Abstract
Gabapentin enacarbil is an extended-release prodrug of gabapentin that is approved in the USA (Horizant®) and Japan (Regnite®) for the treatment of moderate to severe primary restless legs syndrome (RLS) in adults [featured indication]. This article summarizes pharmacological, efficacy and tolerability data relevant to the use of oral gabapentin enacarbil in this indication. In double-blind, multicentre trials, treatment with gabapentin enacarbil 600 mg/day for 12 weeks significantly improved the symptoms of moderate to severe primary RLS in adults. Gabapentin enacarbil also significantly improved RLS pain scores and generally improved sleep and mood outcomes. These data are supported by retrospective pooled analyses of three of these trials (XP081, PIVOT RLS I and PIVOT RLS II), with gabapentin enacarbil generally improving symptoms irrespective of disease severity, associated sleep disturbance or prior dopamine agonist use. Responses to gabapentin enacarbil were sustained in longer-term trials, with lower relapse rates in gabapentin enacarbil than placebo recipients in a longer-term maintenance study. Overall, in short and longer-term trials, relatively few patients discontinued treatment, adverse events were mostly mild to moderate in severity, and somnolence/sedation and dizziness were the most commonly reported adverse events. Notably, there were no reports of augmentation or QT-interval prolongation. Gabapentin enacarbil is an important agent for the treatment of adults with moderate to severe primary RLS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Oral, actively transported, extended-release prodrug of gabapentin |
Only α2δ calcium channel ligand currently approved in the USA and Japan for the treatment of moderate to severe primary RLS in adults |
Improves RLS symptoms, pain, sleep, mood and quality of life compared with placebo |
Generally well tolerated, with most adverse events being mild or moderate in severity |
1 Introduction
Restless legs syndrome (RLS; also known as Willis-Ekbom disease) is a neurological, sensorimotor disorder characterized by an irresistible urge to move the legs that is often accompanied by unpleasant sensations (e.g. crawling, creeping, pain, pulling, stretching) in the legs [1, 2]. RLS may also involve the arms and/or other parts of the body, although the legs are usually affected first and most severely [1]. RLS symptoms are initiated or exacerbated by inactivity or rest and typically occur or worsen in the evening or at night compared with during the day [1]. RLS can occur at any age, affects about twice as many women as men in adults aged >35 years and has a prevalence estimate of up to 15 % in the general adult population [1, 3].
Sleep disturbance is a common aspect of RLS that is reported in ≈75 % of patients who seek treatment and may be attributed to periodic leg movements of sleep [1, 4]. While sleep disturbance may be minimal or non-existent in patients with mild RLS, it is a distressing feature that is commonly seen in patients with moderate to severe RLS that can result in impaired daytime functioning and a reduced quality of life (QOL) [1, 2].
RLS can occur as a primary disorder, generally idiopathic, or secondary to underlying conditions such as iron deficiency anaemia, pregnancy or renal failure [2]. The pathophysiology of RLS is not fully understood but is centred on dopaminergic dysfunction, genetic predisposition, inflammatory mechanisms, immune dysfunction, an alteration in neurotransmitters (e.g. endorphins, hypocretins), and reduced central nervous system iron [2, 5].
Approved treatment options for primary RLS include dopaminergic agents [e.g. pramipexole, ropinirole and rotigotine (patch)] and an α2δ calcium channel ligand (gabapentin enacarbil) for first-line therapy, and opioids (prolonged-release oxycodone/naloxone) for second-line therapy in severe cases [6]. Although dopaminergic medications have been the most extensively investigated and used class of drugs for RLS, they may be associated with treatment-limiting side effects such as daytime sleepiness and impulse control disorders, and they can worsen disease severity through a process called augmentation [4, 7]. Augmentation in RLS is an iatrogenic worsening of disease characterized by an earlier onset of symptoms, an increased intensity of symptoms, a shorter latency to symptoms at rest and the spread of symptoms to previously unaffected areas [7]. International guidelines now recommend α2δ ligands as effective first-line agents for RLS, as they are without the risk of augmentation [8].
This article provides an updated overview of pharmacological, therapeutic efficacy and tolerability data relevant to the use of gabapentin enacarbil [Horizant® (the USA), Regnite® (Japan)] in this indication, previously reviewed in CNS Drugs [9]. Of note, gabapentin enacarbil is also approved in the USA for the management of adults with postherpetic neuralgia (PHN) [10], although this indication is outside the scope of this review.
2 Pharmacodynamic Properties of Gabapentin Enacarbil
Gabapentin enacarbil is a prodrug of gabapentin, and its therapeutic effects are attributed to gabapentin, for which the precise mechanism of action in RLS is unknown [10]. Gabapentin is structurally related to gamma-aminobutyric acid (GABA). However, it does not have an effect on GABA binding, uptake or degradation. According to in vitro studies, gabapentin binds with high affinity to the α2δ subunit of voltage-activated calcium channels, but the therapeutic significance of this binding is unknown. Gabapentin and gabapentin enacarbil have not shown affinities in radioligand binding assays for a number of other common receptors, transporter proteins or ion channels [10].
The effects of gabapentin enacarbil on RLS symptoms, as assessed using the International Restless Legs Scale (IRLS) score and investigator-rated Clinical Global Impression-Improvement (CGI-I) response in phase II and III clinical trials, are discussed in Sect. 4. In a population pharmacodynamic/pharmacokinetic model pooling data from subjects with or without RLS receiving single or multiple doses of gabapentin enacarbil (dosage range 300–2400 mg/day) in 12 phase I to III clinical trials, a dose response/exposure relationship was seen for gabapentin enacarbil and investigator-rated CGI-I scores, but not mean change in IRLS total scores [11].
Gabapentin enacarbil does not affect cardiac repolarization. In three trials in healthy volunteers, gabapentin enacarbil did not have a clinically relevant effect on QT-interval prolongation with single doses across the range of 1200–6000 mg [12–14].
3 Pharmacokinetic Properties of Gabapentin Enacarbil
Gabapentin enacarbil is designed to overcome the pharmacokinetic limitations of gabapentin, which has high inter-patient variability and unpredictable plasma levels that may lead to suboptimal drug exposure because it is absorbed by a saturable, low-capacity solute transporter localized only in the upper small intestine [15]. Gabapentin enacarbil is absorbed and actively transported by at least two high-capacity nutrient transporters, monocarboxylate transporter type-1 (MCT-1) and sodium-dependent multivitamin transporter, expressed throughout the intestinal tract [15].
Following oral administration, gabapentin enacarbil is converted to gabapentin and nontoxic breakdown products (acetaldehyde, carbon dioxide, isobutyric acid) through extensive first-pass hydrolysis by nonspecific esterases (predominantly in enterocytes and to a lesser extent in the liver) [10, 16]. Over the dose range of 300–6000 mg, gabapentin enacarbil provides extended and approximately dose-proportional exposure to gabapentin [10, 11, 14]. Peak plasma gabapentin concentrations are reached in 7.3 h in a fed state and 5.0 h in a fasted state after the administration of gabapentin enacarbil 600 mg. The mean bioavailability of gabapentin after gabapentin enacarbil administration is ≈75 % in a fed state and 42–65 % in a fasted state. Steady state is achieved in 2 days of daily administration. Plasma protein binding is minimal (<3 %), and the apparent volume of distribution of gabapentin is 76 L in subjects receiving gabapentin enacarbil [10].
The released gabapentin is not metabolized to an appreciable extent [10]. The apparent oral clearance of gabapentin from plasma is 6.0–9.3 L/h after the administration of gabapentin enacarbil with food. The plasma clearance of gabapentin is roughly proportional to creatinine clearance. The released gabapentin is excreted almost exclusively by the kidneys unchanged, possibly by active secretion by a renal organic cation transporter type 2 (OCT2). Regardless of food type or intake, the range of gabapentin renal clearance is 5–7 L/h. The elimination half-life of gabapentin is 5.1–6.0 h, irrespective of dose or multiple dosing [10]. The mean recovery of total radioactivity was 94 % in urine and 5 % in faeces after the administration of immediate-release 14C-labeled gabapentin enacarbil [10, 17].
Age (18–64 vs. ≥65 years) and gender do not have a clinically relevant effect on gabapentin enacarbil pharmacokinetics, but elderly patients may experience an age-related decline in renal function that can reduce gabapentin clearance [10]. In patients with moderate or severe renal impairment, the apparent oral clearance of gabapentin was reduced compared with patients without renal impairment (4.2 or 1.7 vs. 6.0–9.3 L/h). The effect of race on gabapentin enacarbil pharmacokinetics has not been studied [10], and trials evaluating the safety and efficacy of gabapentin enacarbil in paediatric patients are ongoing (NCT02633683, NCT02560766, NCT02633657).
Gabapentin enacarbil and gabapentin are not substrates, inducers or inhibitors of major cytochrome P450 enzymes, and gabapentin enacarbil is not a substrate or inhibitor of P-glycoprotein in vitro [10]. Clinically relevant pharmacokinetic interactions are not expected to be seen between gabapentin hydrolyzed from gabapentin enacarbil, and substrates of MCT-1 (e.g. naproxen) or OCT2 (e.g. cimetidine) [10, 18]. Ethanol causes gabapentin enacarbil to be released rapidly from the extended-release tablets, increasing the risk for adverse events [10], and the concomitant administration of morphine and gabapentin enacarbil may also increase the risk of adverse events [10, 19].
4 Therapeutic Efficacy of Gabapentin Enacarbil
4.1 In Short-Term Studies
The short-term (10–12 weeks) efficacy of oral gabapentin enacarbil in the treatment of RLS was investigated in several large (n > 100), double-blind, placebo-controlled, multicentre trials, including two phase II dose-response studies [20, 21] (one of which was in Japanese patients [21]) and four phase III [22–24] or IV [25] trials (parallel-group [22, 24, 25] or crossover [23] design) [Table 1]. Eligible adult patients had moderate to severe primary RLS (IRLS score of ≥15 at baseline) and RLS symptoms for ≥15 days/nights during the month prior to screening (or this symptom frequency before treatment initiation, if the patient was receiving treatment) and symptoms ≥4 days/nights during the 7-day baseline period [20–25].
Although various dosages of gabapentin enacarbil were evaluated (Table 1), this section focuses on data pertaining to gabapentin enacarbil 600 mg/day (i.e. the recommended dosage) wherever possible. In addition to these trials, pooled data are discussed; most pooled data are from analyses of three trials [20, 22, 24] in which 50 % of gabapentin 600 mg/day recipients (n = 161) and 45 % of placebo recipients (n = 244) had severe primary RLS (baseline IRLS total score ≥24) [26].
4.1.1 RLS Symptoms
Treatment for 10–12 weeks with oral gabapentin enacarbil displayed efficacy in relieving the symptoms of moderate to severe primary RLS in adults [20–25]. In phase III [22] and IV [25] trials, recipients of gabapentin enacarbil 600 mg/day had significant reductions (i.e. improvements) in IRLS total score and significantly higher rates of CGI-I response than placebo recipients (Table 1). In the Japanese phase II trial (8825-CL-003), initial analyses indicated that, relative to placebo, this dosage of gabapentin enacarbil did not significantly improve IRLS total score, but was associated with a significantly higher rate of CGI-I response (Table 1); however, post hoc analyses excluding patients who discontinued early in the study showed that gabapentin enacarbil 600 mg/day was superior to placebo (p = 0.012) [21]. Data from the phase II XP081 trial [20] were supportive of these results (Table 1).
These data are generally supported by retrospective pooled data from three phase II or III trials (XP081, PIVOT RLS I and II) [26–28]. Compared with placebo, 12 weeks of treatment with gabapentin enacarbil 600 mg/day significantly improved IRLS total score (adjusted mean change from baseline −13.6 with gabapentin enacarbil vs. −9.3 with placebo; p < 0.0001) and was associated with a significantly higher CGI-I response rate (70.2 vs. 42.2 %; p < 0.0001) in patients with moderate to severe primary RLS [27]. Among patients with severe primary RLS, the between-group differences were also significant for least squares mean (LSM) change from baseline to week 12 in IRLS total score (−16.3 in gabapentin enacarbil 600 mg/day recipients vs. −12.3 in placebo recipients; p < 0.01) and the proportion of responders on the investigator-rated CGI-I scale (64 vs. 42 %; p < 0.01) at week 12 [26]. Gabapentin enacarbil 600 mg/day treatment improved IRLS total scores from baseline to week 12 compared with placebo (p < 0.05) and resulted in significantly more investigator-rated CGI-I responders at week 12 than placebo (p ≤ 0.001), regardless of whether patients did or did not have prior dopamine agonist exposure [28].
4.1.2 Pain
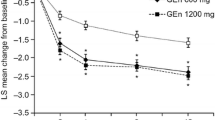
At 12 weeks, gabapentin enacarbil displayed efficacy in relieving RLS pain [scored on an 11-point visual analogue scale (VAS), in which higher scores indicated greater pain intensity] in adults with moderate to severe primary RLS [20, 24]. In PIVOT RLS I, patients with a baseline pain score of ≥4 had a mean reduction in the average daily RLS pain score that was greater with gabapentin enacarbil 1200 mg/day than with placebo (−3.7 vs. −1.9; p < 0.0001) [gabapentin enacarbil 600 mg/day was not evaluated in this trial] [24]. In XP081, the mean reduction from baseline in average daily RLS pain score ranged from −2.8 to −3.3 with gabapentin enacarbil 600–2400 mg/day compared with −2.5 with placebo in patients with moderate to severe RLS [20]. In a subset of patients who had baseline pain scores of ≥4, it was −3.9 to −4.4 with gabapentin enacarbil 600–2400 mg/day compared with −2.4 with placebo [20].
Pooled analyses of data from XP081 and PIVOT RLS I and II supported the efficacy of gabapentin enacarbil 600 mg/day in reducing pain [29, 30]. The LSM reduction in RLS pain scores from baseline to week 12, as assessed with the VAS, was significantly greater with gabapentin enacarbil 600 mg/day than with placebo in patients with moderate to severe RLS (−2.4 vs. −1.6; p < 0.001) and severe RLS (−2.6 vs. −1.7; p < 0.01) [abstract presentation] [29]. An analysis of patients with moderate to severe RLS, using an 11-point pain intensity numerical rating scale, showed consistent results (−3.55 vs. −2.11; p < 0.001) [30]. Between-group differences significantly (p < 0.01) favoured the gabapentin enacarbil group at all assessed time points (weeks 2, 4, 8 and 12) [29, 30]. In combined IRLS-pain responder analyses, which included patients with baseline pain scores of ≥4, moderate to strong correlations were seen between IRLS total score and pain score changes from baseline to week 12 (p < 0.05) [26, 30]. The proportion of patients who were both IRLS responders (i.e. had IRLS total scores of <15, with improvements from baseline of ≥6 at week 12) and pain responders (i.e. had ≥30 % improvement in pain scores) was significantly greater in gabapentin enacarbil 600 mg/day than placebo recipients in patients with moderate to severe primary RLS (p = 0.0003) [30], but not in patients with severe primary RLS (p = 0.05) [26]. Of note, an IRLS-pain responder subanalysis in patients with moderate to severe primary RLS showed significant (p < 0.05) correlations between RLS pain scores and all 10 individual IRLS items for change from baseline to week 12 [30].
4.1.3 Sleep
Oral gabapentin enacarbil treatment for up to 12 weeks generally improved sleep outcomes in adults with moderate to severe primary RLS [20, 22–24]. For instance, in PIVOT RLS II, gabapentin enacarbil 600 mg/day recipients experienced significantly greater improvements (p < 0.03) than placebo recipients in mean change from baseline to week 12 for most Medical Outcomes Study Sleep Scale domains [22]. In addition, all items of the Post-Sleep Questionnaire (PSQ) improved significantly (p < 0.04) from baseline to week 12 with gabapentin enacarbil 600 mg/day compared with placebo. Mean wake time after sleep onset, but not mean daily total sleep time, also improved significantly (p < 0.01) in gabapentin enacarbil 600 mg/day recipients compared with placebo recipients [22].
In pooled analyses (XP081 and PIVOT RLS I and II), gabapentin enacarbil 600 mg/day also improved several sleep parameters [26, 31]. Gabapentin enacarbil 600 mg/day significantly improved overall sleep quality and reduced the number of hours awake at night because of RLS symptoms, compared with placebo (both p ≤ 0.001) [abstract presentation] [31]. In patients with severe primary RLS, significant improvements from baseline to week 12 in all but one PSQ item (overall sleep quality) were seen with gabapentin enacarbil 600 mg/day compared with placebo (p < 0.05) [26]. In terms of RLS rating scale items 4 (severity of sleep disturbance) and 5 (daytime tiredness), the between-group difference significantly (p < 0.05) favoured gabapentin enacarbil 600 mg/day in both of these patient populations [26, 31].
In a pooled analysis of patients with baseline sleep disturbance levels of no to moderate (score of 0–2) or severe to very severe (score of 3–4) on item 4 of the IRLS scale, gabapentin enacarbil 600 mg/day treatment significantly (p < 0.05) improved IRLS total scores, daily RLS pain scores and scores for IRLS items 4, 9 (impact on ability to carry out daily affairs) and 10 (severity of mood disturbance) from baseline to week 12, irrespective of the degree of sleep disturbance [32]. In addition, in both sleep subgroups, gabapentin enacarbil 600 mg/day treatment resulted in significantly more responders on the investigator-rated CGI-I scale (p < 0.01) at week 12 than placebo. When mood was assessed using the Profile of Mood States (POMS) rating scale, which assesses mood states across seven domains, significant improvements were seen in the no to moderate sleep disturbance subgroup (in the depression-dejection and total mood disturbance domains; both p < 0.05) but not in the severe to very severe subgroup [32].
Of note, a polysomnography trial (RXP110908) [23] provided objective confirmation of the efficacy of gabapentin enacarbil in improving sleep disturbance. The trial involved two 4-week crossover periods (separated by a 1-week washout period and each followed by a 7-day taper) in which randomized patients received either gabapentin enacarbil 1200 mg once daily in the first period then placebo in the second period (n = 67) or vice versa (n = 69). Gabapentin enacarbil treatment significantly (p ≤ 0.002) improved sleep endpoints such as wake time during sleep (primary endpoint) and periodic leg movements of sleep associated with arousal per hour of sleep from baseline to weeks 4 and 10 [23].
4.1.4 Other Outcomes
According to pooled data from XP081 and PIVOT RLS I and II, gabapentin enacarbil 600 mg/day also improved mood outcomes, when assessed using IRLS rating scale items 9 (impact on ability to carry out daily affairs) and 10 (severity of mood disturbance) [26, 33]. The differences versus placebo significantly (p < 0.01) favoured gabapentin enacarbil 600 mg/day in patients with moderate to severe primary RLS (−0.3 for item 9 and −0.2 for item 10) [33] and severe primary RLS (−0.4 and −0.3) [26]. Mood outcomes were also evaluated using the subscale scores from the POMS [26] or POMS brief form [33] rating scales. In patients with moderate to severe primary RLS, a significant (p < 0.05) between-group difference in LSM change from baseline to week 12 was seen in favour of gabapentin enacarbil for vigour-activity [33]. In patients with severe primary RLS, POM subscale scores did not differ significantly between gabapentin enacarbil 600 mg/day and placebo recipients at week 12 [26].
Gabapentin enacarbil 600 mg/day improved QOL, as assessed using the RLS QOL questionnaire examining effects on daily life, social life, work life and emotional well-being, according to pooled data from PIVOT RLS I and II [26, 33, 34]. LSM changes from baseline to week 12 in RLS QOL scores were significantly (p < 0.05) more favourable with gabapentin enacarbil 600 mg/day than placebo in patients with moderate to severe RLS (+20.6 vs. +15.5) or severe RLS (+25.8 vs. +20.1) [abstract presentation] [34], with significant benefit also seen with gabapentin enacarbil versus placebo at all earlier time points (weeks 4 and 8) in each of these patient populations [26, 33, 34].
4.2 In Longer-Term Studies
The longer-term efficacy of gabapentin enacarbil in patients with RLS has been investigated in two 52-week, non-comparative, open-label studies [35, 36] [one of which was an extension (n = 581 enrolled) of four short-term trials, including XP081, PIVOT RLS I and PIVOT RLS II [35] and the other a study conducted in Japanese patients (n = 182 enrolled) [36], and the 36-week PIVOT RLS maintenance study (n = 327 enrolled), which employed a placebo-controlled, randomized withdrawal design [37].
Longer-term treatment with gabapentin enacarbil, generally at a dosage of 1200 mg/day, significantly (p < 0.001 vs. baseline) improved RLS symptoms for up to 52 weeks [36] or maintained improvements in RLS symptoms and responder rates seen in short-term studies [35]. Additionally, gabapentin enacarbil was associated with a significantly (p = 0.02) lower relapse rate than placebo in the maintenance of efficacy study [37].
5 Tolerability of Gabapentin Enacarbil
Oral gabapentin enacarbil 600 mg/day was generally well tolerated in patients with moderate to severe primary RLS in clinical trials discussed in Sect. 4. Although several clinical trials investigated the efficacy and safety of gabapentin enacarbil 1200 mg/day, the FDA concluded that this higher dosage provided no added benefit and caused an increase in adverse events compared with gabapentin enacarbil 600 mg/day (the approved dosage for the treatment of moderate to severe RLS in adults; Sect. 6) [10].
In a pooled analysis of clinical trials of 12 weeks’ duration, at least one adverse event was reported in 81 % of gabapentin enacarbil 600 mg/day recipients (n = 163) and 74 % of placebo recipients (n = 245) [27]. Adverse events generally were of mild to moderate severity, occurred upon treatment initiation and resolved within 1–2 weeks. The most commonly reported adverse events (i.e. an incidence of ≥5 % in gabapentin enacarbil recipients and at least double the rate seen with placebo) were somnolence/sedation (20 vs. 5 % in placebo recipients) and dizziness (13 vs. 4 %) [27]. Somnolence/sedation and dizziness persisted in 30 and 20 % of patients during treatment and resulted in treatment discontinuations in 2 and 1 % of patients [10]. Adverse events led to treatment discontinuation in 7 % of gabapentin enacarbil 600 mg/day recipients and 4 % of placebo recipients [10]. Serious adverse events occurred in two gabapentin enacarbil 600 mg/day recipients (cellulitis and intervertebral disc protrusion), but were considered unrelated to treatment [27]. In individual trials, there were no reports of augmentation [20, 22] or clinically relevant changes in laboratory parameters, electrocardiograms or vital signs [20, 22, 24]. In addition, there were no reports of suicidality or fatalities [20, 22, 24].
The tolerability profile of gabapentin enacarbil 600 mg/day in longer-term trials [35–37] was generally similar to that seen in short-term trials. Dizziness and somnolence were the most common adverse events. The majority of adverse events were of mild or moderate severity, and the incidence of adverse events was seen to decline as the trials progressed [35–37]. There were no reports of augmentation [35, 36] or impulse control disorders [35]. Few deaths occurred in the gabapentin enacarbil trials. Those in the 36-week PIVOT RLS maintenance study (one patient) [37] and a 52-week study (one patient) [35], were considered unrelated to treatment. Another death (due to a lymphoma), which occurred in another 52-week study, was considered possibly related (temporally) to gabapentin enacarbil treatment [36].
Gabapentin enacarbil is a prodrug of the antiepileptic drug (AED) gabapentin [10]. AEDs have been associated with an increased risk of suicidal thoughts and behaviour. Since gabapentin enacarbil is a prodrug of gabapentin, which is an AED, such an increased risk of suicidal thoughts and behaviour should be considered in treatment decisions [10]. Regarding the patient mentioned in the preceding paragraph who died as a result of a fall in the 52-week study, acute alcohol intoxication was listed as a contributing factor [35]. However, the death occurred 25 days after the patient concluded ≈1 year of treatment with gabapentin enacarbil 1200 mg/day [35], and there is a comment in the FDA medical review that it should be considered a possible suicide [38]. In the same study, a serious adverse event considered a suicide attempt by ingestion occurred in a patient who had been receiving gabapentin enacarbil 1200 mg/day for 165 days [38]. Patients receiving any AED for any indication must be monitored for the emergence or worsening of depression, any unusual changes in mood or behaviour, and suicidal thoughts or behaviour [10].
Cases of drug reaction with eosinophilia and systemic symptoms (also known as multi-organ hypersensitivity), including life-threatening and fatal cases, have been reported with AEDs, including gabapentin [10]. A patient presenting with early manifestations of hypersensitivity (e.g. fever, lymphadenopathy) should be evaluated immediately, and if an alternative aetiology for the manifestations cannot be established, gabapentin enacarbil treatment should be discontinued [10].
In clinical studies of gabapentin adjunctive therapy in patients with epilepsy aged >12 years (2085 patient-years of exposure to gabapentin), cases of new tumours (10 patients) and worsened pre-existing tumours (11 patients) were reported during treatment or up to 2 years after discontinuation of gabapentin therapy [10]. However, it is not known whether the incidence was or was not affected by gabapentin treatment [10]. Studies in the US and the UK with up to 12 and 15 years of follow up, respectively, did not support a carcinogenic effect with gabapentin use [39]. However, a carcinogenic effect could not be confidently excluded [39].
6 Dosage and Administration of Gabapentin Enacarbil
Gabapentin enacarbil oral extended-release tablets are indicated for the treatment of adults with moderate to severe primary RLS in the USA and Japan (featured indication) [10, 40]. The recommended dosage is 600 mg once daily with food, at around 5 p.m. [10] or after dinner [40]. It is not recommended for patients who need to sleep during the day and stay awake at night [10]. Gabapentin enacarbil tablets should not be chewed, crushed or cut. Of note, gabapentin enacarbil and gabapentin are not interchangeable because their pharmacokinetic profiles differ (Sect. 3) [10].
Gabapentin enacarbil may cause significant impairment in the ability to drive or operate complex machinery [10]. Therefore, patients taking gabapentin enacarbil should not perform these tasks until they have sufficient experience with the drug’s effects [10].
There are no contraindications listed in the US prescribing information [10]. However, treatment with gabapentin enacarbil is not recommended in patients on haemodialysis. In patients with renal impairment not on dialysis, dose adjustments are recommended in accordance with creatinine clearance [10]. In the Japanese prescribing information, contraindications include advanced renal dysfunction with creatinine clearance <30 mL/min [40]. Local prescribing information should be consulted for further, detailed information, including contraindications, precautions, drug interactions, and use in special patient populations.
7 Place of Gabapentin Enacarbil in the Management of Restless Legs Syndrome
The goals of RLS treatment include reducing or curing troublesome RLS symptoms that occur during rest or sleep, reducing RLS-associated sleep disturbance and subsequent daytime fatigue or somnolence, improving QOL and preventing augmentation [2, 7].
Current (2012) guidelines from the American Academy of Sleep Medicine recommend that pramipexole or ropinirole should be used as the standard therapy for RLS, although gabapentin enacarbil and rotigotine can also be considered [41, 42]. These drugs are also included in Level A recommendations in European guidelines (2012) [43], and similarly, the International RLS Study Group (IRLSSG) 2013 guidelines recommended either a dopamine receptor agonist or an α2δ calcium channel ligand as first-line therapy for the long-term treatment of RLS in most patients [44]. However, augmentation has been identified as a serious problem associated with the long-term treatment of RLS with dopamine agonists [8, 41, 44] and has driven guideline changes, with recent (2016) guidance from the combined task force of the IRLSSG, European RLS Study Group and the RLS Foundation recommending α2δ ligands as first-line agents for the treatment of RLS, as they are effective and are without augmentation risk [8].
Gabapentin enacarbil is an α2δ calcium channel ligand designed to overcome the pharmacokinetic limitations of gabapentin, its parent drug (Sect. 3). Gabapentin enacarbil is absorbed and transported by at least two high-capacity nutrient transporters expressed throughout the intestinal tract, and it provides extended and approximately dose-proportional exposure to gabapentin (Sect. 3).
In clinical trials, treatment with gabapentin enacarbil for up to 12 weeks significantly improved the symptoms of moderate to severe primary RLS (Sect. 4). Gabapentin enacarbil also significantly improved RLS pain scores and generally improved sleep and mood outcomes. In pooled analyses of randomized trials of 12 weeks’ duration, gabapentin enacarbil 600 mg/day generally improved the symptoms of primary RLS, irrespective of its severity (moderate to severe or severe), associated sleep disturbance (none to moderate or severe to very severe) or prior dopamine agonist use (Sect. 4).
Gabapentin enacarbil 600 mg/day was generally well tolerated in short- and longer-term trials, with the most commonly reported treatment-related adverse events being somnolence/sedation and dizziness (Sect. 5). Adverse events were mostly mild to moderate in severity and resolved within 1–2 weeks. In addition, there were no reports of augmentation or clinically relevant electrocardiogram changes with gabapentin enacarbil (Sect. 5).
When gabapentin enacarbil was indirectly compared with pramipexole, ropinirole and rotigotine in a mixed treatment comparison of pooled data from 15 randomized, double-blind, placebo-controlled trials in patients with moderate to severe RLS, gabapentin enacarbil ranked third out of the four drugs in relative treatment effect (change in IRLS at week 12 and end of maintenance), with a probability of 15.6 and 18.9 % of being the best out of all compared [45]. Moreover, in terms of CGI-I responder rates, gabapentin enacarbil ranked first, together with pramipexole, in the probability of being the best out of the four treatments compared (46.2 % for gabapentin enacarbil and 44.5 % for pramipexole). However, in order to validate these results, direct-comparison trials are needed [45]. Pharmacoeconomic analyses evaluating the cost effectiveness of gabapentin enacarbil compared with other RLS treatment options would also be of interest.
In conclusion, gabapentin enacarbil is an oral, extended-release prodrug of gabapentin that is effective and generally well tolerated at a dosage of 600 mg/day in adults with moderate to severe primary RLS. Gabapentin enacarbil is an important first-line agent for the treatment of moderate to severe primary RLS without the risk of augmentation.
Data selection sources:
Relevant medical literature (including published and unpublished data) on gabapentin enacarbil was identified by searching databases including MEDLINE (from 1946), PubMed (from 1946) and EMBASE (from 1996) [searches last updated 25 April 2016], bibliographies from published literature, clinical trial registries/databases and websites. Additional information was also requested from the company developing the drug.
Search terms: Gabapentin enacarbil, Regnite, Horizant, ASP-8825, XP-13512, restless legs, Wittmaack-Ekbom, Willis-Ekbom.
Study selection: Studies in patients with restless legs syndrome who received gabapentin enacarbil. When available, large, well designed, comparative trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
References
Allen RP, Picchietti DL, Garcia-Borreguero D, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria-history, rationale, description, and significance. Sleep Med. 2014;15(8):860–73.
Nagandla K, De S. Restless legs syndrome: pathophysiology and modern management. Postgrad Med J. 1053;2013(89):402–10.
Ohayon MM, O’Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16(4):283–95.
Comella CL. Treatment of restless legs syndrome. NeuroTherapeutics. 2014;11(1):177–87.
Ondo WG. Restless legs syndrome: pathophysiology and treatment. Curr Treat Options Neurol. 2014;16(11):317.
Trenkwalder C, Winkelmann J, Inoue Y, et al. Restless legs syndrome-current therapies and management of augmentation. Nat Rev Neurol. 2015;11(8):434–45.
Rios Romenets S, Postuma RB. Treatment of restless legs syndrome. Curr Treat Options Neurol. 2013;15(4):396–409.
Garcia-Borreguero D, Silber MH, Winkelman JW, et al. Guidelines for the first-line treatment of restless legs syndrome/Willis-Ekbom disease, prevention and treatment of dopaminergic augmentation: a combined task force of the IRLSSG, EURLSSG, and the RLS-foundation. Sleep Med. 2016;21:1–11.
Scott LJ. Gabapentin enacarbil: in patients with restless legs syndrome. CNS Drugs. 2012;26(12):1073–83.
US FDA. Horizant (gabapentin enacarbil) extended-release tablets: US prescribing information. 2013. http://www.fda.gov. Accessed 26 Apr 2016.
Lal R, Sukbuntherng J, Luo W, et al. Population pharmacokinetics and pharmacodynamics of gabapentin after administration of gabapentin enacarbil. J Clin Pharmacol. 2013;53(1):29–40.
Davy M, Upward J, Arumugham T, et al. Cardiac repolarization with gabapentin enacarbil in a randomized, double-blind, placebo- and active-controlled, crossover thorough QT/QTc study in healthy adults. Clin Ther. 2013;35(12):1964–74.
Chen D, Lal R, Zomorodi K, et al. Evaluation of gabapentin enacarbil on cardiac repolarization: a randomized, double-blind, placebo- and active-controlled, crossover thorough QT/QTc study in healthy adults. Clin Ther. 2012;34(2):351–62.e3.
Lal R, Sukbuntherng J, Luo W, et al. Pharmacokinetics and tolerability of single escalating doses of gabapentin enacarbil: a randomized-sequence, double-blind, placebo-controlled crossover study in healthy volunteers. Clin Ther. 2009;31(8):1776–86.
Cundy KC, Branch R, Chernov-Rogan T, et al. XP13512 [(±)-1-([(α-isobutanoyloxyethoxy)carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: I. Design, synthesis, enzymatic conversion to gabapentin, and transport by intestinal solute transporters. J Pharmacol Exp Ther. 2004;311(1):315–23.
Cundy KC, Sastry S, Luo W, et al. Clinical pharmacokinetics of XP13512, a novel transported prodrug of gabapentin. J Clin Pharmacol. 2008;48(12):1378–88.
Lal R, Sukbuntherng J, Ho J, et al. A phase I, single-dose study of the disposition of 14C-radiolabeled gabapentin enacarbil in healthy male volunteers. Int J Clin Pharmacol Ther. 2011;49(2):109–15.
Lal R, Sukbuntherng J, Luo W, et al. Clinical pharmacokinetic drug interaction studies of gabapentin enacarbil, a novel transported prodrug of gabapentin, with naproxen and cimetidine. Br J Clin Pharmacol. 2010;69(5):498–507.
Chen C, Upward J, Arumugham T, et al. Gabapentin enacarbil and morphine administered in combination versus alone: a double-blind, randomized, pharmacokinetic, and tolerability comparison. Clin Ther. 2015;37(2):349–57.
Lal R, Ellenbogen A, Chen D, et al. A randomized, double-blind, placebo-controlled, dose-response study to assess the pharmacokinetics, efficacy, and safety of gabapentin enacarbil in subjects with restless legs syndrome. Clin Neuropharmacol. 2012;35(4):165–73.
Inoue Y, Hirata K, Uchimura N, et al. Gabapentin enacarbil in Japanese patients with restless legs syndrome: a 12-week, randomized, double-blind, placebo-controlled, parallel-group study. Curr Med Res Opin. 2013;29(1):13–21.
Lee DO, Ziman RB, Perkins AT, et al. A randomized, double-blind, placebo-controlled study to assess the efficacy and tolerability of gabapentin enacarbil in subjects with restless legs syndrome. J Clin Sleep Med. 2011;7(3):282–92.
Winkelman JW, Bogan RK, Schmidt MH, et al. Randomized polysomnography study of gabapentin enacarbil in subjects with restless legs syndrome. Mov Disord. 2011;26(11):2065–72.
Kushida CA, Becker PM, Ellenbogen AL, et al. Randomized, double-blind, placebo-controlled study of XP13512/GSK1838262 in patients with RLS. Neurology. 2009;72(5):439–46.
GlaxoSmithKline. Result summary: study 114025. 2014. http://www.gsk-clinicalstudyregister.com/files2/114025-Clinical-Study-Result-Summary.pdf. Accessed 26 Apr 2016.
Lee DO, Buchfuhrer MJ, Garcia-Borreguero D, et al. Efficacy of gabapentin enacarbil in adult patients with severe primary restless legs syndrome. Sleep Med. 2015;19:50–6.
VanMeter SA, Kavanagh ST, Warren S, et al. Dose response of gabapentin enacarbil versus placebo in subjects with moderate-to-severe primary restless legs syndrome: an integrated analysis of three 12-week studies. CNS Drugs. 2012;26(9):773–80.
Ondo WG, Hermanowicz N, Garcia Borreguero D, et al. Effect of prior exposure to dopamine agonists on treatment with gabapentin enacarbil in adults with moderate-to-severe primary restless legs syndrome: pooled analyses from 3 randomized trials. J Clin Mov Disord. 2015. doi:10.1186/s40734-015-0018-3.
Hermanowicz N, Buchfuhrer M, Wynn D, et al. The effect of gabapentin enacarbil (GEn) on pain outcomes in adults with moderate-to-severe and severe primary restless legs syndrome (RLS): pooled analyses from 3 randomized controlled trials [abstract no. P7.294 plus poster]. In: 67th Annual Meeting of the American Academy of Neurology; 2015.
Hermanowicz N, Ellenbogen A, Irving G, et al. The effect of gabapentin enacarbil on pain associated with moderate-to-severe primary restless legs syndrome in adults: pooled analyses from three randomized controlled trials. CNS Drugs. 2016. doi:10.1007/s40263-016-0333-8.
Ahmed M, Hays R, Poceta J, et al. The effect of gabapentin enacarbil on individual items of the international restless legs scale and post-sleep questionnaire in patients with moderate-to-severe primary restless legs syndrome: pooled analyses from 3 randomized trials [abstract no. 0634]. Sleep. 2014;37(abstract supplement):A221.
Bogan RK, Lee DO, Buchfuhrer MJ, et al. Treatment response to sleep, pain, and mood disturbance and their correlation with sleep disturbance in adult patients with moderate-to-severe primary restless legs syndrome: pooled analyses from 3 trials of gabapentin enacarbil. Ann Med. 2015;47(3):269–77.
Avidan AY, Lee D, Park M, et al. The effect of gabapentin enacarbil on quality of life and mood outcomes in a pooled population of adult patients with moderate-to-severe primary restless legs syndrome. CNS Drugs. 2016. doi:10.1007/s40263-016-0329-4.
Avidan A, Isaacson S, Jaros M, et al. The effect of gabapentin enacarbil (GEn) on quality-of-life (QoL) outcomes in adult patients with moderate-to-severe and severe primary restless legs syndrome (RLS): pooled analyses from two 12-week trials [abstract no. P7.299]. Neurology. 2015;84(14 Suppl).
Ellenbogen AL, Thein SG, Winslow DH, et al. A 52-week study of gabapentin enacarbil in restless legs syndrome. Clin Neuropharmacol. 2011;34(1):8–16.
Inoue Y, Uchimura N, Kuroda K, et al. Long-term efficacy and safety of gabapentin enacarbil in Japanese restless legs syndrome patients. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36(2):251–7.
Bogan RK, Cramer Bornemann MA, Kushida CA, et al. Long-term maintenance treatment of restless legs syndrome with gabapentin enacarbil: a randomized controlled study. Mayo Clin Proc. 2010;85(6):512–21.
US FDA. Horizant (gabapentin enacarbil) medial review. 2011. https://www.fda.gov. Accessed 26 Apr 2016.
Irizarry MC, Webb DJ, Boudiaf N, et al. Risk of cancer in patients exposed to gabapentin in two electronic medical record systems. Pharmacoepidemiol Drug Saf. 2012;21(2):214–25.
Pharmaceuticals and Medical Devices Agency. Regnite® tablets: Japanese prescribing information (in Japanese). 2015. http://www.pmda.go.jp/english/. Accessed 26 Apr 2016.
Aurora RN, Kristo DA, Bista SR, et al. The treatment of restless legs syndrome and periodic limb movement disorder in adults-an update for 2012: practice parameters with an evidence-based systematic review and meta-analyses: an American Academy of Sleep Medicine clinical practice guideline. Sleep. 2012;35(8):1039–62.
Aurora RN, Kristo DA, Bista SR, et al. Update to the AASM clinical practice guideline: “The treatment of restless legs syndrome and periodic limb movement disorder in adults-an update for 2012: practice parameters with an evidence-based systematic review and meta-analyses”. Sleep. 2012;35(8):1037.
Garcia-Borreguero D, Ferini-Strambi L, Kohnen R, et al. European guidelines on management of restless legs syndrome: report of a joint task force by the European Federation of Neurological Societies, the European Neurological Society and the European Sleep Research Society. Eur J Neurol. 2012;19(11):1385–96.
Garcia-Borreguero D, Kohnen R, Silber MH, et al. The long-term treatment of restless legs syndrome/Willis-Ekbom disease: evidence-based guidelines and clinical consensus best practice guidance: a report from the International Restless Legs Syndrome Study Group. Sleep Med. 2013;14(7):675–84.
Sun Y, van Valkenhoef G, Morel T. A mixed treatment comparison of gabapentin enacarbil, pramipexole, ropinirole and rotigotine in moderate-to-severe restless legs syndrome. Curr Med Res Opin. 2014;30(11):2267–78.
Acknowledgments
During the peer review process, the manufacturer of gabapentin enacarbil was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Esther Kim and Emma Deeks are salaried employees of Adis/Springer, are responsible for the article content and declare no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: R. K. Bogan, SleepMed Inc., School of Medicine, University of South Carolina, Columbia, SC, USA; K. Suzuki, Department of Neurology, Dokkyo Medical University, Mibu, Tochigi, Japan.
Rights and permissions
About this article
Cite this article
Kim, E.S., Deeks, E.D. Gabapentin Enacarbil: A Review in Restless Legs Syndrome. Drugs 76, 879–887 (2016). https://doi.org/10.1007/s40265-016-0584-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-016-0584-1