (Carmona-Galindo et al. 2013). It often grows as a wild plant in polluted and degraded soils. Castor bean has a major role in remediation and improving the green cover by stabilizing nutrient cycling (Singh et al. 2012). In addition, castor bean is often abundant along watercourses and floodplains, fallow lands, and roadsides with a high tolerance for growth under harsh environmental conditions, such as low rainfall and high temperature (Yashim et al. 2016). Castor bean is one of the medicinal and bioenergy plants in Egypt (Mamoucha et al. 2016) and used for biodiesel production for its high oil content (> 42%) of castor seeds (Ren et al. 2017; Palconite et al. 2018). Conversely, the plant is said to be one of the most poisonous due to the existence of a naturally occurring protein, ricin, however, it has some very important phytochemical constituents such as flavonoids, saponins, alkaloids, and steroids (Mamoucha et al. 2016). It has the potential to be used as biopreservative in food industries and applied as an efficient cancer therapy strategy (Javanshir et al. 2020). Castor bean in semi-arid subtropical agro-climatic conditions is considered a fast growing oil crop with high phytoremediation potential (Shi and Cai 2009). It remediates high concentrations of Cu, Zn, Mn, Pb, and Cd from metalliferous substrates (Olivares et al. 2013). The propagation is by seeds at depth of 3–6 cm and seeding ranging between 11.2 and 15.5 kg/ha, depending on seed size and type of spacing (Salihu et al. 2014).
Phytoremediation is the use of hyper-accumulating plants to remove pollutants from the environment or render them harmless with low costs and eco-friendly nature (Rajkumar and Freitas 2008). It gained enormous momentum for environmental decontamination and particularly for using castor bean (Kiran and Prasad 2017). The selection of appropriate candidate species is of great importance for successful rehabilitation of contaminated soils. This species should be tolerant to the stress caused by the pollutants and must produce high biomass under several limitations (Kiran and Prasad 2017). In this context, castor bean grows with a fast rate and able to accumulate heavy metals in heavily polluted soils (Shi and Cai 2009; Huang et al. 2011). Furthermore, it has industrial and economic importance as an excellent and a cash crop in modern agriculture along with its phytoremediation of heavy metal polluted soils (Vamerali et al. 2010; Yashim et al. 2016). Moreover, castor bean contains some distinguished inedible features, high biomass production, and tolerance to both biotic and abiotic stresses such as heavy metals, salinity, drought, and pests (Zhang et al. 2015; Bauddh et al. 2015). According to Salihu et al. (2014), the annual total world production is 1,314,193 MT, produced on a total area of 1,369,720 ha, while the average seed yields never exceeded 1200 kg ha−1.
Heavy metal pollution is a global problem that is considered one of the major toxic environmental pollution causing global disaster (Ananthi et al. 2012). In recent years, metal hyperaccumulators are of great interest because of their potential application as phytoremediator of contaminated soils (Tang et al. 2009). For successful phytoremediation, hyperaccumulators and accumulators have to be screened in heavy metal contaminated soils (Ananthi et al. 2012). As an important nonedible oilseed crop, castor bean makes up for the disadvantages of phytoremediation due to its relatively high growth rate and biomass compared to other hyperaccumulators (Huang et al. 2017), high economic value, and strong ability to accumulate and tolerate high heavy metal levels (Zhou et al. 2020). So, the present study aims to assess the uptake capability of heavy metals and its impact on the growth dynamics of castor bean, in terms of size structure, density, number of branches and leaves, leaves area, and normalized vegetation index (NDVI), along the different habitats in Qalyubia Province, Egypt.
Materials and methods
Study area
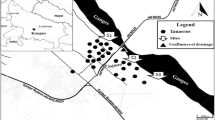
The study area is considered as anthropogenic produced (urban) or naturally regenerating habitats that occur in all kinds of disturbed ground. The study area is located at the south Nile Delta in Qalyubia Province (30° 18′ 0″ N, 31° 15′ 0″ E), which is known for its agricultural crop production and industrial processes. The province is a major source of production of many economic crops such as maize, cotton, wheat, and citrus, in addition the most extensive industrial activities such as electrical cables, plastic, automobile, oil refining, minerals, and fertilizer industries. In the study area, rainfall occurs from October to May (the cold season), while it is practically rare at summer; and the total annual rainfall is 31.7 total year−1. The average annual minimum and maximum temperatures are 12.1 °C and 27.6 °C, respectively. The value of mean relative humidity is 59.6% according to the Egyptian Meteorological Organization in 2018.
Growth measurements
Plant samples were collected from four different habitats: (a) urban (residential area), (b) canal banks receiving the wastes from anthropogenic activities, (c) field edges, and (d) drain banks that obtain agricultural and sewage wastes (Fig. 1). Sampling was done during the spring season (March 2018). All the selected populations were healthy and with no physical harm. In this area, castor bean makes up pure stands. Forty quadrats (5 × 5 m) were chosen to study the micro-variations of castor bean in the chosen habitats. Ten quadrats that distributed on two sites were selected in each habitat. In each quadrat, all individuals of castor bean were counted to calculate plant density (i.e., number of individuals per unit area). For each individual in the quadrat, the number of branches and number of leaves/branch were counted, while the leaf area was determined by trace paper method.
Ninety individuals from the study habitats were selected for measuring their growth criteria. For each individual, its tree height (H) and average crown diameter (D) were measured (based on 3 measurements ind.−1), and its volume was valued as a cylinder. Size index of each individual was calculated as (H + D)/2 (modified from Crisp and Lange, 1976) and used to categorize castor bean population in each habitat into 6 size classes (< 1.5 m, 1.5–2.0 m, 2.0–3.0 m, 3.0–4.0 m, > 4.0 m). Absolute frequency of castor bean individuals per size class and mean tree height and crown diameter per individual tree in each size class were then computed. The size-frequency distribution of the whole population was also determined. According to Shaltout et al. (2015), tree height to tree diameter ratio (H/D) per individual was estimated.
Plant analysis
In each castor bean habitat, three composite plant samples (leaves), from three different individuals, were composed and transported to the laboratory in plastic bags. Samples were washed thoroughly with tap water and rinsed with deionized water, left to air-dry, and subsequently oven-dry at 65 °C till constant weight. The samples were ground into fine powder in an agate mortar.
Oven-dried samples were ground in stainless steel blender into a fine powder and then were sifted by a 2mm sieve. One gram of ground sample was digested in 20 ml tri-acid mixture of nitric acid:sulfuric acid:perchloric acid (5:1:1, v/v/v) till the mixture looked highly transparent. Next, the plant digested solutions were filtered and diluted to 25 ml with double deionized water (Allen 1989). Total N was determined by Kjeldahl method, while total P was determined by applying a molybdenum blue method using a spectrophotometer (CECIL CE 1021). Calcium and potassium were assessed using flame photometer (CORNING M410), whereas magnesium (Mg), iron (Fe), copper (Cu), manganese (Mn), zinc (Zn), and nickel (Ni) were assessed using atomic absorption (Shimadzu AA-6200). All these procedures are described by Allen (1989).
For plant chlorophyll and carotenoid analysis, the three castor bean leaves were collected from each quadrat and were mixed to make three composite samples from each habitat. Estimation of chlorophylls and carotenoids was measured spectrophotometrically using aqueous acetone as the three wave lengths 453, 644, and 663 nm were applied according to the method of Allen (1989):
where A is the absorbance. The values were then expressed as (mg g−1 fresh wt.).
Analysis normalized vegetation index
The normalized difference vegetation index (NDVI) technique is a very suitable technique to detect the vegetation and to identify the difference between the vegetation of two different types. Temporal and spatial changes of vegetated areas are well demarcated through NDVI technique. NDVI index values ranged from −1.0 to 1.0, but the values of vegetation usually ranged between 0.1 and 0.7. The higher levels of healthy vegetation cover are associated with higher index values. NDVI maps were produced using data collected by the moderate resolution imaging spectroradiometer (Landsat 8). For Landsat 8 OLI data, band 4 and band 5 are required for generating NDVI.
The NDVI method has been measured according to this equation:
where RED and NIR are the reflectances of red and near-infrared, respectively in the Landsat satellite data (Yongnian et al. 2010; Guha 2016).
Soil analysis
Three composite soil samples were composed from each habitat including the soil at a profile 0–50 cm. 1:5 soil-water extracts were prepared for the estimation of different parameters. These extracts were used to determine total nitrogen and total phosphorus using micro-Kjeldahl method and a spectrophotometer (CECIL CE 1021) by applying molybdenum blue method, respectively. Calcium and magnesium were determined by titration against 0.01 mol l−1 versenate solution (EDTA disodium salt) using murexide and eriochrome black T as indicators, while potassium was determined using flame photometer. Iron, zinc, copper, manganese, and nickel were estimated using atomic absorption spectrophotometer (Shimadzu AA-6200). All the above-mentioned procedures are outlined in more details by Allen (1989). The apparatus setting and operational conditions were by the manufacturers’ specifications.
Statistical analysis
One-way ANOVA was applied to calculate the significance of variations in the soil, plant growth variables, elements, organic components, and nutritive variables in relation to habitat type. Pearson simple linear correlation coefficient (R) was calculated for evaluating the type of relationship between plant height and diameter (SPSS 2012). In addition, simple linear regression analysis was carried out to assess the relationship between mean NDVI and density (ind. ha−1). A bioaccumulation factor (BF) index is the capability of the plant to accumulate a particular metal with definite to its concentration in the soil substrate (Ghosh and Singh 2005); it was measured as follows: BF = Cplant/Csoil, where Cplant and Csoil symbolize the heavy metal concentrations in the plant and soil, respectively.
Results
Population growth traits
Castor bean was recorded in pure stands or associated with few species such as Chenopodium murale L., Convolvulus arvensis L., Sonchus oleraceus L., and Malva parviflora L. (authors’ observation). The growth measurements of castor bean individuals showed significant variations among the different habitats (Table 1). The highest plant density (13,200 individual ha−1) was recorded along the canal banks, while the lowest (960 individual ha−1) was recorded along the field edges. In addition, the highest total mean of the individual’s height (4.86 m) was recorded for the individuals of the urban areas, while the lowest (2.40 m) was in the drain bank. On the other hand, the individuals had their highest mean crown diameter (3.72 m) in urban areas, while the lowest (0.97 m) was recorded in the canal bank. The correlations between an individual’s height and diameter of castor bean individuals (N = 90) were positively significant in all habitats with the highest correlation coefficient (R = 0.79) in urban habitat and the lowest (R = 0.72) in the drain banks. Moreover, the plant exhibited higher height/diameter ratios more than unity in all habitats with the highest value (2.58) for canal bank plants, while the lowest (1.30) was observed in plants from urban areas.
The individuals of castor bean had its highest size index (4.29) and volume (55.29 m3) in urban areas, while the lowest was recorded in canal bank plants (1.70 and 1.39 m3, respectively). Additionally, the variations in the number of branches, number of leaves per branch, and the leaf area of castor bean were significant among the different habitats (Table 1). The highest number of branches (6.17 branch ind.−1) was recorded in drain banks, while the lowest (3.30 branch ind.−1) was obtained in urban habitat. On the other hand, plants from urban areas had the highest number of leaves (211.5 leaves branch−1) and leaf area (562.67 cm2).
The NDVI and its relationship with plant density
The NDVI map of castor bean sites in Qalyubia Province (Fig. 2) illustrated that the white portion within the vegetation cover is indicated a higher NDVI (0.7–1). In comparison, the bright gray is indicated moderate NDVI (0.45–0.6) and the dark gray is indicated lower NDVI (0.1–0.4) (Fig. 2). The mean NDVI values at the four sampled castor bean sites ranged between 0.32 and 0.608 (Fig. 2). The simple linear regression analysis between mean NDVI and density showed a significant positive relationship and relatively strong coefficient of determination (R2 = 0.66, p > 0.05) (Fig. 3).
Size-frequency distributions
The size-frequency distributions of castor bean populations that collected from different habitats showed positively skewed size distribution as illustrated in (Fig. 4). The highest size frequency (47.3%) was recorded in the class 1.5–2 m, while the lowest (4.3%) was recorded in the class of the largest individuals (> 4 m). The height and diameter were positively correlated (r = 0.77) with the highest values (5.78 and 3.78 m) in class > 4 m, and the lowest (2.01 and 0.79 m) were recorded for plants of the smallest class (< 1.5 m).
Plant analysis
Inorganic nutrients
The analysis of the inorganic elements of castor bean showed significant differences in Ca, N, P, K, and Mg between plant shoots from the different habitats (Table 2). It was indicated that the aboveground tissues from urban areas contributed the highest values of total P and K (0.26 and 0.43 mg kg−1, respectively), and the lowest value of total Ca and Mg (0.02 and 0.43 mg kg−1). However, the field edge plants had the lowest total N, P, and K contents (0.13, 0.07, and 0.31 mg kg−1). Moreover, the highest contents of plant Ca, N, and Mg (1.53, 0.36, and 0.71 mg kg−1) were recorded in drain banks, canal banks, and field edges, respectively.
Heavy metals analysis
The analysis of heavy metal concentration of the aboveground tissues of castor bean showed great variations among the different habitats (Table 3). The plant tissues from the individuals grown along the field edges had the highest concentrations of Fe and Mn (17082.67 and 683.61 mg kg−1), while those from canal banks had the highest concentrations of Zn and Cu (145.98 and 50.92 mg kg−1). Also, the highest Ni concentration (27.14 mg kg−1) was recorded in the drain plants. Moreover, the lowest concentrations of Zn, Cu, and Ni (64.29, 40.21, and 17.20 mg kg−1) were recorded in the tissues of urban plants, while the lowest Fe and Mn concentrations (5089.31 and 405.75 mg kg−1) were recorded in canal bank plants.
Pigment analysis
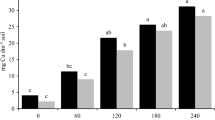
The pigment analysis of the green leaves of castor bean showed great variation among the different habitats (Fig. 5). Plants grown along the drain banks had the highest contents of chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids (0.90, 0.94, 1.84, and 7.15 g g−1), while those from the canal banks had the lowest values (0.65, 0.34, 0.99, and 5.90 g g−1, respectively).
Soil analysis
The analysis of the inorganic elements of castor bean soils indicated significant differences in Ca, N, P, K, and Mg between plant shoots from the different habitats as illustrated in Table 4. The results indicated that the urban soils contributed the highest values of total P (0.49 mg kg−1), but the lowest values of Ca, K, and Mg were 0.11, 1.82 and 0.23 mg kg−1, respectively. In addition, the highest Ca and total N contents were recorded in canal bank soils with values of 0.74 and 5.41 mg kg−1, while the highest Mg value (0.55 mg kg−1) was recorded along the drain banks. Moreover, the field edge soils had the lowest values of total N and P (3.9 and 0.26 mg kg−1, respectively).
The analysis of heavy metal concentration in the soils of castor bean showed great variations among the different habitats (Table 4). The drain bank soils had the highest concentrations of Fe and Mn (1012.96 and 103.66 mg kg−1), while those from canal banks had the highest concentrations of Zn and Cu (63.27 and 12.57 mg kg−1). However, the highest Ni concentration (2.61 mg kg−1) and lowest Mn concentration (36.35 mg kg−1) were recorded in the urban soils. Moreover, the lowest concentrations of Fe, Zn, Cu, and Ni (466.12, 37.51, 5.23, and 1.35 mg kg−1, respectively) were recorded in the soil of the field edges.
Bioaccumulation of heavy metals
The descriptive statistical results of the bioaccumulation factor (BF) for heavy metals from soil to plant shoot (Fig. 6) indicated that all investigated metals had BF more than one. The BF of heavy metals fell in the order of Fe (17.04) > Ni (11.75) > Mn (8.47) > Cu (5.47) > Zn (1.91).
Discussion
Many factors affect plant growth creating natural variability and productivity including an integration of these interactions (Severino et al. 2017). The differences in growth rates due to resource heterogeneity, age, and competition cause directly or indirectly changes in the status of a plant population through its density and size variations (Galal 2011). The largest density of castor bean in the present study was registered on the renewable water resource canal banks, sedimentation and reduction in soil erosion, which favor the seed germination, seedling emergence, and stability of the production. On the other hand, the lowest plant density was recorded along field edges with lower soil moisture contents due to the dense grass cover. Moreover, the low plant density in part, due to low temperature, reduced growing season, and soil moisture content, as in particular to eco-physiological bonds (Galal 2011). In this study, the lower size index, volume, and number of leaves of castor bean was recorded along canal banks characterized by high soil heavy metal concentration, especially Zn, Cu, and Ni, the highest values were recorded along field edges with lower heavy metal concentration. Shehata and Galal (2020) and Slima and Ahmed (2020) reported that heavy metals have bad effects on several morphological and physiological features, which lead to inhibiting the growth of most plant species. According to Yruela (2005) and Huang et al. (2018, 2020), the excess Cu concentration inhibits plant growth and impairs important cellular processes (i.e., photosynthetic electron transport). Sarwar et al. (2017) observed that growth reduction through the decreasing photosynthetic rate and chlorophyll content can be affected by heavy metals, while Hadi et al. (2014) and Galal et al. (2017) recorded that the harmful effects of heavy metals on plant growth reduce plant height, root length, and leaf area. According to Mizoi and Yamaguchi-Shinozaki (2013), the plants may have a survival strategy under risky conditions of heavy metals via induced stunted growth.
Remotely sensed data is usually readily available over large areas, so it has been widely used for assessing vegetation structural diversity (Ozdemir and Karnieli 2011; Halmya et al. 2019) and used as a source of biophysical information of several tree plants species. Mohammadi et al. (2010) recorded a correlation of many vegetation parameters with remote sensed reflectance patterns. Granero-Belinchon et al. (2020) used vegetation indices for monitoring of urban tree vegetation phenological dynamics.
According to Rocchini et al. (2015), Meng et al. (2015), and Feilhauer et al. (2012), the NDVI index has a role to detect species diversity, changes in species composition, and species distribution, due to the reflectance of vegetation has different nature in red bands than the near-infrared (NIR) of the electromagnetic spectrum. Normalized difference vegetation index (NDVI) is perhaps the most widely used proxy (Tucker et al. 1986). The sites of this study had young/growing and also mature trees with bright gray cover in canal bank, urban, and drain bank habitats, which reflected greater NDVI (implying healthy vegetation), while mature trees in the field edges had the lowest number of individuals indicated lower NDVI. Similarly, Kovacs et al. (2005), Satyanarayana et al. (2011), and Essa (2017) used NDVI to indicate healthy mangrove populations. Moreover, the simple linear regression analysis between mean NDVI and plant density indicated a significant positive and relatively strong relationship (R2 = 0.66, p > 0.05). Previous study, Essa (2017) on the management of natural resources and environmental research, found important links between tree numbers and NDVI indexes.
Plant size is the main indicator of competitive ability and the vegetation structure according to several comparative studies (Gaudet and Keddy 1988, 1995; Galal 2011; Galal et al. 2016a). The present study indicated that castor bean attained its highest stem height, diameter, size index, and volume at urban habitat characterized with lower plant density. This result matched with François et al. (2016), who reported a reverse relationship between plant density of jack pine tree and its volume. In addition, the population structure such as plant size distribution has significant importance for evolution and conservation. For instance, the distribution of plant size has usually shown an impact on survival (Wesselingh et al. 1997) and may be linked to inter- and intra-specific rivalry (Galal et al. 2016a). In the present study, the size distribution of castor bean populations was positively skewed and could be highly reproductive and quickly expanding populations. These distributions can also show an elevated youth death rate (Harper 1977), but nevertheless, they seem to characterize long-term stability, since in most stable populations one could expect an excess of juveniles over mature individuals (Shaltout et al. 2015; Galal et al. 2016a). Additionally, heterogeneity of resources, competition, the development rate, and genetic variation are also the cause of these distribution patterns due to growth rate changes (Weiner and Solbrig 1984; Farahat et al. 2015).
The H/D variation could be an indicative of the adaptation and competition of plants to their habitats or the competition of shrubs for resources (Canham et al. 2006). The H/D ratio is important for determining the plant status in different habitats that was greater than unity for castor bean individuals from all the habitats. Results also showed that individuals are motivated to evolve vertically rather than horizontally, which is a plant approach to provide a secure adaptation or just a passive reaction to environmental gradients such as light. Similar results were postulated by Eid (2002) on Ipomea carnea Jacq., Slima (2006) on Pluchea dioscoridis (L.) DC. in the Nile delta, Galal (2011) on Tamarix nilotica (Ehrenb.) Bunge, Shaltout et al. (2015) on Phlomis aurea Decne., and Galal et al. (2016a) on Calotropis procera (Aiton) W.T. Aiton. Moreover, the highest H/D ratio was recorded along canal banks associated with the highest plant density. According to Galal et al. (2016a) and Lentz (1998), plant vertical expansion may be assigned to the high density and then high intraspecific competition of these plants.
Soils with high metal levels usually received anthropogenic and industrial waste (Alyemeni and Almohisen 2014; Galal et al. 2016b). In this study, the soil of urban habitats is rich in nutrients (N, P, and K) and Ni, though the highest levels of Cu and Zn were in the canal bank. According to Huang et al. (2018) and Zhou et al. (2019), the high contents of N and P in soil significantly increased the growth and dry weight (DW) of castor bean plants. The concentrations of the investigated metals, except Fe in drain bank soil, were at the normal level according to UNEP/WHO (2002) and Chiroma et al. (2014). Castor bean can grow easily in the wasteland soils having multiple stresses like salinity, drought as well as heavy metals (Bauddh and Singh 2012).
The bioavailability of the metals in both internal (plant) and external (soil-associated) is the most effective factors on plant heavy metal absorption (Eltaher et al. 2019). The concentration of Fe in castor bean leaves ranged from 6188.00 mg kg−1 at drain banks to 17,082.67 mg kg−1 at field edge. Das (2000) revealed that the ordinary iron ranged between 50 and 250 mg kg−1 in plants, as a result, it can be suggested that iron concentration in castor bean from the studied habitats exceeded the normal range. In addition, Mn concentration ranged between 405.75 mg kg−1 at canal bank and 683.60 mg kg−1 at field edges, which exceeded the safe range (50–500 mg kg−1) of Allen (1989). Moreover, Zn concentration in castor bean leaves ranged from 64.29 mg kg−1 at urban to 145.98 mg kg−1 at canal bank. Furthermore, Cu can cause lethal effects when the aboveground part (stem or leaves) concentrate levels exceeding 20 mg kg−1 (Ren et al. 2017). This study demonstrated a range of Cu ranged from 40.21 mg kg−1 at urban to 50.92 mg kg−1 in canal bank, which indicated the toxicity of leaves with Cu from all habitats. Similar results were reported by Huang et al. (2020) and Zhou et al. (2020) on the same plant.
The measuring of plant capacity to absorb heavy metals from the soil is by the bioaccumulation factor (BF) (Bi et al. 2010; Ratko et al. 2011; Xiao et al. 2011). BF of the investigated heavy metals was greater than 1. The order of BF for R. communis leaves to take up five heavy metals were Fe > Ni > Mn > Cu > Zn, which matched with that revealed by Al-Farraj and Al-Wabel (2007), Galal et al. (2016b) on C. procera, and Galal and Shehata (2015) on Plantago major L. In addition, BF > 1 can refer to the capacity of plants for metal accumulation (Zu et al. 2005). The calculated BF > 1 might indicate that castor bean can be considered a bioaccumulator for the investigated heavy metals. According to Bauddh et al. (2015), castor bean has been established to possess excellent capability to extract greater concentrations of toxic metals like Pb, Cu, Ni, etc. The castor bean has the ability for heavy metals accumulation under natural conditions with powerful phytoextraction (Yashim et al. 2016; Zhou et al. 2019). Finally, the elevated accumulation potential of castor bean is compatible for Fe, Ni, Mn, and Cu; it can be used as a biomonitor for soil pollution. As reported by Bauddh et al. (2015), castor bean seems to be a sustainable crop for phytoremediation purposes because it has commercial value worldwide and can be used globally to remediate the contaminated areas.
Conclusion
The present study showed that castor bean attained its highest stem height, diameter, size index, and volume at urban habitat characterized with lower plant density. It had positively skewed size-frequency distribution. Castor bean had a high bioaccumulation potential for heavy metals, whereas the high BF of this species to heavy metals makes it a promising biomonitor as well as phytoremediator since it is a non-food multiple industrial crop. Moreover, the NDVI indicated that castor bean from the studied habitats except field edges represented healthy populations. Finally, when utilizing castor bean in all medicinal uses, the above highlights should be taken into consideration. Strict monitoring strategy for the polluted medicinal plant habitats is recommended for public health protection in developing countries.
References
Al-Farraj AS, Al-Wabel ML (2007) Heavy metals accumulation of some plant species grown. J Appl Sci 7(8):1170–1175
Allen SE (ed) (1989) Chemical analysis of ecological materials, 2nd edn. Blackwell Scientific Publications, Oxford 368 pp
Alyemeni MN, Almohisen IA (2014) Traffic and industrial activities around Riyadh cause the accumulation of heavy metals in legumes: a case study. Saudi J biol Sci 21(2):167–172
Ananthi TAS, Meerabai RS, Krishnasamy R (2012) Potential of Ricinus communis L. and Brassica juncea (L.) Czern. under natural and induced Pb phytoextraction. Univ J Environ Res Tech 2(5):429–438
Bauddh K, Singh RP (2012) Growth, tolerance efficiency and phytoremediation potential of Ricinus communis (L.) and Brassica juncea (L.) in salinity and drought affected cadmium contaminated soil. Ecotoxicol Environ Saf 85:13–22
Bauddh K, Singh K, Singh B, Singh RP (2015) Ricinus communis: a robust plant for bio-energy and phytoremediation of toxic metals from contaminated soil. Ecol Eng 84:640–652
Bi X, Ren L, Gong M, He Y, Wang L, Ma Z (2010) Transfer of cadmium and lead from soil to mangoes in an uncontaminated area, Hainan Island, China. Geoderma 155:115–120
Bullock JM, Silvertown J, Clear Hill B (1996) Plant demographic responses to environmental variation: distinguishing between effects on age structure and effects on age-specific vital rates. J Ecol 84:733–743
Canham CD, Papaik MJ, Uriarte M, McWilliams WH, Jenkins JC, Twery MJ (2006) Neighborhood analysis of tree competition along environmental gradients in New England forests. Ecol Appl 16:540–554
Carmona-Galindo VD, Hinton-Hardin D, Kagihara J, Pascua MRT (2013) Assessing the impact of invasive species management strategies on the population dynamics of castor bean (Ricinus communis L., Euphorbiaceae) at two southern California coastal habitats. Nat Areas J 33(2):222–226
Chiroma TM, Ebewele RO, Hymore FK (2014) Comparative assessment of heavy metal levels in soil, vegetables and urban grey waste water used for irrigation in Yola and Kano. Int Refer J Eng Sci 3(2):01–09
Das DK (2000) Micronutrients: their behaviour in soils and plants, 1st edn. Administrative Office, New Delhi 75 pp
Eid EM (2002) Population ecology of Ipomoea carnea Jacq. in the Nile Delta region. M.Sc. Thesis, Tanta Univ., Tanta, 118 pp
Eltaher GT, Ahmed DA, El-Beheiry M, Sharaf El-Din A (2019) Biomass estimation and heavy metal accumulation by Pluchea dioscoridis (L.) DC. in the Middle Nile Delta, (Egypt): perspectives for phytoremediation. South Afr J Bot 127:153–166
Essa BS (2017) Integrated field studies and remote sensing approach for mapping seagrasses and mangrove habitats, Red Sea, Egypt. PhD Thesis, Botany Depart. Faculty of women, Ain Shams University
Farahat EA, Galal TG, El-Midany M, Hassan LM (2015) Effect of urban habitat heterogeneity on functional traits plasticity of the invasive species Calotropis procera (Aiton) W.T. Aiton. Rend Fis Acc Lincei 26:193–201
Feilhauer H, He KS, Rocchini D (2012) Modelling species distribution using niche-based proxies derived from composite bioclimatic variables and MODIS NDVI. Remote Sens 4:2057–2075
François H, Cornelia K, Pierre-Yves P, Alexis A, Guy P, Jean M (2016) Effect of tree spacing on tree level volume growth, morphology, and wood properties in a 25-year-old Pinus banksiana plantation in the boreal forest of Quebec. Forests 7:276
Galal TM (2011) Size structure and dynamics of some woody perennials along elevation gradient in Wadi Gimal, Red Sea coast of Egypt. Flora 206:638–645
Galal TM, Shehata HS (2015) Bioaccumulation and translocation of heavy metals by Plantago major L. grown in contaminated soils under the effect of traffic pollution. Ecol Indic 48:244–251
Galal TM, Farahat EA, El-Midany MM, Hassan LM (2016a) Demography and size structure of the giant milkweed shrub Calotropis procera (Aiton) W.T. Aiton. Rend Fis Acc Lin 27:341–349
Galal TM, Farahat EA, El-Midany MM, Hassan LM (2016b) Nutrients and heavy metals accumulation by the giant milkweed Calotropis procera (Aiton) WT Aiton in urbanized areas. Egypt Rend Fis Acc Lin 27(2):241–250
Galal TM, Gharib FA, Ghazi SM, Mansour KH (2017) Metal uptake capability of Cyperus articulatus L. and its role in mitigating heavy metals from contaminated wetlands. Environ Sci Pollut Res 24:21636–21648
Gaudet CL, Keddy PA (1988) A comparative approach to predicting competitive ability from plant traits. Nature 334:242–243
Gaudet CL, Keddy PA (1995) Competitive performance and species distribution in shoreline plant communities: a comparative approach. Ecol 76:280–291
Ghosh M, Singh SP (2005) A comparative study of cadmium phytoextraction by accumulator and weed species. Environ Pollut 133:365–371
Granero-Belinchon C, Adeline K, Lemonsu A, Briottet X (2020) Phenological dynamics characterization of alignment trees with Sentinel-2 Imagery: a vegetation indices time series reconstruction methodology adapted to urban areas. Remote Sens 12:639
Guha S (2016) Capability of NDVI technique in detecting mangrove vegetation. Int J Advan Biol Res 6(2):253–258
Hadi F, Hussain F, Hussain M, Sanaullah AA, Ur Rahman S, Ali N (2014) Phytoextraction of Pb and Cd; the effect of urea and EDTA on Cannabis sativa growth under metals stress. Int J Agron Agri Res 5(3):30–39
Halmya MW, Fawzy M, Ahmed DA, Saeed MN, Awad MA (2019) Monitoring and predicting the potential distribution of alien plant species in arid ecosystem using remotely-sensed data. Rem Sens Appl: Soci Environ 13:69–84
Harper JL (1977) Population biology of plants. Academic Press, London
Huang H, Yu N, Wang L, Gupta DK, He Z, Wang K, Zhu Z, Yan X, Li T, Yang XE (2011) The phytoremediation potential of bioenergy crop Ricinus communis for DDTs and cadmium co-contaminated soil. Bioresour Technol 102(23):11034–11038
Huang GY, Jin Y, Zheng J, Kang W, Hu HQ, Liu YH, Zou T (2017) Accumulation and distribution of copper in castor bean (Ricinus communis L.) callus cultures in vitro. Plant Cell Tiss Organ Cult (PCTOC) 128(1):177–186
Huang GY, Rizwan MS, Ren C, Guo G, Fu QL, Zhu J, Hu HQ (2018) Influence of phosphorus fertilization on copper phytoextraction and antioxidant defenses in castor bean (Ricinus communis L.). Environ Sci Pollut Res 25(1):115–123
Huang G, Zhou X, Guo G, Ren C, Hu H (2020) Variations of dissolved organic matter and Cu fractions in rhizosphere soil induced by the root activities of castor bean. Chemosphere 254:126800
Javanshir A, Karimi E, Maragheh AD, Tabrizi MH (2020) The antioxidant and anticancer potential of Ricinus communis L. essential oil nanoemulsions. J Food Measur Charact 14(3):1356–1365
Kiran BR, Prasad MNV (2017) Ricinus communis L. (castor bean), a potential multi-purpose environmental crop for improved and integrated phytoremediation. The EuroBiotech J 1(2):101–116
Kovacs JM, Wang J, Flores-Verdugo F (2005) Mapping mangrove leaf area index at the species level using IKONOS and LAI-2000 sensors for the Agua Brava Lagoon, Mexican Pacific. Estuarine. Coast Shelf Sci 62:377–384
Lentz DR (1998) Petrogenetic evolution of felsic volcanic sequences associated with Phanerozoic volcanic-hosted massive sulphide systems: the role of extensional geodynamics. Ore Geol Rev 12(5):289–327
Mamoucha S, Tsafantakis N, Fokialakis N, Christodoulakis NS (2016) Structural and phytochemical investigation of the leaves of Ricinus communis. Aust J Bot 65(1):58–66
Manpreet R, Hitesh D, Bharat P, Shivani S (2012) Ricinus communis L. a review. Int J Pharm Tech Res 4:1706–1711
Meng R, Dennison PE, Huang C, Moritz MA, D’Antonio C (2015) Effects of fire severity and post-fire climate on short-term vegetation recovery of mixed-conifer and red fir forests in the Sierra Nevada Mountains of California. Remote Sens Environ 171:311–325
Mizoi J, Yamaguchi-Shinozaki K (2013) Molecular approaches to improve rice abiotic stress tolerance. In: Yang Y (ed) Rice protocols. Methods in molecular biology (Methods and protocols), vol 956. Humana Press, Totowa, pp 269–283
Mohammadi J, Joibary SS, Yaghmaee F, Mahiny AS (2010) Modelling forest stand volume and tree density using Landsat ETM+ data. Int J Remote Sens 31:2959–2975
Olivares AR, Carrillo-González R, González-Chávez MD, Hernández RM (2013) Potential of castor bean (Ricinus communis L.) for phytoremediation of mine tailings and oil production. J Environ Manag 114:316–323
Ozdemir I, Karnieli A (2011) Predicting forest structural parameters using the image texture derived from WorldView-2 multispectral imagery in a dryland forest, Israel. Int J Appl Earth Obs Geoinf 13:701–710
Palconite CL, Edrolin AC, Lustre SNB, Manto AA, Caballero JRL, Tizo MS, Ido AL, Arazo RO (2018) Optimization and characterization of bio-oil produced from Ricinus communis seeds via ultrasonic-assisted solvent extraction through response surface methodology. Sustain Environ Res 28(6):444–453
Rajkumar M, Freitas H (2008) Influence of metal resistant-plant growth-promoting bacteria on the growth of Ricinus communis in soil contaminated with heavy metals. Chemosphere 71(5):834–842
Ratko K, Snĕzana B, Dragica OP, Ivana B, Nada D (2011) Assessment of heavy metal content in soil and grasslands in national park of the lake plateau of the NP Durmitor Montenegro. Afr J Biotechnol 10:5157–5165
Ren C, You JW, Qi YB, Huang GY, Hu HQ (2017) Effects of sulfur on toxicity and bioavailability of Cu for castor (Ricinus communis L.) in Cu-contaminated soil. Environ Sci Pollut Res 24(35):27476–27483
Rocchini D, Hernández-Stefanoni JL, He KS (2015) Advancing species diversity estimate by remotely sensed proxies: a conceptual review. Ecol Inform 25:22–28
Sagarin RD, Gaines SD, Gaylord B (2006) Moving beyond assumptions to understand abundance distributions across the ranges of species. Trends Ecol Evol 21(9):524–530
Salihu BZ, Gana AK, Gbadeyan T, Alabi MB (2014) Castor oil plant (Ricinus communis L.): a potential oil crop for agribusiness in Africa. Int J Appl Res Technol 3(8):29–35
Sarwar N, Imran M, Shaheen MR, Ishaque W, Kamran MA, Matloob A, Hussain S (2017) Phytoremediation strategies for soils contaminated with heavy metals: modifications and future perspectives. Chemosphere 171:710–721
Satyanarayana B, Mohamad KA, Idris IF, Mohd-Lokman H, Dahdouh-Guebas F (2011) Assessment of mangrove vegetation based on remote sensing and ground-truth measurements at Tumpat, Kelantan Delta, East Coast of Peninsular Malaysia. Int J Remote Sens 32:1635–1650
Severino LS, Auld DL, Vale LS, Marques LF (2017) Plant density does not influence every castor plant equally. Ind Crop Prod 107:588–594
Shaltout K, Ahmed DA, Shabana H (2015) Population structure and dynamics of the endemic species Phlomis aurea Decne in different habitats in southern Sinai Peninsula, Egypt. Glob Ecol Conser 4:505–515
Shehata HS, Galal TM (2020) Trace metal concentration in planted cucumber (Cucumis sativus L.) from contaminated soils and its associated health risks. J Consum Protec Food Saf 15:205–217
Shi G, Cai Q (2009) Cadmium tolerance and accumulation in eight potential energy crops. Biotechnol Adv 27(5):555–561
Singh A, Mittal S, Shrivastava R, Dass S, Srivastava JN (2012) Biosynthesis of silver nanoparticles using Ricinus communis L. leaf extract and its antibacterial activity. J Nanomater Biostruct 7:1157–1163
Slima DF (2006) Sociological behaviour and variability among Pluchea dioscoridis (L.) DC. populations in Nile Delta. M.Sc. Thesis, Menoufia University, Shebin El–Kom
Slima DF, Ahmed DA (2020) Trace metals accumulated in pea plant (Pisum sativum L.) as a result of irrigation with wastewater. J Soil Sci Plant Nutr. https://doi.org/10.1007/s42729-020-00341-8
SPSS (2012) SPSS version 20.0. SPSS Inc., Chicago
Tang YT, Qiu RL, Zeng XW, Ying RR, Yu FM, Zhou XY (2009) Lead, zinc, cadmium hyperaccumulation and growth stimulation in Arabis paniculata Franch. Environ Exp Bot 66:126–134
Tucker CJ, Fung Y, Keeling CD, Gammon RH (1986) Relationship between atmospheric CO2 variations and a satellite-derived vegetation index. Nature 319:195–199
UNEP/WHO (2002) Environmental health criteria 226. Palladium, Geneva
Vamerali T, Bandiera M, Mosca G (2010) Field crops for phytoremediation of metal-contaminated land. A review. Environ Chem Lett 8(1):1–17
Weiner J, Solbrig OT (1984) The meaning and measurement of size hierarchies in plant populations. Oecol 61:334–336
Weiss EA (2000) Oilseed crops, 2nd edn. Blackwell Science Ltd., Oxford
Wesselingh RA, Klinkhamer PG, De Jong TJ, Boorman LA (1997) Threshold size for flowering in different habitats: effects of size-dependent growth and survival. Ecol 78(7):2118–2132
Worbs S, Köhler K, Pauly D, Avondet MA, Schaer M, Dorner MB, Dorner BG (2011) Ricinus communis intoxications in human and veterinary medicine—a summary of real cases. Toxins 3(10):1332–1372
Xiao R, Bai J, Zhang H, Gao H, Liua X, Wilkes A (2011) Changes of P, Ca, Al and Fe contents in fringe marshes along a pedogenic chronosequence in the Pearl River Estuary, South China. Cont Shelf Res 31:739–747
Yashim ZI, Agbaji EB, Gimba CE, Idris SO (2016) Phytoremediation potential of Ricinus communis L. (castor oil plant) in Northern Nigeria. Int J Plant Soil Sci 10(5):1–8
Yongnian Z, Wei H, Benjamin ZF, Honghui Z, Huimin (2010) Study on the urban heat island effects and its relationship with surface biophysical characteristics using MODIS Imageries. Geospat Inform Sci 13(1):1–7
Yruela I (2005) Copper in plants. Braz J Plant Physiol 17(1):145–156
Zhang D, Stenger A, Harou PA (2015) Policy instruments for developing planted forests: theory and practices in China, the U.S., Brazil, and France. J Forest Econ 21:223–237
Zhou X, Huang G, Liang D, Liu Y, Hu H (2019) Influence of nitrogen forms and application rates on the phytoextraction of copper by castor bean (Ricinus communis L.). Environ Sci Pollut Res 247:125965
Zhou X, Wang S, Liu Y, Huang G, Hu H (2020) Coupling phytoremediation efficiency and detoxification to assess the role of P in the Cu tolerant Ricinus communis L. Chemosphere 247:125965
Zu YQ, Li Y, Chen JJ, Chen HY, Qin L, Schvartz C (2005) Hyperaccumulation of Pb, Zn and Cd in herbaceous plants grown on lead-zinc mining area in Yunnan. Chin Environ Int 31:755–762
Acknowledgements
The authors extend their appreciation to the scientific research deanship for funding this article by Taif University Research Supporting Project number (TURSP-2020/199), Taif University, Taif, Saudi Arabia. They also thank Dr. Nashwa Maghraby, professor of the geographic department, Faculty of Women for Arts, Science and Education, Ain Shams University, Cairo, Egypt; for her assistance in Meteorological information of the study area.
Availability of data and materials
Not applicable.
Author information
Authors and Affiliations
Contributions
TG proposed the study, analyzed the data, and revised the manuscript; BE carried out the field work, analyzed plant and soil, made the remote sensing, and wrote the manuscript; and HE analyzed the data and revised the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Galal, T.M., Essa, B. & Al-Yasi, H. Heavy metals uptake and its impact on the growth dynamics of the riparian shrub Ricinus communis L. along Egyptian heterogenic habitats. Environ Sci Pollut Res 28, 37158–37171 (2021). https://doi.org/10.1007/s11356-021-13383-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13383-x