Abstract
The Yangzi River has been seriously affected by intensive anthropogenic activities in recent years. In this study, the levels and distribution, risk assessment, and source apportionment of heavy metals in sediments were investigated in the Yangtze River (Anqing section). The heavy metal concentration and chemical characteristics (Cu/Pb/Zn/Cd/Cr/As/Hg) of 16 samples over four sections were measured using Atomic Fluorescence Spectrometry and Graphite Furnace Atomic Absorption Spectrometry. The metal concentration in the study area showed variance in different sampling sites. The mean heavy metal content was also higher than the background value. Vertical analysis of metal concentration showed the decreasing order from surface sediment. The analysis of Hakanson’s potential risk assessment indicated that the total potential ecological risk of these elements amounted to a considerable pollution level in the study area. The result of geo-accumulation index indicated that the risk of the studied metals had the following decreasing order: Cd > Hg > Pb > Cu > Zn > As > Cr. Based on the result of enrichment factor and principal component analysis, Cr, As, Hg, and partly Zn, Cu, and Cd mostly originated from natural sources. Pb and partly Cu and Zn mostly originated from gas companies, industries, and traffic sources, whereas Cd and partly Zn represented agricultural activities, such as fertilizer and pesticide consumption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to the toxicity, persistence, and bioaccumulation in the environment, heavy metal contamination has attracted much more attention and becomes a worldwide environmental concern in recent years (Yong et al. 2013). In the last 40 years, because of the rapid industrialization of China, heavy metals have been discharged into the environment through all kinds of natural and anthropogenic inputs, whether intentionally or not.
Sediments in river ecosystems, as a reservoir or sink for heavy metals, can act as a source of heavy metals for aquatic organisms when environmental conditions change (Mihajlovic et al. 2014), which may accumulate to toxic concentrations and have toxic effects on benthic fauna and other aquatic organisms (Li et al. 2017; Zhao et al. 2014). Previous studies have shown that significant amounts of heavy metals (such as Cu/Pb/Zn/Cd/Cr/As/Hg) have accumulated in river sediments in highly urbanized areas and show a relatively high ecological risk (Alves et al. 2014; Hu et al. 2014; Martínez and Poleto 2014; Xun and Xuegang 2015). Heavy metals can be bio-accumulated and biomagnified through the food chain, resulting in elevated levels in freshwater organisms (Rajmohan et al. 2014), such as fish, which ultimately does harm human health. Therefore, the source apportionment and risk assessment of heavy metals in sediments are essential in understanding the possible changes of sediment quality caused by intensive anthropogenic activities (Massey et al. 2013; Yang et al. 2014; Yuan et al. 2014; Wang et al. 2014a, b; Martínez-Graña et al. 2016).
Understanding the sources and assessment of pollutants in aquatic sediments is important for the pollution control. Multivariate statistical methods are useful in assessing the inter-relationships among measured data and are widely used in geochemical studies (Deng et al. 2014; Wang et al. 2014a, b). Statistical approaches, such as principal component analysis (PCA), are frequently used for the apportionment of contamination sources by grouping sampling sites and metals (Wu et al. 2014; Yuan et al. 2014; Wang et al. 2014a, b; Zhang et al. 2017); enrichment factors are often calculated to evaluate the status of anthropogenic discharge into the sediment (Cui et al. 2014a, b; Zang et al. 2017). Risk assessments of the environmental pollution are also critical for sediment analysis. Numerous indexes have been developed to assess the environmental risk of heavy metals in river surface sediment based on the total content, bioavailability, and toxicity (Cui et al. 2014a, b; Rajmohan et al. 2014). The geo-accumulation index (Igeo) of individual heavy metals in sediment are calculated using their total content, background value (Ma et al. 2016, 2017). Potential ecological risk index was used wildly to assess the comprehensive risk of multiple heavy metals in sediment (RI) (Martínez and Poleto 2014; Alves et al. 2014).
The Yangtze River basin is now causing serious environmental pollution problems with heavy metals being discharged into the river. Anqing, a city located at midstream of The Yangtze River, is a highly industrialized city with a large number of factories, such as petrochemical plants, electroplating plants, steel mills, and so on. Heavy metals contamination in river sediments from these areas has occurred over the last several decades (Chen et al. 2013). It is very urgent and important to maintain the sustainable development of the study area by strengthening the understanding of the level and risk of heavy metals pollution in the river sediments. However, until now few studies that assess the ecological risks posed by heavy metals contamination and their spatial and temporal differentiations of river sediments have been reported in the Yangtze River Anqing section, where it is closely related to abundant human activities.
Therefore, it is important to investigate heavy metals distributions, the degree of pollution, and the potential ecological risk in this area. The main objectives of this study were as follows: (1) to determine the levels and distribution of the toxic heavy metals in sediment; (2) to explore the degree of contamination and pollution impacts using comprehensive pollution indicators; and (3) to investigate the nature and origin of the contamination of the river sediments.
Materials and methods
Study area and sediment sampling
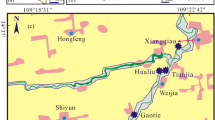
In this study, the sediment samples from 16 sampling sites in the study area were collected in April, 2014. The sampling sites are shown in Fig. 1. Among those sampling sites, section A was around agricultural area, section B was closed to residential area, section C was around industrial regions, and section D was around natural area. A composite sample was collected at each site by mixing 4–5 random samples. Composite sediments (0–20 cm) were taken using a grab sampler. Vertical samples were collected by sedimentary column sampler; the core samples (0–100 cm) were cut apart every 10 cm. All samples were sealed in clean plastic bags and delivered to the laboratory immediately.
Chemical analysis
The sediment samples were air-dried, homogenized, ground, and sieved with a 100-mesh nylon screen with the stones and plant fragments removed (Yuan et al. 2014; Cui et al. 2014a, b; Hu et al. 2014). Coarse particles were used to determine pH and electrical conductivity (EC), and the fine particles were used to analyze sediment organic matter and heavy metals. pH was measured in a suspension ratio of 1:5 sample to distilled water using an Artorius PB-10 Standard pH meter after 30 min of agitation. EC was measured in a 1:5 ratio of soil to water suspension using a Model DDS-307 Conductometer after equilibration for 30 min and subsequent filtering. Soil organic carbon was determined through wet oxidation at 185 °C with a mixture of potassium dichromate and titration with ferrous ammonium sulfate. A coefficient factor of 1.724 was used to convert organic carbon to organic matter (Xun and Xuegang 2015).
To analysis of heavy metals Cu, Pb, Zn, Cd, and Cr, 0.2 g of dry sediment was weighed in Teflon beakers with an added mixture of concentrated HF-HCLO4-HNO3 (5:5:2) and was then digest in a microwave oven, analyzed by Graphite furnace AAS. The sample solutions were then filtered and adjusted to 25 ml with deionized water. As and Hg were analyzed using Atomic Fluorescence Spectrometry (AFS). Fe was measured using Graphite Furnace Atomic Absorption Spectrometry (GF-AAS) (Sakan et al. 2009; Varol and Şen 2009).
Pollution and potential ecological risk assessment
Geo-accumulation index
The Geo-Accumulation Index (Igeo) is defined by the equation:
where Cn is the concentration of heavy metals examined in sediment and Bn is the background concentration of the heavy metals. Factor K was defined 1.5 as the background matrix correction factor caused by lithospheric effects. The Igeo consists of seven classes that are listed in Table 1.
Potential ecological risk index
The Hakanson Pollution Index, reflecting the concentration of a specific heavy metals in the sediment, is used to evaluate the pollution of heavy metals in the sediment (Bianchini et al. 2012). The potential ecological risk of heavy metals is classified into five levels according to the values of Eri and RI (Table 2). The formula for the single heavy metals is:
where Cfi is the pollution coefficient for heavy metal i, which reflects the characters of the pollution of the investigated region but cannot reveal the ecological effects and hazards. Ci is the measured values of heavy metals in surface sediments. Cni is the background values for calculation. Background concentrations of heavy metals were set as 21.02, 26.39, 74.33, 0.07, 63.71, 9.13, 0.02, and 10.14 mg/kg for Cu, Pb, Zn, Cd, Cr, As, and Hg, respectively.
RI is used separately to assess the pollution degree and biological effect of multiple heavy metals pollution in sediment
where Eri is the pollution degree of a single heavy metals in sediment, and Tri is the toxic response factor for a given substance (e.g., Cu = 5, Pb = 5, Zn = 1, Cd = 30, Cr = 2, As = 10, and Hg = 80). Four classes of RI are defined as followed Table 2.
Statistical analysis
Enrichment factors
Enrichment factors (EFs) were wildly used to analyze the source of heavy metals in sediment and were calculated by comparing the sediment sample to an uncontaminated reference material (Alves et al. 2014; de Andrade Lima and Bernardez 2017). The uncontaminated soil representing the background was chosen as the reference material. Meanwhile Al was chosen as the reference element in the EFs calculation. EFs was calculated by:
where xi and x0 are the concentrations of the individual metal in the sample and the background soil, respectively. Ali and Al0 are the concentrations of Al in the sample and the background soil, respectively. The degree of contamination is usually graded based on the EF values: EF < 2, minimal enrichment; EF 2–5, moderate enrichment; EF 5–20, significant enrichment; EF 20–40, high enrichment; and EF > 40, extreme enrichment. In addition, we use EF = 1.5 to recommended as an evaluation standard because when EF < 1.5, the trace metal may be entirely from natural sources, whereas when EF ≥ 1.5, a considerable part of the trace metal may originate from anthropogenic source. Background concentrations of heavy metals were set as 21.02, 26.39, 74.33, 0.07, 63.71, 9.13, 0.02, and 10.14 mg/kg for Cu, Pb, Zn, Cd, Cr, As, and Hg, respectively.
Principal component analysis
Principal component analysis (PCA) was used to investigate the association among the seven heavy metals and infer the hypothetical source of heavy metals (i.e., nature and anthropogenic) (Wang et al. 2014a, b; Duan et al. 2015). The components of the PCA were rotated using a varimax rotation. The Kaiser–Meyer–Olkin and Bartlett’s sphericity tests were performed to examine the appropriateness of the data for PCA. ArcGIS (version 10.2) and SPSS statistical software (version 19.0) were used for all the data analyses.
Results and discussion
Physical and chemical characteristics of surface water and sediment
The mean pH, EC, DO, CODmn, BOD5, ammonia nitrogen, TN, and TP values of the surface water and the pH, EC, and TOC of the sediment in every section are shown in Table 3.
As shown in Table 3, the pH of the surface water and sediment ranged from 7.61 to 7.65 and 7.98 to 8.13, respectively. Slightly variance was observed in the pH of the sediment, indicating that the sediment studied in this study was alkaline and had different of sediment acidifications. Different sediment compositions, such as grain-size distribution, natural heavy metals concentrations, and sediment pH can influence the mobilization of heavy metals. In addition, Sakan et al. (2009) had confirmed that the mobilization of heavy metals is limited and that the plant uptake effect can be decreased. Sediment properties have effects on surface water; the speed of heavy metals stored in sediment released into the water is also inferenced by the sediment properties, such as pH, EC, and TOC. In the study area, the properties of the surface water properties showed the same trend, at sampling sites C, as a drain outlet site in the city showed the worst situation among all the sites. EC and organic matter have an important role in the physical, biological, and chemical properties of sediment that can affect stability, nutrient availability, porosity, and cation exchange capacity. Therefore, the chemical and physical properties between surface water and sediment are related which is very important to analysis.
Spatial distribution characteristics of heavy metal pollution
Table 4 shows a summary of the statistics for the monitoring results, including the concentration ranges, mean, standard deviation (SD), and variable coefficient (CV) of the seven measured heavy metals (Cu, Pb, Zn, Cd, Cr, As, and Hg). The results of the Kolmogorov–Smirnov test (p < 0.01) show that the contents of the elements in the sediment fit a normal distribution.
The concentration of the seven heavy metals, shown in Table 4, varies markedly. The mean average concentration of the heavy metals exhibits the following decreasing order: Zn > Cr > Pb > Cu > As > Cd > Hg. Large variances among the sites are observed for Pb (18.24–144.43 mg/kg) and Zn (50.55–351.24). There are no significant differences between the four sampled areas among most heavy metals. The average concentrations of Cu, Zn, and Hg are almost twice as high as the background values. The sample section C has the highest values, and section D also suffers from high levels. The highest concentrations of Pb and Cd are located in sample site C-2, whereas the lowest are in site B-1 and site B-4. The average concentrations of these two elements are almost three times of their background values. The average concentrations of Cr and As reach the content of the background values. The sites with the highest concentrations are also located in section C, and those with the lowest concentrations are located in section D.
In addition, these heavy metals have similar distribution patterns; all seven elements have the highest concentration in sample section C, where the main drain outlet of SINOPEC is located. Sample section A, which is located in the upstream of the study area, shows relatively low concentration of heavy metals. This may be because no industries were located here, given that this area is an agriculture zone. However, the overuse of fertilizers has caused the concentration of Zn, Cr, and As in some sites to be higher than average. The section B and section D may have the lowest heavy metal concentrations because the water intake of the city is close to section B, whereas section D is located downstream of the study area with no industries or residents there. Water self-purification may also a reason for the low concentration of heavy metals in section D. These results suggest that the sediment in the study areas had received anthropogenic discharge.
Vertical distribution characteristics of the heavy metal pollution were analyzed in this study (Fig. 2). The results show that these heavy metals have a relatively high content at 10–20 cm, which clearly demonstrates an anthropogenic contribution. The Cd and As contents in the 20–30 cm layer of the core profile increase gradually and remarkably, which means that external sources contribute the most content. It also shows that most of the samples in vertical concentration exceed the local background values, except at section D. The results showed that Cd, Zn, Cu, and Pb occur at high concentrations in section C, especially in the 10–20 cm layer. Many paper-making industries, petrochemical plants, steel industries, and small workshops, which do not have waste water treatment equipment, are distributed in section C.
With the analysis of vertical heavy metal monitoring, Pb and Cd show the highest enrichment extent in the sediment and originate mainly from anthropogenic activities (Bolan et al. 2017; Han et al. 2017; Li et al. 2017), such as industrial wastewater and traffic emissions. The Pb and Cd contents with the detected average value of 126.84 mg/kg in section C, which has a petrochemical factory, is much higher than its background value. Given that Pb is added to gasoline, the use of gasoline-powered machines and oil spill disasters represents an anthropogenic source of Pb and can cause high pollution levels. The values of Cu and Hg follow closely and are mostly attributed to agriculture waste water influenced by farmland, aquaculture, and paddy field areas located to the west of the city. Zn, Cr, and As show a slight enrichment especially for Cr, which indicates less input from anthropogenic activities, such as fertilizer and pesticide consumption (Agarwal et al. 2009; El Azhari et al. 2017).
Risk assessment of heavy metal contamination in sediment
The concentrations of heavy metals in sediment were used to assess the quality of life in an environment under the risk of heavy metal exposure. Geo-accumulation and potential ecological risk assessment were used to assess the risk of the study area.
Geo-accumulation index assessment in sediment
According to the comparison between the obtained mean Igeo and the Igeo classification in Table 1, the mean values exhibit in the following decreasing order: Cd > Hg > Pb > Cu > Zn > As > Cr. Cr and As remain in Class 0 (practically unpolluted), and Cu, Pb, Zn, Cd, and Hg in Class 1 (unpolluted to moderately polluted), whereas Cd is nearly Class 2 (moderately polluted). The spatial distribution of Igeo and the class for each heavy metal show that only Cd and Hg have moderate to heavy levels of pollution, while Cr and As had practically unpolluted to moderate levels of pollution. The remaining metals show no level to low levels of pollution in all locations. The moderately polluted areas for Cd occur in sample site C-2, and most Class 2 levels of pollution occur in section C, where the main drain outlet of the city is located. As for Hg, 7 of the 16 locations are over Class 2 (including Class 2); section C shows most samples which exceed. The vicinity of section A and section B shows moderate levels of pollution of As. The moderately polluted areas that are mostly located in section C should be identified as the priority regions for environmental monitoring and management. The Igeo of Cd and Hg in the most samples are significantly higher than the background values in local sediment. Cd presents moderate to significant level, these show that the sources of Cd, Hg, and Pb the sediment are related to the local anthropogenic activities (Fig. 3).
The potential ecological risk index assessment in sediment
The pollution index (Cfi) and the potential ecological risk index (RI) of the seven heavy metals in the surface sediment of the Yangtze River in the Anqing section were calculated using the method described by Hakanson (1980) and Kucuksezgin (2008). The results are shown in Table 5.
Cu, Pb, and Hg vary from 0.41 to 3.44, 0.69 to 4.81, and 0.36 to 6.21, respectively. The highest concentration of Cu was located in C-1, while the highest value of Pb was located in C-2, probably because it is the drain outlet of SINOPEC. The mean Cfi values of Cu, Pb, and Hg are 2.13, 2.42, and 2.67, respectively, indicating a moderate level of sediment pollution in the Anqing section. However, Hg shows extremely high levels of pollution. For Zn, Cr, and As, the mean values of Cfi also show moderate levels of pollution. However, the values are lower than Cu, Pb, and Hg. The values of Zn, Cr, and As vary from 0.68 to 4.73, 0.81 to 2.60, and 0.38 to 3.85, respectively. Half of the locations in section A show low contaminations of Zn and Cr, while section D has low pollution level of As, indicating low pollution levels in this position. The Cfi values of Cd vary from 1.04 to 6.75 with a mean value of 3.14, indicating high level pollution. Sample section B and section D, which located in a water protection zone with very low vehicular traffic, have relatively low contamination, while section C has the highest values of contamination. This result varies from other research results: Wang found there was no obvious pollution of As in the Yangtze River (Wang et al. 2014a, b); Wang hui observed that the pollution index of Cd values indicated low pollution in the mainstream (Li et al. 2013).
Eri of the seven heavy metals and metalloids exhibit the following decreasing order: Hg > Cd > As > Pb > Cu > Cr > Zn. The total RI of the seven heavy metals in the surface sediment of the study area is classified as considerable pollution. Among the seven metals, Cu, Pb, Zn, Cr, and As have a potential ecological risk below 40, indicating a low risk level. By contrast, the Eri of Cd and Hg are 94.25 and 213.57, which indicate considerable and high potential ecological risk levels, respectively. As shown in Table 6, 2 of 16 samples had an RI value below 150 and 4 of 16 samples had an RI value between 150 and 300, indicating low and moderate risk. 56% samples have defined as considerable to high potential for ecological risk, and one sample site (C-2) is over 600, indicating an extremely high potential risk. The distribution of the total potential ecological risk exhibits the following order: C > B > A > D. The SINOPEC drain outlet may be the reason why section C has the highest concentration among all the 16 samples. The concentrations of Cd and Pb in the most samples are significantly higher than the background values in local sediment. Cd presents moderate to significant potential ecologic risk index; these show that the sources of Cd, Pb, and Hg sediment are related to the local anthropogenic activities (Mihajlovic et al. 2014).
Source identification in study area
Enrichment factors
The reliability of the EFs analysis will depend on the chosen reference element. Therefore, the reference element must be conservative and natural origin. The reasons that we chose Al as the reference list are as follow: first, the content of Al in crustal rocks is generally stable; moreover, Al has a similar conservative behavior during chemical weathering, and it has few anthropogenic sources of Al in the environment. Meanwhile, Al was ascertained as being a natural and conservative element in this region. The EFs for the individual heavy metals in the sediment samples are shown in Fig. 4.
The EF values show apparent differences among the heavy metals and exhibit the following sequence: Cd > Hg > Pb > Cu > Zn > As > Cr. The mean EF value of Cd and Hg are the highest in sediment, reaching 3.10 and 2.98 on average, respectively, indicating significant enrichment. Cr has the lowest average EF value implying moderate enrichment in the study area. Most sediment samples show low EF values, except in the section C.
The analysis of the EFs of all the heavy metals for the individual samples that reveals a marked change in the middle of the study area, which locates at sample section C, presents a stronger enrichment than the other sampling sites. The EF values in the sample D sites decrease in the downstream because of river self-purification and dilution.
EF values were interpreted with the support of the classification (Macdonald et al. 2000; Lu et al. 2017; Tepanosyan et al. 2017); sediment with higher metal enrichment values are influenced by anthropogenic discharge. The result shows that the enrichment in section C is attributable to the dense population and gas industry, suggesting that the sediment was severely enriched. Enrichment of Pb can be attributed to the outlet of a gas factory; previous studies that also reported elevated concentrations of Pb in the city are influenced by SINOPEC, a national gas factory set in the downtown city. Section B shows a relatively high enrichment value located in a residential area and may have been polluted by domestic sewage (Liu et al. 2016).
Principal component analysis
Principal component analysis (PCA) was used to investigate the association among the seven heavy metals that were studied and infer the hypothetical source of heavy metals (i.e., nature and anthropogenic) (Wang et al. 2014a, b; Duan et al. 2015).
The analysis results of PCA for the heavy metal contents are presented in Fig. 5; PCA was analyzed both in heavy metal contents and sampling sites. With the different heavy metals, the PCA of the entire data reveals three principal components with eigenvalues > 1, which explains about 85.16% of the total variance in the sediment quality data. Three major components were identified in this study. The first component explains 39.33% of the total variance and consists of Zn, Cd, and Cr. These three elements were extensively caused by producing stainless steel and traffic; also with the outlet by the gas industry, the high-value zone of Pb, Zn, and Cr from study area is located in the section C where the petrochemical factory is. Results also indicate that Zn and Pb have the same sources in the area. Pb, Cr, and Cd are extensively used to produce stainless steel and gas industry (Li et al. 2004), which can also be confirmed by the spatial distributions of Cd, Cu, and Pb. The high-value zones of Pb, Cr, and Cu were located in section C and show they might mainly come from human sources. Cu is deficiency to minimal enrichment, indication that they mainly came from natural sources, which partly stems from anthropogenic sources (Li et al. 2004; Yi et al. 2011; Chen et al. 2013).
The second component explains 27.76% of the total variance and correlated with Hg and Pb, which showed that PC1 versus PC2 corresponds to the distribution of heavy metals in the surface sediment (Fig. 5a). Figure 5 also indicates the existence of close relationships among Cd, Zn, and Cu, which are positively associated and displayed over Axis X, which also indicate that Cr, As, and Hg have the same sources. The third component explains 18.08% of the total variance with Cu, which indicates that Cu has a natural source.
Cu is a component of alloys used in mechanical parts due to its desirable qualities such as corrosive resistance and strength (Hu et al. 2014; Guo et al. 2015). Pb is also represented in brass automotive radiators due to its high corrosive resistance and high thermal conductivity (Yang et al. 2011), and is often used in car lubricants (Hu et al. 2014; Guo et al. 2015). Pb alloy and galvanized board are widely used in motor vehicles (Zhang et al. 2010). Pb is also added to tires during the vulcanizing process, comprises from 0.3 to 4.5% of the resulting tire tread (Doni et al. 2015; Gołdyn et al. 2015). Deterioration of the mechanical parts in vehicles over time results in Cu and Pb being emitted to the surrounding environment (Abdel-Satar and Goher 2015; Giri and Singh 2015; Xun and Xuegang 2015; Massey et al. 2016). Cu and Zn being released into the urban environment is a result of wear of automobile oil pumps or corrosion of metal parts which come into contact the oil (Cui et al. 2015; Tepanosyan et al. 2017; Yu et al. 2017).
Cu is not correlated with the other heavy metals considered and it is separated from the first two principal components in the PCA, which showed that Pb has a different source than other heavy metals. The metals are extensively used in alloys, coating materials, paints, and pigments. This is because the reconstruction of the section C produced the pollution. It also suggests that some metals in the study area are originated from the building construction or car pollution, as well as weathering and corrosion of building materials (Acosta et al. 2014; Brito et al. 2015).
Meanwhile, the results of PCA analysis for sampling sites are also presented in Fig. 5b. The results also indicated that PCA led to a reduction of the initial dimension of the dataset to the two components which explained 96.74% of the data variation. The first component explained 51.54% of the total variance and was loaded highly by sampling sites A2, B2, B4, C1, and C2. The second component accounted for 45.2% of the total variance and loaded highly by A1, A3, A4, C4, and D3. The reason of sampling sites B and C contributes to high variance that may be caused by the discharge of the chemicals in Sinopec.
Conclusions
In this study, the levels and distribution, risk assessment, and source apportionment of heavy metals in sediments were investigated in the Yangtze River (Anqing section), China. The main conclusions were as following:
-
1.
The concentration of heavy metals showed different spatial variations and pollution levels in the study area. The mean concentration of all the heavy metals in the surface sediment is higher than the background values. The sampling sites which are close to urbanized areas and industrial areas generally have higher potential ecological risks among the seven studied heavy metals.
-
2.
The heavy metal contamination in the sediment of the study area was assessed through RI, Igeo. The total pollution status reaches considerable degrees of potential ecological risk, especially in the section C. Cd and Hg were determined to be the main contamination indicators, given their higher toxicity and high concentrations than those of the other heavy metals. Pb also shows a relatively high level of contamination. Therefore, Pb, Hg, and Cd were considered as the most important elements in the environment in the study area.
-
3.
Based on multivariate statistical analyses, the enrichment degree of the studied metals exhibits the following decreasing order: Cd > Hg > Pb > Cu > Zn > As > Cr. Cr, As and partly Zn, Cu mostly originate from natural sources. Pb and partly Cu and Zn mainly originate from gas companies, industry, and traffic, whereas Cd and partly Zn represent agricultural activities, such as fertilizer and pesticide consumption.
The results presented in this study can facilitate planning, risk assessment, and decision making in the environmental management of the study area. However, more detailed studies in the study area would be required to draw such a conclusion.
References
Abdel-Satar AM, Goher ME (2015) Heavy metals fractionation and risk assessment in surface sediments of Qarun and Wadi El-Rayan Lakes, Egypt. Environ Monit Assess 187:346–352
Acosta JA, Faz A, Kalbitz K, Jansen B, Martínez-Martínez S (2014) Partitioning of heavy metals over different chemical fraction in street dust of Murcia (Spain) as a basis for risk assessment. J Geochem Explor 144:298–305
Agarwal T, Khillare PS, Shridhar V, Ray S (2009) Pattern, sources and toxic potential of PAHs in the agricultural soils of Delhi, India. J Hazard Mater 163:1033–1039
Alves RIS, Sampaio CF, Nadal M, Schuhmacher M, Domingo JL, Segura-Muñoz SI (2014) Metal concentrations in surface water and sediments from Pardo River, Brazil: human health risks. Environ Res 133:149–155
Bianchini G, Natali C, Di Giuseppe D, Beccaluva L (2012) Heavy metals in soils and sedimentary deposits of the Padanian Plain (Ferrara, Northern Italy): characterisation and biomonitoring. J Soil Sedim 12:1145–1153
Bolan S, Kunhikrishnan A, Seshadri B, Choppala G, Naidu R, Bolan NS, Ok YS, Ming Z, Chunguang L, Feng L (2017) Sources, distribution, bioavailability, toxicity, and risk assessment of heavy metal(loid)s in complementary medicines. Environ Int 108:103–118
Brito EMS, De la Cruz Barrón M, Caretta CA, Goñi-Urriza M, Andrade LH, Cuevas-Rodríguez G, Malm O, Torres JPM, Simon M, Guyoneaud R (2015) Impact of hydrocarbons, PCBs and heavy metals on bacterial communities in Lerma River, Salamanca, Mexico: investigation of hydrocarbon degradation potential. Sci Total Environ 521:1–10
Chen Y, Huang B, Hu W, Weindorf DC, Yang L (2013) Environmental assessment of closed greenhouse vegetable production system in Nanjing, China. J Soils Sedim 13:1418–1429
Cui B, Zhu M, Jiang Y, Jiang Y, Cao H (2014a) Identification of the sources of metals and arsenic in river sediments by multivariate analysis and geochemical approaches. J Soils Sedim 14:1456–1468
Cui J, Zang S, Zhai D, Wu B (2014b) Potential ecological risk of heavy metals and metalloid in the sediments of Wuyuer River basin, Heilongjiang Province, China. Ecotoxicology 23:589–600
Cui X, Sun X-L, Hu P-J, Yuan C, Luo Y-M, Wu L-H, Christie P (2015) Concentrations of heavy metals in suburban horticultural soils and their uptake by Artemisia selengensis. Pedosphere 25(6):878–887
de Andrade Lima LRP, Bernardez LA (2017) Characterization of the soil contamination around the former primary lead smelter at Santo Amaro, Bahia, Brazil. Environ Earth Sci. https://doi.org/10.1007/s12665-017-6791-6
Deng G, Yang W, Zhou G, Li Y, Liu S (2014) Heavy metals and polycyclic aromatic hydrocarbons in sediments from the Shenzhen River, South China. Environ Sci Pollut R 21:10594–10600
Doni S, Macci C, Peruzzi E, Iannelli R, Masciandaro G (2015) Heavy metal distribution in a sediment phytoremediation system at pilot scale. Ecol Eng 81:146–157
Duan Y, Shen G, Tao S, Hong J, Chen Y, Xue M, Li T, Su S, Shen H, Fu X, Meng Q, Zhang J, Zhang B, Han X, Song K (2015) Characteristics of polycyclic aromatic hydrocarbons in agricultural soils at a typical coke production base in Shanxi, China. Chemosphere 127:64–69
El Azhari A, Rhoujjati A, El Hachimi ML, Ambrosi J (2017) Pollution and ecological risk assessment of heavy metals in the soil-plant system and the sediment-water column around a former Pb/Zn-mining area in NE Morocco. Ecotox Environ Safe 144:464–474
Giri S, Singh AK (2015) Human health risk and ecological risk assessment of metals in fishes, shrimps and sediment from a tropical river. Int J Environ Sci Technol 12:2349–2362
Gołdyn B, Chudzińska M, Barałkiewicz D, Celewicz-Gołdyn S (2015) Heavy metal contents in the sediments of astatic ponds: influence of geomorphology, hydroperiod, water chemistry and vegetation. Ecotox Environ Safe 118:103–111
Guo W, Huo S, Xi B, Zhang J, Wu F (2015) Heavy metal contamination in sediments from typical lakes in the five geographic regions of China: distribution, bioavailability and risk. Ecol Eng 81:243–255
Hakanson L (1980) An ecological risk index for aquatic pollution control.a sedimentological approach. Water Res 14(8):975–1001
Han L, Gao B, Lu J, Zhou Y, Xu D, Gao L, Sun K (2017) Pollution characteristics and source identification of trace metals in riparian soils of Miyun Reservoir, China. Ecotox Environ Safe 144:321–329
Hu Y, Wang D, Wei L, Song B (2014) Heavy metal contamination of urban topsoils in a typical region of Loess Plateau, China. J Soil Sedim 14:928–935
Kucuksezgin F, Uluturhan E, Batki H (2008) Distribution of heavy metals in water, particulate matter and sediments of Gediz River (Eastern Aegean). Environ Monit Assess 141(1–3):213–225
Li X, Lin Lee S, Wong S, Shi W, Thornton I (2004) The study of metal contamination in urban soils of Hong Kong using a GIS-based approach. Environ Pollut 129:113–124
Li F, Huang J, Zeng G, Yuan X, Li X, Liang J, Wang X, Tang X, Bai B (2013) Spatial risk assessment and sources identification of heavy metals in surface sediments from the Dongting Lake, Middle China. J Geochem Explor 132:75–83
Li Y, Long L, Ge J, Yang L, Cheng J, Sun L, Lu C, Yu X (2017) Presence, distribution and risk assessment of polycyclic aromatic hydrocarbons in rice-wheat continuous cropping soils close to five industrial parks of Suzhou, China. Chemosphere 184:753–761
Liu G, Niu J, Guo W, An X, Zhao L (2016) Ecological and health risk-based characterization of agricultural soils contaminated with polycyclic aromatic hydrocarbons in the vicinity of a chemical plant in China. Chemosphere 163:461–470
Lu J, Li A, Huang P (2017) Distribution, sources and contamination assessment of heavy metals in surface sediments of the South Yellow Sea and northern part of the East China Sea. Mar Pollut Bull 124:470–479
Ma L, Wang L, Jia Y, Yang Z (2016) Arsenic speciation in locally grown rice grains from Hunan Province, China: spatial distribution and potential health risk. Sci Total Environ 438–444
Ma L, Wang L, Tang J, Yang Z (2017) Arsenic speciation and heavy metal distribution in polished rice grown in Guangdong Province, Southern China. Food Chem 233:110–116
Macdonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Con Tox 39:20–31
Martínez LLG, Poleto C (2014) Assessment of diffuse pollution associated with metals in urban sediments using the geoaccumulation index (I geo). J Soil Sedim 14:1251–1257
Martínez-Graña AM, Boski T, Goy JL, Zazo C, Dabrio CJ (2016) Coastal-flood risk management in central Algarve: vulnerability and flood risk indices (South Portugal). Ecol Indic 71:302–316
Massey DD, Kulshrestha A, Taneja A (2013) Particulate matter concentrations and their related metal toxicity in rural residential environment of semi-arid region of India. Atmos Environ 67:278–286
Massey DD, Habil M, Taneja A (2016) Particles in different indoor microenvironments-its implications on occupants. Build Environ 106:237–244
Mihajlovic J, Giani L, Stärk H, Rinklebe J (2014) Concentrations and geochemical fractions of rare earth elements in two different marsh soil profiles at the North Sea, Germany. J Soil Sedim 14:1417–1433
Rajmohan N, Prathapar SA, Jayaprakash M, Nagarajan R (2014) Vertical distribution of heavy metals in soil profile in a seasonally waterlogging agriculture field in Eastern Ganges Basin. Environ Monit Assess 186:5411–5427
Sakan SM, Đorđević DS, Manojlović DD, Predrag PS (2009) Assessment of heavy metal pollutants accumulation in the Tisza river sediments. J Environ Manage 90:3382–3390
Tepanosyan G, Sahakyan L, Belyaeva O, Maghakyan N, Saghatelyan A (2017) Human health risk assessment and riskiest heavy metal origin identification in urban soils of Yerevan, Armenia. Chemosphere 184:1230–1240
Varol M, Şen B (2009) Assessment of surface water quality using multivariate statistical techniques: a case study of Behrimaz Stream, Turkey. Environ Monit Assess 159:543–553
Wang L, Yang L, Kong L, Li S, Zhu J, Wang Y (2014a) Spatial distribution, source identification and pollution assessment of metal content in the surface sediments of Nansi Lake, China. J Geochem Explor 140:87–95
Wang Z, Chen L, Zhang H, Sun R (2014b) Multivariate statistical analysis and risk assessment of heavy metals monitored in surface sediment of the Luan river and its tributaries, China. Hum Ecol Risk Assess 20:1521–1537
Wu B, Wang G, Wu J, Fu Q, Liu C (2014) Sources of heavy metals in surface sediments and an ecological risk assessment from two adjacent Plateau reservoirs. PLoS One 9:e102101
Xun Y, Xuegang L (2015) Heavy Metals in sediment from Bei Shan river: distribution, relationship with soil characteristics and multivariate assessment of contamination sources. B Environ Contam Tox 95:56–60
Yang Z, Han H, Zhao Q (2011) Soil erosion control degree of the project of converting farmland to forest in mountainous areas at China’s Southwest border: a case study in Mangshi, Yunnan Province. J Mt Sci 8(6):845–854
Yang J, Chen L, Liu L, Shi W, Meng X (2014) Comprehensive risk assessment of heavy metals in lake sediment from public parks in Shanghai. Ecotox Environ Safe 102:129–135
Yi Y, Yang Z, Zhang S (2011) Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environ Pollut 159:2575–2585
Yong SK, Bolan N, Lombi E, Skinner W (2013) Synthesis and characterization of thiolated chitosan beads for removal of Cu(II) and Cd(II) from wastewater. Water Air Soil Pollut 224:1720
Yu S, Hong B, Ma J, Chen Y, Xi X, Gao J, Hu X, Xu X, Sun Y (2017) Surface sediment quality relative to port activities: a contaminant-spectrum assessment. Sci Total Environ 596:342–350
Yuan X, Zhang L, Li J, Wang C, Ji J (2014) Sediment properties and heavy metal pollution assessment in the river, estuary and lake environments of a fluvial plain, China. Catena 119:52–60
Zang F, Wang S, Nan Z, Ma J, Zhang Q, Chen Y, Li Y (2017) Accumulation, spatio-temporal distribution, and risk assessment of heavy metals in the soil-corn system around a polymetallic mining area from the Loess Plateau, northwest China. Geoderma 305:188–196
Zhang C, Luo Q, Geng C, Li Z (2010) Stabilization treatment of contaminated soil: a field-scale application in Shanghai, China. Front Environ Sci Eng China 4(4):395–404
Zhang Y, Chu C, Li T, Xu S, Liu L, Ju M (2017) A water quality management strategy for regionally protected water through health risk assessment and spatial distribution of heavy metal pollution in 3 marine reserves. Sci Total Environ 599–600:721–731
Zhao H, Ma F, Zhang Y, Guo J (2014) Monitoring and assessment of mining subsidence in a metal mine in China. Environ Eng Manag J 13(12):3015–3024
Acknowledgements
This work was supported by the National Key Research and Development Program of China (Grant number 2016YFC0502705). We would like to express our thanks to the anonymous reviewers for their helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, Y., Huang, H., Sun, T. et al. Comprehensive risk assessment and source apportionment of heavy metal contamination in the surface sediment of the Yangtze River Anqing section, China. Environ Earth Sci 77, 493 (2018). https://doi.org/10.1007/s12665-018-7621-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-018-7621-1