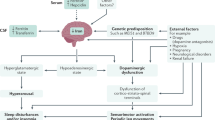
Opinion statement
Restless legs syndrome (RLS) is a complicated sensory-motor syndrome. The pathology is increasingly understood, but a clear physiologic understanding still remains elusive. The most robust findings remain reduced central nervous system (CNS) iron and some perturbation in dopaminergic systems. Other neurotransmitter systems are also like involved, and the phenotype may result from distinct pathophysiologic processes. Treatment of RLS is often very successful, and treatment goals should be high. Dopamine agonists may most robustly improve pure urge to move and certainly periodic limb movements. They do not directly improve sleep, and long-term use is limited by augmentation. Alpha-2-delta ligand drugs such as gabapentin enacarbil and pregabalin improve RLS, presumably in a less specific manner. These drugs increase slow wave sleep and improve pain, but have less impact on leg movements. Mu specific opioids also robustly improve RLS and are probably underutilized in severe cases. Intravenous iron inconsistently but sometimes considerably improves RLS and can be considered in refractory cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction: clinical RLS
Restless legs syndrome (RLS) is clinically defined by the presence of four criteria: 1) an urge to move the limbs with or without uncomfortable sensations, 2) worsening at rest, 3) improvement with activity, 4) worsening in the evening or at night [1•]. Additional criteria now stipulate that these symptoms not be caused by another etiology. The diagnosis of RLS is exclusively based on those symptoms. The patient subjective descriptions, however, are quite varied and tend to be suggestible and education dependent. The sensation is always unpleasant, but not necessarily “painful.” It is usually deep within the legs and commonly between the knee and ankle. Patients usually deny any “burning” or “pins and needles” sensations, commonly experienced in neuropathies or nerve entrapments, although neuropathic pain and RLS can co-exist. Most patients report transient symptomatic improvement by walking, although other movements also help. Other therapeutic techniques reported by patients include rubbing or pressure, stretching, and hot water. In general, harsh sensory stimuli can mitigate RLS. Cognitive or emotional activating circumstances (i.e., arguing) also often reduce RLS. Other clinical features typical for RLS include the tendency for symptoms to gradually worsen with age, improvement with dopaminergic treatments, a positive family history, and periodic limb movements while asleep (PLMS).
PLMS are defined by the American Academy of Sleep Medicine as “periodic episodes of repetitive and highly stereotyped limb movements that occur during sleep.” They classically occur every 20–40 seconds, mostly in Stage I and II sleep. PLMS are common in the general population, and incidence increases with age. However, they are seen in more than 80 % of RLS patients [2]. Therefore, most people with RLS have PLMS, but many patients with isolated PLMS do not have RLS. PLMS are associated with an autonomic spike just before the movement, with transient increase in both blood pressure and pulse. This is increasingly scrutinized, as it is postulated to account for the correlation between PLMS and RLS, and risk of cardiovascular disease seen in some [3–6], but not all studies [7, 8]. Dopaminergics used to treat PLMS also blunt the autonomic response, as well as the actual movement [9]. In contrast, K-complexes and arousals that usually precede the PLMS may persist even if PLMS are reduced [10].
Although some children report classic RLS symptoms that meet inclusion criteria, other complain of “growing pains” [11, 12], and some appear to present with an attention deficit hyperactivity disorder (ADHD) phenotype. Diagnostic criteria for RLS in children is less well validated, but emphasizes supportive criteria such as a family history of RLS, sleep disturbances, the presence of PLMS, and typical descriptors used by children [13].
RLS is very common. Studies in predominantly Caucasian populations consistently show that between 5 and 15 % of people have RLS, which is clinically significant in 2–3 % [14]. In general, northern European countries demonstrate the highest prevalence, followed by Germanic/Anglo-Saxon, and then Mediterranean countries. The prevalence tends to decline the farther east one progresses, dropping to less than 1 % in Singapore. People from African have never been specifically studied, but anecdotally, African Americans only rarely present with RLS. Women usually have higher RLS rates. However, this female preponderance is lost in null-parous women [15, 16].
Pathophysiology of RLS
Patholophysiologic studies show a number of abnormalities in patients with RLS; however, our understanding is far from complete. Abnormalities in circadian physiology, iron, dopamine, glutamate, and opioid systems within the central nervous system (CNS) have been identified. Peripheral nervous systems show abnormalities in sensory perception. Several genetic alleles increase the risk of RLS, which has a strong genetic component. A number of other conditions in which RLS is more commonly seen also provide pathophysiologic clues.
Iron
The most robust and consistent observation is reduced CNS iron stores, even in the setting of normal systemic iron studies. CSF ferritin is lower in RLS cases [17], and specially sequenced magnetic resonance imaging (MRI) studies show reduced iron stores in the striatum and red nucleus [18, 19]. CNS ultrasonography is also able to identify RLS based on reduced iron echogenicity in the substantia nigra (SN) [20, 21]. Most importantly, pathologic data in RLS autopsied brains show reduced H-ferritin staining, iron staining, and increased transferrin stains, but also reduced transferrin receptors [22]. Mitochondrial ferritin, however, is increased in neurons in the SN but not putamen of RLS subjects [23].
The reduced transferrin receptor finding is especially important, because globally reduced iron stores would normally upregulate transferrin receptors. Therefore, it appears that primary RLS has reduced intracellular iron indices associated with a perturbation of homeostatic mechanisms that regulate iron influx or efflux from the cell. Intracellular iron regulation is very complex; however, subsequent staining of RLS brains has shown reduced levels of iron regulatory protein-type 1 (IRB-1) [22]. This potentiates or inhibits (depending on feedback mechanisms involving iron atoms themselves) the production of ferritin molecules, which are the main iron storage proteins in the CNS as well as the periphery, and transferrin receptors, which facilitate intracellular iron transport. Whether reduced iron manifests the symptoms of RLS, or is just an epiphenomenon, is not known.
Dopamine
CNS dopaminergic systems are most implicated in RLS by the vigorous symptom improvement seen with dopaminergics. The normal circadian dopaminergic variation is also augmented in patients with RLS [24, 25]. However, there is little evidence to suggest any dopaminergic deficiency in patients with RLS. Pathologic studies have not found loss of dopamine cells or dopamine in the striatum or SN [26, 27]. Evidence of neurodegeneration markers, including Lewy bodies, plaques or tangles, is also absent [28]. Dopamine marker pathologic studies have been difficult to interpret. SN tyrosine hydroxylase, the rate-limiting step in dopamine synthesis, both phosphorylated (active) and non-phosphorylated (inactive), is increased in RLS subjects compared to controls [29]. There was some decrease in Dopamine-2 receptors in the putamen, but no change in dopamine-1 receptors. Vesicular monoamine transporter (VMAT), a marker of dopamine storage, and other monoamine markers were normal.
Brain imaging studies are inconsistent and show modest or no abnormalities [30–33]. Briefly, dopamine precursor studies show normal or reduced levels, dopamine transporter studies have been normal or reduced, and dopamine receptor studies show reduced, normal, or increased activity. Two studies using different ligands from the same group are interpreted as showing increased dopamine turnover [34]. It should be noted that functional brain imaging studies mostly reflect activity in the striatum, the largest dopaminergic area. Other smaller dopaminergic areas, which may be involved in RLS, are too small to image.
Other indirect evidence suggests increased dopamine turnover in RLS. Cerebrospinal fluid (CSF) 3- Ortho-methyldopa (OMD) is increased in RLS subjects, suggesting either increased dopamine metabolism in general, or specifically increased monoamine oxidase (MAO)-B activity, which metabolizes dopamine to 3-OMD [35]. These data have led some to speculate that RLS is a disease of hyperdopaminergic function. However, this does not explain the dramatic and immediate benefit of dopaminergics.
The identification of a specific anatomical site in RLS remains another puzzle. The spinal cord and sub-cortex are implicated in studies of RLS onset after stroke and spinal cord injuries [36]. More specifically, involvement of the little-studied diencephalospinal dopaminergic tract, originating from the A11-A14 nuclei, might explain some RLS features. It is involved in anti-nociception, is near circadian control centers, and would explain why legs are involved more than arms. A preliminary animal model with A11 lesions demonstrated increased standing episodes, which improved after the administration of ropinirole, a dopamine agonist [37]. Subsequent studies of this model in mice, with and without dietary iron deprivation, also demonstrate increased movement, as measured in laser marked cages, in the lesioned animals [38]. This hyperkinesis is normalized by D2 agonists such as ropinirole and pramipexole, but not by the D1 agonist SKF, which actually increased movement further. This small area is not visible with dopamine imaging and would probably not contribute meaningfully to CSF dopamine studies. No careful study examining spinal cord dopamine metabolites/receptors exists. Human pathologic studies of the dopaminergic neurons of the A11 system, however, have not shown any cell loss [39].
There are several potential interactions between iron and dopamine systems. First, iron is a co-factor for tyrosine-hydroxylase (TH), which is the rate-limiting step in the production of dopamine. However, TH activity is actually increased in humans with RLS, so this is unlikely. Second, iron is a component of the dopamine type-2 (D2) receptor. Iron deprivation in rats results in a 40–60 % reduction of D2 post-synaptic striatal, but not spinal cord receptors [40, 41]. The effect in the striatum is quite specific, as other neurotransmitter systems, including D1 receptors, are not affected. However, the dramatic and immediate response to dopamine agonists would be difficult to explain in the setting of receptor deficiency. Third, iron is necessary for Thy1 protein regulation. This cell adhesion molecule, which is robustly expressed on dopaminergic neurons, is reduced in brain homogenates in iron-deprived mice [42] and in brains of patients with RLS [43]. Thy1 regulates vesicular release of monoamines, including dopamine [44]. It also stabilizes synapses and suppresses dendritic growth [45]. Further work on this possible connection is warranted.
Opioid systems
Opioid pathways are implicated by clinical improvement seen with narcotics and pathological data that show reduced Beta-endorphin positive cells (37.5 %, p = 0.006, effect size 2.16) and Met-enkephalin positive cells (26.4 %, p = 0.028, effect size 1.58) in six RLS patients compared to six controls [26]. Dopamine activity was normal in this study. Positron emission tomography (PET) imaging studies using non-specific opioid ligands did not differentiate between human RLS and controls, but did correlate with severity within the RLS group, suggesting that the more severe the RLS, the greater the release of endogenous opioids within the medial pain system [46].
Afferent systems are also implicated [47, 48]. Stiasny-Kolster reported that pin-prick pain ratings (static hyperalgesia) in RLS patients were significantly elevated in the lower limb, whereas pain to light touch (allodynia = dynamic mechanical hyperalgesia) was normal. They felt this type of hyperalgesia was probably mediated by central sensitization to A-delta fiber high-threshold mechanoreceptor input, a hallmark sign of the hyperalgesia type of neuropathic pain.
Miscellaneous studies
Recently, (1)H magnetic resonance spectroscopy (MRS) showed increased glutaminergic activity in the thalamus of RLS patients [49]. This correlated with increased arousal, but not PLMS. This study, however, was contradicted by a later report by Winkelman et al., who found increased gamma-aminobutyric acid (GABA) in the thalamus using MR spectroscopy [50].
A single cerebrospinal fluid (CSF) proteomics trial of only five subjects found four proteins that were increased in RLS (Cystatin C, Lipocalin-type Prostaglandin D2 Synthase, Vitamin D binding Protein, and beta-Hemoglobin) and two proteins (Apolipoprotein A1 and alpha-1-acid Glycoprotein) that were decreased [51].
Functional MRI studies show increased activity in the cerebellum and thalamus during the sensory component, which also included the red nucleus and brainstem reticular formation during the sensory/motor component [52]. MRI voxel-based morphometry has shown several inconsistent abnormalities, of which increased pulvinar grey matter seems the most robust [53].
RLS genetics
In 40–60 % of cases, a family history of RLS can be found, although this is often not initially reported by the patient [54]. Twin studies also show a very high concordance rate [55, 56]. Most pedigrees suggest an autosomal dominant (AD) pattern [57], although an autosomal recessive (AR) pattern with a very high carrier rate is possible. Many linkages have been identified in traditional familial studies, but in no case has a specific gene mutation been identified [Table 1]. A number of candidate genes, including those involved with dopamine metabolism, Parkinson’s disease, neurodegeneration, and systemic iron regulation, have generally been unrevealing [74]. To date, six risk factor genes, identified through genome-wide association studies, have been published [Table 2]. Identification of the physiology of these genes is ongoing. Several of these are thought to be developmental genes and at least one is now associated with iron regulation. Recently, these genes did not show any association in Asian uremic RLS patients [75]. As with other common conditions, the genetics of RLS are complex and probably involve the presence of multiple at risk genetic alleles.
Pathophysiologic clues from associated conditions
The most common diseases associated with RLS include renal failure, iron deficiency, neuropathy, myelopathy, pregnancy, multiple sclerosis, and possibly Parkinson’s disease and essential tremor. Many other additional reported associations are less well established. The exact relationship between these conditions and RLS is not known, but several patterns of interest exist.
Neuropathy and myelopathy both result in deafferentation. This is intuitive, since sensory stimulation (pain, heat, etc.) improves RLS. Alteration in iron homeostasis is seen in systemic iron deficiency, uremia, and pregnancy. Systemic iron deficiency correlates with RLS, mostly in those with an older age of onset and in those who lack a family history of RLS, suggesting it is a true secondary cause, resulting in similar symptoms to those with a presumed genetic cause of RLS. Data on whether iron deficiency specifically correlates with RLS in uremia are sparse. Uremia is a complex biochemical condition and most subjects on dialysis receive aggressive iron supplementation. RLS seems to improve with successful kidney transplantation, but not with dialysis [76–79]. RLS in pregnancy correlates with low iron measures in some studies [80•, 81]. Increased progesterone may also be culpable.
The relationship between Parkinson’s disease (PD) and RLS has been extensively studied. Almost all studies of PD patients show increased prevalence of RLS, but in most cases, PD precedes RLS onset, although some studies suggest RLS occurs early in the course of PD [81, 82]. There is no evidence that early onset familial RLS is a risk factor for the subsequent development of PD, and in fact, some data suggest RLS may prevent PD [83]. The preponderance of data suggests that RLS in PD has a distinct pathophysiology from idiopathic RLS.
Several medications are also known to exacerbate existing RLS or possibly precipitate RLS themselves. The most notable of these include antihistamines, dopamine antagonists, including many anti-nausea medications, mirtazapine, possibly tricyclic antidepressants, and serotonergic reuptake inhibitors (SSRIs). Sedating antihistamines, which cross the blood brain barrier, most strongly exacerbate RLS. Many RLS patients have experienced this, especially since they are marketed as sleep aids. SSRIs are more associated with PLMS than RLS. Interestingly, dopamine antagonists do not usually worsen RLS and the dopamine release inhibitor tetrabenazine does not exacerbate RLS [84, 85].
Treatment of RLS
Multiple medications have demonstrated efficacy in well-designed clinical trials, and several treatment guidelines and evidence based reviews are published or in preparation [86, 87•, 88•, 89]. The best data support the use of dopaminergics, alpha2delta ligands, opioids, and iron. With the possible exception of iron, all are felt to provide only symptomatic relief, rather than any “curative” effect [Table 2]. Therefore, treatment should only be initiated when the benefits are felt to justify any potential side effects and costs. Treatment decisions also need to consider the chronicity and general progressive course of RLS. Over time, both dosing and drug changes may be required to maximize benefit and minimize the risk of side effects. The quality of RLS symptoms and the timing of symptoms need to be considered to optimize treatment results.
Dopaminergics
Dopamine agonists (DA) are the most investigated, consistently effective treatments for RLS. Most placebo-controlled trials usually show a 3–6 point improvement over placebo on the International RLS Rating Scale (IRLS). The large placebo response in RLS trials should be noted, and mitigate the importance of data that are not placebo controlled [90]. The improvement with DA is immediate and often very dramatic. No evidence favors any particular DA. Ropinirole [91–93], pramipexole [94–96], and rotigotine patches [97, 98] are the best studied and approved for use by European and American agencies. Pergolide [91, 99–102], bromocriptine [103], apomorphine [104], cabergoline [105] and lisuride [106] are also effective, but less commonly used. The dopamine precursor levodopa also effectively treats RLS and is officially licensed for use in some European countries [107, 108]; however, several comparative studies have favored DA over levodopa. [100, 109, 110]
Polysomnogram studies of DA consistently demonstrate dramatic improvement in PLMS, but only modest or no improvement in other sleep parameters. The effect is immediate, so titration to the smallest effective dose can be fairly rapid. Acute adverse events of DA in RLS studies are generally milder than in PD studies, perhaps owing to the lower dose or differences in the disease state. In contrast to PD, hallucinations and hypotension rarely occur in RLS, and daytime sedation may lesson rather than increase. Nausea remains the most common adverse event. Edema, impulse control disorders and nasal congestion still occur. The rotigotine 24-hour patch can cause an application site rash. Immediate release oral DA work best if administered at least 90 minutes before the onset of symptoms. Based on pharmacokinetics, many people may benefit from more than one dose, despite the formal indications, which recommend dosing 1–3 hours before bed. Extended release preparations of pramipexole and ropinirole are also very effective, but have not been formally studied in RLS.
Although the three approved DA have very similar receptor affinity profiles (all have a relatively greater D3:D2 receptor affinity ratio compared to endogenous dopamine), there may be intra-subject variability among them [Table 2]. Therefore, failure of one agent should not preclude attempts at another. There are differences in duration of effect: 24-hour rotigotine patch>followed by extended release pramipexole>extended release ropinirole>pramipexole>ropinirole>L-dopa. L-dopa, however, is the most rapidly absorbed, and therefore may be considered for as-needed use.
Augmentation
The long-term use of DA for RLS is more problematic, as some subjects develop tolerance and others can develop very problematic augmentation [87•]. Augmentation is defined by an earlier phase shift of symptom onset, an increased intensity of symptoms, increased anatomic involvement, or less relief with movement [111]. Levodopa has the worst augmentation, often within six months, and is much less used because of this problem [112]. Augmentation with dopamine agonists is modest at one year (2–9 %), but seems to increase linearly over time [113–116]. There is some open label non-controlled data suggesting that the rotigotine patch may have less augmentation than oral agents [115]. Whether this results from preventing the actual physiologic process of augmentation, or by intrinsically treating augmentation by already having medicine in the system during the day, is not known. Risk factors for augmentation have been inconsistent, but include lower serum ferritin, a higher dose of a dopaminergic, worse RLS, a family history of RLS, and absence of neuropathy [112, 117, 118].
Although augmentation has been highly associated with dopaminergics, despite a few case reports seen with other classes of agents, only recently has a study confirmed that augmentation was higher with pramipexole (0.5 mg) than with the non-dopaminergic pregabalin at 52 weeks, although this study was complicated by a very high dropout rate [113].
The mechanisms behind augmentation are still not understood and there is no standard treatment strategy to address it. Taking the DA earlier, fractionating the dose, or switching to a longer-acting DA and taking it earlier will usually treat earlier onset RLS symptoms initially, but the augmentation may continue to worsen over time. Eventually, stopping the offending agent may be necessary, but is very difficult to accomplish without a severe exacerbation of symptoms. This usually lasts 1–2 weeks, then subsides and the RLS reverts back into the baseline nocturnal symptoms. In our experience, only high potency narcotics can treat this withdrawal phase.
Alpha-2-delta ligands
Gabapentin and pregabalin have affinity for the alpha-2-(delta) sub-unit of the sodium channel. Both have been used to treat seizures and various painful conditions. Gabapentin enacarbil (an extended release preparation of a gabapentin precursor with markedly improved absorption throughout the gastrointestinal tract) [119, 120] and pregabalin have both improved RLS symptoms in large, Class I, controlled, multi-center trials [121–125]. Smaller studies also support the use of relatively high dose gabapentin [126]. Gabapentin enacarbil is approved by American agencies, but pregabalin is not, despite similar efficacy results. Compared to DA, these drugs show more improvement of sleep architecture, mostly by increasing the percentage of slow wave sleep, but with less improvement of PLMS. Overall efficacy on the IRLS tends to be similar when comparing different trials. One large comparator trial that did directly evaluate high dose pregabalin vs. lower dose pramipexole found that at 12 weeks, both groups were superior to placebo, but not significantly different from each other [110]. There was a trend favoring pregabalin, but also a higher dropout rate due to adverse events.
Both DA and alpha-2 delta ligands are considered first line therapies for RLS. Anecdotally, the alpha-2 delta agents better improve associated painful conditions and sleep, whereas DA may better improve pure urge to move and PLMS (motor component of RLS). Therefore, the specific RLS phenotype and associated conditions may be considered when picking an agent. Combined use has not been formally studied, but is common in practice. Of note, it is difficult to switch directly from a DA to an alpha-2 delta ligand in the setting of augmentation, as they tend not to adequately treat symptoms unless there has been a washout period in between the DA and the alpha-2-delta drug.
Opioid medications
Opioid medications, also known as narcotics, have long been known to successfully treat RLS. Open label trials consistently demonstrate good initial and long-term results, without difficulty with tolerance, dependence or addiction [127]. There exist, however, only a modest amount of controlled data that demonstrate efficacy [128, 129]. In one recent, well-designed large placebo controlled trial, prolonged release oxycodone-naloxone was evaluated [130]. The dose of oxycodone ranged from 10 to 40 mg/day in two divided doses, and was titrated to effect. Improvement at 12 weeks was 7 points (IRLS score) superior to placebo and adverse events are what would be expected with an opioid (nausea, sedation, constipation). Intrathecal spinal morphine pumps have also been used in severe refractory cases [131].
There is impressive long-term, open label data with methadone (a μ specific opioid agonist) in RLS patients who have failed dopamine agonists due to lack of efficacy, adverse events, or severe augmentation [132, 133]. Overall, methadone at doses from 5 to 20 mg/day (often about 10 mg/day) markedly benefits most refractory RLS patient without augmentation, tolerance, or evidence for dependency. Patients taking methadone dropped out within the first three months due to expected side effects, but for those who initially tolerated the medication, long-term tolerability and efficacy was excellent.
Interestingly, there is some evidence suggesting the effect of opioids is independent of their analgesic effect, although a detailed understanding is lacking [134]. Mu-opioid drugs, which potentiate dopamine release and upregulate dopamine receptors, all appear effective, whereas Kappa receptor drugs, like meperidine, that inhibit dopamine release are not effective [135]. Overall, opioid medications may be underutilized when managing chronic and severe RLS.
Iron
Although open label oral iron supplementation has been reported to improve RLS, there is very little controlled data for oral iron supplementation, which has not significantly improved RLS in trials [136, 137]. In some of these reports, however, the oral iron did not markedly increase iron levels, and this fact highlights the limitations related to absorption and tolerance. Many different iron salts and organic preparations exist. Tolerability and absorption may vary among them, but no formal data support superiority of any preparation. It is also ideal to take oral iron without other foods or divalent metals. It is generally recommended to take oral iron supplements with RLS, especially if iron studies [ferritin (<50) or iron binding percentage (<20 %)] is even modestly low, but there is surprisingly little formal data. Oral iron can cause constipation, bloating, and other abdominal discomfort, and so is sometimes poorly tolerated.
Intravenous iron markedly increases iron stores and has been studied in both RLS with and without iron deficiency. Results are mixed, but mostly support the use of some intravenous iron preparations. Both rational explanations and empirical data specifically support the use of iron dextran for RLS. This preparation stays in the serum longer than other preparations, and it appears to take days to transport the iron into the CNS, where it presumably is treating RLS. High molecular weight iron dextran has been most associated with anaphylactic reactions, but this does not seem to occur with low molecular weight preparations, which are now recommended [138]. The most commonly prescribed dose is 1 gm. A controlled trial of iron sucrose (a common preparation) failed to show benefit [139]. Intravenous iron is increasingly used to treat RLS, especially refractory cases. Concerns about toxic effects of very high intravenous dosing are reasonable, but to date no animal data suggest dangerous effects of this treatment.
Miscellaneous treatments
Despite their past widespread use, there is little data to support the use of benzodiazepines for RLS. In the opinion of most experts, benzodiazepines do help facilitate sleep, but seldom improve RLS cardinal features. These can be used successfully in mild cases of RLS and as adjunct therapy for residual insomnia.
Numerous other agents, including other anti-epileptic and muscle relaxant medications, such as levetiracetam, carbamazepine, clonidine, baclofen, tramadol, and magnesium, have been reported to help RLS, but suffer from limited data and cannot be recommended as either first-line or second-line therapy.
Physical measures that increase activity or create a sensory stimulus [140] can also improve RLS, but are often problematic when one desires sleep. One such vibrating device was recently approved by American agencies for RLS treatment. General exercise and stretching techniques are also advocated. Botulinum toxin injections into leg muscles are not effective [141]. Deep brain stimulation (DBS) into the thalamic ventral intermediate nucleus [142] does not help, but a single case of DBS into the globus pallidus internus (GPi) showed benefit [143].
Treatment of RLS in specific populations: pediatrics and renal failure
The treatment of pediatric RLS is much less studied. In open label reports and anecdotally, both oral and intravenous iron often improve symptoms, much more consistently than in adult RLS [144–147]. We recommend aggressive oral iron treatment in this population. A single controlled trial supports the use of L-dopa [148]. Anecdotal experience also supports the use of gabapentin.
Uremic RLS seems to respond to similar medications as idiopathic RLS [149]. Anecdotally, higher doses of medications are often required to achieve remission. Successful kidney transplant is the definitive treatment.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the national institutes of health. Sleep Med. 2003;4(2):101–19. Most accepted definition of RLS.
Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lesperance P. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord. 1997;12(1):61–5.
Giannaki CD, Zigoulis P, Karatzaferi C, Hadjigeorgiou GM, George KP, Gourgoulianis K, et al. Periodic limb movements in sleep contribute to further cardiac structure abnormalities in hemodialysis patients with restless legs syndrome. J Clin Sleep Med. 2013;9(2):147–53.
Li Y, Walters AS, Chiuve SE, Rimm EB, Winkelman JW, Gao X. Prospective study of restless legs syndrome and coronary heart disease among women. Circulation. 2012;126(14):1689–94.
Walters AS, Moussouttas M, Siddiqui F, Silveira DC, Fuentes K, Wang L, et al. Prevalence of stroke in restless legs syndrome: initial results point to the need for more sophisticated studies. Open Neurol J. 2010;4:73–7.
Winkelman JW, Shahar E, Sharief I, Gottlieb DJ. Association of restless legs syndrome and cardiovascular disease in the sleep heart health study. Neurology. 2008;70(1):35–42.
Winter AC, Schurks M, Glynn RJ, Buring JE, Gaziano JM, Berger K, et al. Vascular risk factors, cardiovascular disease, and restless legs syndrome in women. Am J Med. 2013;126(3):220–7. 227 e221-222.
Szentkiralyi A, Volzke H, Hoffmann W, Happe S, Berger K. A time sequence analysis of the relationship between cardiovascular risk factors, vascular diseases and restless legs syndrome in the general population. J Sleep Res. 2013;22(4):434–42.
Manconi M, Ferri R, Zucconi M, Clemens S, Rundo F, Oldani A, et al. Effects of acute dopamine-agonist treatment in restless legs syndrome on heart rate variability during sleep. Sleep Med. 2011;12(1):47–55.
Manconi M, Ferri R, Zucconi M, Bassetti CL, Fulda S, Arico D, et al. Dissociation of periodic leg movements from arousals in restless legs syndrome. Ann Neurol. 2012;71(6):834–44.
Ekbom KA. Growing pains and restless legs. Acta Paediatr Scand. 1975;64(2):264–6.
Rajaram SS, Walters AS, England SJ, Mehta D, Nizam F. Some children with growing pains may actually have restless legs syndrome. Sleep. 2004;27(4):767–73.
Picchietti DL, Bruni O, de Weerd A, Durmer JS, Kotagal S, Owens JA, et al. Pediatric restless legs syndrome diagnostic criteria: an update by the international restless legs syndrome study group. Sleep Med. 2013;14(12):1253–9.
Yeh P, Walters AS, Tsuang JW. Restless legs syndrome: a comprehensive overview on its epidemiology, risk factors, and treatment. Sleep Breath. 2012;16(4):987–1007.
Berger K, Luedemann J, Trenkwalder C, John U, Kessler C. Sex and the risk of restless legs syndrome in the general population. Arch Intern Med. 2004;164(2):196–202.
Pantaleo NP, Hening WA, Allen RP, Earley CJ. Pregnancy accounts for most of the gender difference in prevalence of familial RLS. Sleep Med. 2010;11(3):310–3.
Earley CJ, Connor JR, Beard JL, Malecki EA, Epstein DK, Allen RP. Abnormalities in csf concentrations of ferritin and transferrin in restless legs syndrome. Neurology. 2000;54(8):1698–700.
Allen RP, Barker PB, Wehrl F, Song HK, Earley CJ. Mri measurement of brain iron in patients with restless legs syndrome. Neurology. 2001;56(2):263–5.
Astrakas LG, Konitsiotis S, Margariti P, Tsouli S, Tzarouhi L, Argyropoulou MI. T2 relaxometry and fmri of the brain in late-onset restless legs syndrome. Neurology. 2008;71(12):911–6.
Schmidauer C, Sojer M, Stocckner H, Hogel B, Wenning W, Poewe W. Brain parenchyma sonography differentiates rls patients from normal controls and patients with parkinson's disease. Mov Disord. 2005;20(supple10):S43.
Godau J, Klose U, Di Santo A, Schweitzer K, Berg D. Multiregional brain iron deficiency in restless legs syndrome. Mov Disord. 2008;23(8):1184–7.
Connor JR, Wang XS, Patton SM, Menzies SL, Troncoso JC, Earley CJ, et al. Decreased transferrin receptor expression by neuromelanin cells in restless legs syndrome. Neurology. 2004;62(9):1563–7.
Snyder AM, Wang X, Patton SM, Arosio P, Levi S, Earley CJ, et al. Mitochondrial ferritin in the substantia nigra in restless legs syndrome. J Neuropathol Exp Neurol. 2009;68(11):1193–9.
Garcia-Borreguero D, Larrosa O, Granizo JJ, de la Llave Y, Hening WA. Circadian variation in neuroendocrine response to l-dopa in patients with restless legs syndrome. Sleep. 2004;27(4):669–73.
Earley CJ, Hyland K, Allen RP. Circadian changes in csf dopaminergic measures in restless legs syndrome. Sleep Med. 2006;7(3):263–8.
Walters AS, Ondo WG, Zhu W, Le W. Does the endogenous opiate system play a role in the restless legs syndrome? A pilot post-mortem study. J Neurol Sci. 2009;279(1–2):62–5.
Connor JR, Boyer PJ, Menzies SL, Dellinger B, Allen RP, Ondo WG, et al. Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology. 2003;61(3):304–9.
Pittock SJ, Parrett T, Adler CH, Parisi JE, Dickson DW, Ahlskog JE. Neuropathology of primary restless leg syndrome: absence of specific tau- and alpha-synuclein pathology. Mov Disord. 2004;19(6):695–9.
Connor JR, Wang XS, Allen RP, Beard JL, Wiesinger JA, Felt BT, et al. Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain. 2009;132(Pt 9):2403–12.
Staedt J, Stoppe G, Kogler A, Riemann H, Hajak G, Munz DL, et al. Nocturnal myoclonus syndrome (periodic movements in sleep) related to central dopamine d2-receptor alteration. Eur Arch Psychiatry Clin Neurosci. 1995;245(1):8–10.
Trenkwalder C, Walters AS, Hening WA, Chokroverty S, Antonini A, Dhawan V, et al. Positron emission tomographic studies in restless legs syndrome. Mov Disord. 1999;14(1):141–5.
Turjanski N, Lees AJ, Brooks DJ. Striatal dopaminergic function in restless legs syndrome: 18f-dopa and 11c-raclopride pet studies. Neurology. 1999;52(5):932–7.
Tribl GG, Asenbaum S, Happe S, Bonelli RM, Zeitlhofer J, Auff E. Normal striatal d2 receptor binding in idiopathic restless legs syndrome with periodic leg movements in sleep. Nucl Med Commun. 2004;25(1):55–60.
Earley CJ, Kuwabara H, Wong DF, Gamaldo C, Salas RE, Brasic JR, et al. Increased synaptic dopamine in the putamen in restless legs syndrome. Sleep. 2013;36(1):51–7.
Allen RP, Connor JR, Hyland K, Earley CJ. Abnormally increased csf 3-ortho-methyldopa (3-omd) in untreated restless legs syndrome (rls) patients indicates more severe disease and possibly abnormally increased dopamine synthesis. Sleep Med. 2009;10(1):123–8.
Lee SJ, Kim JS, Song IU, An JY, Kim YI, Lee KS. Poststroke restless legs syndrome and lesion location: anatomical considerations. Mov Disord. 2009;24(1):77–84.
Ondo WG, He Y, Rajasekaran S, Le WD. Clinical correlates of 6-hydroxydopamine injections into a11 dopaminergic neurons in rats: a possible model for restless legs syndrome. Mov Disord. 2000;15(1):154–8.
Qu S, Le W, Zhang X, Xie W, Zhang A, Ondo WG. Locomotion is increased in a11-lesioned mice with iron deprivation: a possible animal model for restless legs syndrome. J Neuropathol Exp Neurol. 2007;66(5):383–8.
Earley CJ, Allen RP, Connor JR, Ferrucci L, Troncoso J. The dopaminergic neurons of the a11 system in rls autopsy brains appear normal. Sleep Med. 2009;10(10):1155–7.
Ben-Shachar D, Finberg JP, Youdim MB. Effect of iron chelators on dopamine d2 receptors. J Neurochem. 1985;45(4):999–1005.
Zhao H, Zhu W, Pan T, Xie W, Zhang A, Ondo WG, et al. Spinal cord dopamine receptor expression and function in mice with 6-ohda lesion of the a11 nucleus and dietary iron deprivation. J Neurosci Res. 2007;85(5):1065–76.
Ye Z, Connor JR. Identification of iron responsive genes by screening cdna libraries from suppression subtractive hybridization with antisense probes from three iron conditions. Nucleic Acids Res. 2000;28(8):1802–7.
Wang X, Wiesinger J, Beard J, Felt B, Menzies S, Earley C, et al. Thy1 expression in the brain is affected by iron and is decreased in restless legs syndrome. J Neurol Sci. 2004;220(1–2):59–66.
Jeng CJ, McCarroll SA, Martin TF, Floor E, Adams J, Krantz D, et al. Thy-1 is a component common to multiple populations of synaptic vesicles. J Cell Biol. 1998;140(3):685–98.
Shults CW, Kimber TA. Thy-1 immunoreactivity distinguishes patches/striosomes from matrix in the early postnatal striatum of the rat. Brain Res Dev Brain Res. 1993;75(1):136–40.
von Spiczak S, Whone AL, Hammers A, Asselin MC, Turkheimer F, Tings T, et al. The role of opioids in restless legs syndrome: an [11c] diprenorphine pet study. Brain. 2005;128(Pt 4):906–17.
Stiasny-Kolster K, Magerl W, Oertel WH, Moller JC, Treede RD. Static mechanical hyperalgesia without dynamic tactile allodynia in patients with restless legs syndrome. Brain. 2004;127(Pt 4):773–82.
Stiasny-Kolster K, Pfau DB, Oertel WH, Treede RD, Magerl W. Hyperalgesia and functional sensory loss in restless legs syndrome. Pain. 2013;154(8):1457–63.
Allen RP, Barker PB, Horska A, Earley CJ. Thalamic glutamate/glutamine in restless legs syndrome: increased and related to disturbed sleep. Neurology. 2013;80(22):2028–34.
Winkelman JW, Schoerning L, Platt S, Jensen J. Restless legs syndrome and central nervous system gamma-aminobutyric acid: preliminary associations with periodic limb movements in sleep and restless leg syndrome symptom severity. Sleep Med (2014).
Patton SM, Cho YW, Clardy TW, Allen RP, Earley CJ, Connor JR. Proteomic analysis of the cerebrospinal fluid of patients with restless legs syndrome/willis-ekbom disease. Fluids Barriers CNS. 2013;10(1):20.
Bucher SF, Seelos KC, Oertel WH, Reiser M, Trenkwalder C. Cerebral generators involved in the pathogenesis of the restless legs syndrome. Ann Neurol. 1997;41(5):639–45.
Etgen T, Draganski B, Ilg C, Schroder M, Geisler P, Hajak G, et al. Bilateral thalamic gray matter changes in patients with restless legs syndrome. Neuroimage. 2005;24(4):1242–7.
Ondo W, Jankovic J. Restless legs syndrome: clinicoetiologic correlates. Neurology. 1996;47(6):1435–41.
Ondo WG, Vuong KD, Wang Q. Restless legs syndrome in monozygotic twins: clinical correlates. Neurology. 2000;55(9):1404–6.
Xiong L, Jang K, Montplaisir J, Levchenko A, Thibodeau P, Gaspar C, et al. Canadian restless legs syndrome twin study. Neurology. 2007;68(19):1631–3.
Winkelmann J, Muller-Myhsok B, Wittchen HU, Hock B, Prager M, Pfister H, et al. Complex segregation analysis of restless legs syndrome provides evidence for an autosomal dominant mode of inheritance in early age at onset families. Ann Neurol. 2002;52(3):297–302.
Stefansson H, Rye DB, Hicks A, Petursson H, Ingason A, Thorgeirsson TE, et al. A genetic risk factor for periodic limb movements in sleep. N Engl J Med. 2007;357(7):639–47.
Winkelmann J, Schormair B, Lichtner P, Ripke S, Xiong L, Jalilzadeh S, et al. Genome-wide association study of restless legs syndrome identifies common variants in three genomic regions. Nat Genet. 2007;39(8):1000–6.
Schormair B, Kemlink D, Roeske D, Eckstein G, Xiong L, Lichtner P, et al. Ptprd (protein tyrosine phosphatase receptor type delta) is associated with restless legs syndrome. Nat Genet. 2008;40(8):946–8.
Desautels A, Turecki G, Montplaisir J, Brisebois K, Sequeira A, Adam B, et al. Evidence for a genetic association between monoamine oxidase a and restless legs syndrome. Neurology. 2002;59(2):215–9.
Winkelmann J, Lichtner P, Schormair B, Uhr M, Hauk S, Stiasny-Kolster K, et al. Variants in the neuronal nitric oxide synthase (nnos, nos1) gene are associated with restless legs syndrome. Mov Disord. 2008;23(3):350–8.
Desautels A, Turecki G, Montplaisir J, Xiong L, Walters AS, Ehrenberg BL, et al. Restless legs syndrome: confirmation of linkage to chromosome 12q, genetic heterogeneity, and evidence of complexity. Arch Neurol. 2005;62(4):591–6.
Bonati MT, Ferini-Strambi L, Aridon P, Oldani A, Zucconi M, Casari G. Autosomal dominant restless legs syndrome maps on chromosome 14q. Brain. 2003;126(Pt 6):1485–92.
Chen S, Ondo WG, Rao S, Li L, Chen Q, Wang Q. Genomewide linkage scan identifies a novel susceptibility locus for restless legs syndrome on chromosome 9p. Am J Hum Genet. 2004;74(5):876–85.
Pichler I, Schwienbacher C, Zanon A, Fuchsberger C, Serafin A, Facheris MF, et al. Fine-mapping of restless legs locus 4 (rls4) identifies a haplotype over the spats2l and kctd18 genes. J Mol Neurosci. 2012;49(3):600–5.
Levchenko A, Provost S, Montplaisir JY, Xiong L, St-Onge J, Thibodeau P, et al. A novel autosomal dominant restless legs syndrome locus maps to chromosome 20p13. Neurology. 2006;67(5):900–1.
Sas AM, Di Fonzo A, Bakker SL, Simons EJ, Oostra BA, Maat-Kievit AJ, et al. Autosomal dominant restless legs syndrome maps to chromosome 20p13 (rls-5) in a dutch kindred. Mov Disord. 2010;25(11):1715–22.
Lohmann-Hedrich K, Neumann A, Kleensang A, Lohnau T, Muhle H, Djarmati A, et al. Evidence for linkage of restless legs syndrome to chromosome 9p: are there two distinct loci?[see comment]. Neurology. 2008;70(9):686–94.
Levchenko A, Montplaisir JY, Asselin G, Provost S, Girard SL, Xiong L, et al. Autosomal-dominant locus for restless legs syndrome in french-canadians on chromosome 16p12.1. Mov Disord. 2009;24(1):40–50.
Skehan EB, Abdulrahim MM, Parfrey NA, Hand CK. A novel locus for restless legs syndrome maps to chromosome 19p in an irish pedigree. Neurogenetics. 2012;13(2):125–32.
Balaban H, Bayrakli F, Kartal U, Pinarbasi E, Topaktas S, Kars HZ. A novel locus for restless legs syndrome on chromosome 13q. Eur Neurol. 2012;68(2):111–6.
Weissbach A, Siegesmund K, Bruggemann N, Schmidt A, Kasten M, Pichler I, et al. Exome sequencing in a family with restless legs syndrome. Mov Disord. 2012;27(13):1686–9.
Vilarino-Guell C, Soto AI, Young JE, Lin SC, Uitti RJ, Wszolek ZK, et al. Susceptibility genes for restless legs syndrome are not associated with parkinson disease. Neurology. 2008;71(3):222–3.
Lin CH, Chen ML, Wu VC, Li WY, Sy HN, Wu SL, et al. Association of candidate genetic variants with restless legs syndrome in end stage renal disease: a multicenter case-control study in taiwan. Eur J Neurol. 2014;21(3):492–8.
Huiqi Q, Shan L, Mingcai Q. Restless legs syndrome (rls) in uremic patients is related to the frequency of hemodialysis sessions. Nephron. 2000;86(4):540.
Yasuda T, Nishimura A, Katsuki Y, Tsuji Y. Restless legs syndrome treated successfully by kidney transplantation--a case report. Clin Transpl (1986) 138.
Winkelmann J, Stautner A, Samtleben W, Trenkwalder C. Long-term course of restless legs syndrome in dialysis patients after kidney transplantation. Mov Disord. 2002;17(5):1072–6.
Azar SA, Hatefi R, Talebi M. Evaluation of effect of renal transplantation in treatment of restless legs syndrome. Transplant Proc. 2007;39(4):1132–3.
Manconi M, Govoni V, De Vito A, Economou NT, Cesnik E, Casetta I, et al. Restless legs syndrome and pregnancy. Neurology. 2004;63(6):1065–9. Largest study of RLS during pregnancy.
Tunc T, Karadag YS, Dogulu F, Inan LE. Predisposing factors of restless legs syndrome in pregnancy. Mov Disord. 2007;22(5):627–31.
Ondo WG, Vuong KD, Jankovic J. Exploring the relationship between parkinson disease and restless legs syndrome. Arch Neurol. 2002;59(3):421–4.
Ondo W, Chen S, Dragan E. Does idiopathic restless legs syndrome delay onset and reduce severity of parkinson’s disease. Mov Disord. 2014;29(Supple1):S303.
Ondo WG, Jia JJ. Effect of tetrabenazine use on restless legs syndrome. Sleep Med. 2010;11(9):958–9.
Jagota P, Asawavichienjinda T, Bhidayasiri R. Prevalence of neuroleptic-induced restless legs syndrome in patients taking neuroleptic drugs. J Neurol Sci. 2012;314(1–2):158–60.
Garcia-Borreguero D, Ferini-Strambi L, Kohnen R, O'Keeffe S, Trenkwalder C, Hogl B, et al. European guidelines on management of restless legs syndrome: report of a joint task force by the European federation of neurological societies, the European neurological society and the European sleep research society. Eur J Neurol. 2012;19(11):1385–96.
Garcia-Borreguero D, Kohnen R, Silber MH, Winkelman JW, Earley CJ, Hogl B, et al. The long-term treatment of restless legs syndrome/willis-ekbom disease: evidence-based guidelines and clinical consensus best practice guidance: a report from the international restless legs syndrome study group. Sleep Med. 2013;14(7):675–84. Only review of long-term management of disease.
Silber MH, Becker PM, Earley C, Garcia-Borreguero D, Ondo WG. Willis-ekbom disease foundation revised consensus statement on the management of restless legs syndrome. Mayo Clin Proc. 2013;88(9):977–86. Recent review of treatment options.
Hornyak M, Scholz H, Kohnen R, Bengel J, Kassubek J, Trenkwalder C. What treatment works best for restless legs syndrome? Meta-analyses of dopaminergic and non-dopaminergic medications. Sleep Med Rev. 2014;18(2):153–64.
Ondo WG, Hossain MM, Gordon MF, Reess J. Predictors of placebo response in restless legs syndrome studies. Neurology. 2013;81(2):193–4.
Trenkwalder C, Garcia-Borreguero D, Montagna P, Lainey E, de Weerd AW, Tidswell P, et al. Ropinirole in the treatment of restless legs syndrome: results from the treat rls 1 study, a 12 week, randomised, placebo controlled study in 10 European countries. J Neurol Neurosurg Psychiatry. 2004;75(1):92–7.
Walters AS, Ondo WG, Dreykluft T, Grunstein R, Lee D, Sethi K. Ropinirole is effective in the treatment of restless legs syndrome. Treat rls 2: a 12-week, double-blind, randomized, parallel-group, placebo-controlled study. Mov Disord. 2004;19(12):1414–23.
Bogan R, Connolly G, Rederich G. Ropinirole is effective, well tolerated treatment for moderate-to-severe rls: results of a u.S. Study. Mov Disord. 2005;20(supple10):S61.
Oertel WH, Stiasny-Kolster K, Bergtholdt B, Hallstrom Y, Albo J, Leissner L, et al. Efficacy of pramipexole in restless legs syndrome: a six-week, multicenter, randomized, double-blind study (effect-rls study). Mov Disord. 2007;22(2):213–9.
Trenkwalder C, Stiasny-Kolster K, Kupsch A, Oertel WH, Koester J, Reess J. Controlled withdrawal of pramipexole after 6 months of open-label treatment in patients with restless legs syndrome. Mov Disord. 2006;21(9):1404–10.
Partinen M, Hirvonen K, Jama L, Alakuijala A, Hublin C, Tamminen I, et al. Efficacy and safety of pramipexole in idiopathic restless legs syndrome: a polysomnographic dose-finding study-the prelude study. Sleep Med. 2006;7(5):407–17.
Trenkwalder C, Benes H, Poewe W, Oertel WH, Garcia-Borreguero D, de Weerd AW, et al. Efficacy of rotigotine for treatment of moderate-to-severe restless legs syndrome: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2008;7(7):595–604.
Oertel WH, Benes H, Garcia-Borreguero D, Geisler P, Hogl B, Saletu B, et al. Efficacy of rotigotine transdermal system in severe restless legs syndrome: a randomized, double-blind, placebo-controlled, six-week dose-finding trial in europe. Sleep Med. 2008;9(3):228–39.
Earley CJ, Yaffee JB, Allen RP. Randomized, double-blind, placebo-controlled trial of pergolide in restless legs syndrome. Neurology. 1998;51(6):1599–602.
Staedt J, Wassmuth F, Ziemann U, Hajak G, Ruther E, Stoppe G. Pergolide: Treatment of choice in restless legs syndrome (rls) and nocturnal myoclonus syndrome (nms). A double-blind randomized crossover trial of pergolide versus l-dopa. J Neural Transm (Budapest). 1997;104(4-5):461–8.
Trenkwalder C, Brandenburg U, Hundemer H, Lledo A, Quail D. A randomized long-term placebo controlled multicenter trial of pergolide in the treatment of restless legs syndrome with central evaluation of polysomnographic data. Neurology. 2001;56(Supple 3):A5.
Wetter TC, Stiasny K, Winkelmann J, Buhlinger A, Brandenburg U, Penzel T, et al. A randomized controlled study of pergolide in patients with restless legs syndrome. Neurology. 1999;52(5):944–50.
Walters AS, Hening WA, Kavey N, Chokroverty S, Gidro-Frank S. A double-blind randomized crossover trial of bromocriptine and placebo in restless legs syndrome. Ann Neurol. 1988;24(3):455–8.
Reuter I, Ellis CM, Ray Chaudhuri K. Nocturnal subcutaneous apomorphine infusion in parkinson's disease and restless legs syndrome. Acta Neurol Scand. 1999;100(3):163–7.
Happe S, Trenkwalder C. Role of dopamine receptor agonists in the treatment of restless legs syndrome. CNS Drugs. 2004;18(1):27–36.
Benes H. Transdermal lisuride: short-term efficacy and tolerability study in patients with severe restless legs syndrome. Sleep Med. 2006;7(1):31–5.
Benes H, Kurella B, Kummer J, Kazenwadel J, Selzer R, Kohnen R. Rapid onset of action of levodopa in restless legs syndrome: a double-blind, randomized, multicenter, crossover trial. Sleep. 1999;22(8):1073–81.
Saletu M, Anderer P, Hogl B, Saletu-Zyhlarz G, Kunz A, Poewe W, et al. Acute double-blind, placebo-controlled sleep laboratory and clinical follow-up studies with a combination treatment of rr-l-dopa and sr-l-dopa in restless legs syndrome. J Neural Transm. 2003;110(6):611–26.
Trenkwalder C, Benes H, Grote L, Happe S, Hogl B, Mathis J, et al. Cabergoline compared to levodopa in the treatment of patients with severe restless legs syndrome: results from a multi-center, randomized, active controlled trial. Mov Disord. 2007;22(5):696–703.
Pellecchia MT, Vitale C, Sabatini M, Longo K, Amboni M, Bonavita V, et al. Ropinirole as a treatment of restless legs syndrome in patients on chronic hemodialysis: an open randomized crossover trial versus levodopa sustained release. Clin Neuropharmacol. 2004;27(4):178–81.
Garcia-Borreguero D, Kohnen R, Hogl B, Ferini-Strambi L, Hadjigeorgiou GM, Hornyak M, et al. Validation of the augmentation severity rating scale (asrs): a multicentric, prospective study with levodopa on restless legs syndrome. Sleep Med. 2007;8(5):455–63.
Hogl B, Garcia-Borreguero D, Kohnen R, Ferini-Strambi L, Hadjigeorgiou G, Hornyak M, et al. Progressive development of augmentation during long-term treatment with levodopa in restless legs syndrome: results of a prospective multi-center study. J Neurol. 2010;257(2):230–7.
Allen RP, Chen C, Garcia-Borreguero D, Polo O, DuBrava S, Miceli J, et al. Comparison of pregabalin with pramipexole for restless legs syndrome. N Engl J Med. 2014;370(7):621–31.
Garcia-Borreguero D, Hogl B, Ferini-Strambi L, Winkelman J, Hill-Zabala C, Asgharian A, et al. Systematic evaluation of augmentation during treatment with ropinirole in restless legs syndrome (willis-ekbom disease): results from a prospective, multicenter study over 66 weeks. Mov Disord. 2012;27(2):277–83.
Benes H, Garcia-Borreguero D, Ferini-Strambi L, Schollmayer E, Fichtner A, Kohnen R. Augmentation in the treatment of restless legs syndrome with transdermal rotigotine. Sleep Med. 2012;13(6):589–97.
Inoue Y, Hirata K, Hayashida K, Hattori N, Tomida T, Garcia-Borreguero D. Efficacy, safety and risk of augmentation of rotigotine for treating restless legs syndrome. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40:326–33.
Ondo W, Romanyshyn J, Vuong KD, Lai D. Long-term treatment of restless legs syndrome with dopamine agonists. Arch Neurol. 2004;61(9):1393–7.
Silber MH, Girish M, Izurieta R. Pramipexole in the management of restless legs syndrome: an extended study. Sleep. 2003;26(7):819–21.
Yaltho TC, Ondo WG. The use of gabapentin enacarbil in the treatment of restless legs syndrome. Ther Adv Neurol Disord. 2010;3(5):269–75.
Merlino G, Serafini A, Young JJ, Robiony F, Gigli GL, Valente M. Gabapentin enacarbil, a gabapentin prodrug for the treatment of the neurological symptoms associated with disorders such as restless legs syndrome. Curr Opin Investig Drugs. 2009;10(1):91–102.
Allen R, Chen C, Soaita A, Wohlberg C, Knapp L, Peterson BT, et al. A randomized, double-blind, 6-week, dose-ranging study of pregabalin in patients with restless legs syndrome. Sleep Med. 2010;11(6):512–9.
Garcia-Borreguero D, Larrosa O, Williams AM, Albares J, Pascual M, Palacios JC, et al. Treatment of restless legs syndrome with pregabalin: a double-blind, placebo-controlled study. Neurology. 2010;74(23):1897–904.
Lee DO, Ziman RB, Perkins AT, Poceta JS, Walters AS, Barrett RW. A randomized, double-blind, placebo-controlled study to assess the efficacy and tolerability of gabapentin enacarbil in subjects with restless legs syndrome. J Clin Sleep Med. 2011;7(3):282–92.
VanMeter SA, Kavanagh ST, Warren S, Barrett RW. Dose response of gabapentin enacarbil versus placebo in subjects with moderate-to-severe primary restless legs syndrome: an integrated analysis of three 12-week studies. CNS Drugs. 2012;26(9):773–80.
Inoue Y, Hirata K, Uchimura N, Kuroda K, Hattori N, Takeuchi M. Gabapentin enacarbil in japanese patients with restless legs syndrome: a 12-week, randomized, double-blind, placebo-controlled, parallel-group study. Curr Med Res Opin. 2013;29(1):13–21.
Garcia-Borreguero D, Larrosa O, de la Llave Y, Verger K, Masramon X, Hernandez G. Treatment of restless legs syndrome with gabapentin: a double-blind, cross-over study. Neurology. 2002;59(10):1573–9.
Walters AS, Winkelmann J, Trenkwalder C, Fry JM, Kataria V, Wagner M, et al. Long-term follow-up on restless legs syndrome patients treated with opioids. Mov Disord. 2001;16(6):1105–9.
Walters AS, Wagner ML, Hening WA, Grasing K, Mills R, Chokroverty S, et al. Successful treatment of the idiopathic restless legs syndrome in a randomized double-blind trial of oxycodone versus placebo. Sleep. 1993;16(4):327–32.
Kaplan PW, Allen RP, Buchholz DW, Walters JK. A double-blind, placebo-controlled study of the treatment of periodic limb movements in sleep using carbidopa/levodopa and propoxyphene. Sleep. 1993;16(8):717–23.
Trenkwalder C, Benes H, Grote L, Garcia-Borreguero D, Hogl B, Hopp M, et al. Prolonged release oxycodone-naloxone for treatment of severe restless legs syndrome after failure of previous treatment: a double-blind, randomised, placebo-controlled trial with an open-label extension. Lancet Neurol. 2013;12(12):1141–50.
Lindvall P, Hariz GM, Blomstedt P. Overall self-perceived health in restless legs treated with intrathecal morphine. Acta Neurol Scand. 2012;127(4):268–73.
Ondo WG. Methadone for refractory restless legs syndrome. Mov Disord. 2005;20(3):345–8.
Silver N, Allen RP, Senerth J, Earley CJ. A 10-year, longitudinal assessment of dopamine agonists and methadone in the treatment of restless legs syndrome. Sleep Med. 2011;12(5):440–4.
Walters AS. Review of receptor agonist and antagonist studies relevant to the opiate system in restless legs syndrome. Sleep Med. 2002;3(4):301–4.
Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244(3):1067–80.
Trotti LM, Bhadriraju S, Becker LA. Iron for restless legs syndrome. Cochrane Database Syst Rev. 2012;5:CD007834.
Lee CS, Lee SD, Kang SH, Park HY, Yoon IY. Comparison of the efficacies of oral iron and pramipexole for the treatment of restless legs syndrome patients with low serum ferritin. Eur J Neurol. 2013;21(2):260–6.
Cho YW, Allen RP, Earley CJ. Lower molecular weight intravenous iron dextran for restless legs syndrome. Sleep Med. 2013;14(3):274–7.
Earley CJ, Horska A, Mohamed MA, Barker PB, Beard JL, Allen RP. A randomized, double-blind, placebo-controlled trial of intravenous iron sucrose in restless legs syndrome. Sleep Med. 2009;10(2):206–11.
Lettieri CJ, Eliasson AH. Pneumatic compression devices are an effective therapy for restless legs syndrome: a prospective, randomized, double-blinded, sham-controlled trial. Chest. 2009;135(1):74–80.
Nahab FB, Peckham EL, Hallett M. Double-blind, placebo-controlled, pilot trial of botulinum toxin a in restless legs syndrome. Neurology. 2008;71(12):950–1.
Ondo W. Vim deep brain stimulation does not improve pre-existing restless legs syndrome in patients with essential tremor. Parkinsonism Relat Disord. 2006;12(2):113–4.
Ondo WG, Jankovic J, Simpson R, Jimenez-Shahed J. Globus pallidus deep brain stimulation for refractory idiopathic restless legs syndrome. Sleep Med. 2012;13(9):1202–4.
Tilma J, Tilma K, Norregaard O, Ostergaard JR. Early childhood-onset restless legs syndrome: symptoms and effect of oral iron treatment. Acta Paediatr. 2013;102(5):e221–6.
Grim K, Lee B, Sung AY, Kotagal S. Treatment of childhood-onset restless legs syndrome and periodic limb movement disorder using intravenous iron sucrose. Sleep Med. 2013;14(11):1100–4.
Mohri I, Kato-Nishimura K, Kagitani-Shimono K, Kimura-Ohba S, Ozono K, Tachibana N, et al. Evaluation of oral iron treatment in pediatric restless legs syndrome (rls). Sleep Med. 2012;13(4):429–32.
Amos LB, Grekowicz ML, Kuhn EM, Olstad JD, Collins MM, Norins NA, et al. Treatment of pediatric restless legs syndrome. Clin Pediatr (Phila). 2013;53(4):331–6.
England SJ, Picchietti DL, Couvadelli BV, Fisher BC, Siddiqui F, Wagner ML, et al. L-dopa improves restless legs syndrome and periodic limb movements in sleep but not attention-deficit-hyperactivity disorder in a double-blind trial in children. Sleep Med. 2011;12(5):471–7.
de Oliveira MM, Conti CF, Valbuza JS, de Carvalho LB, do Prado GF. The pharmacological treatment for uremic restless legs syndrome: Evidence-based review. Mov Disord. 2010;25(10):1335–42.
Compliance with Ethics Guidelines
Conflict of Interest
William G. Ondo reports grants and personal fees from Lundbeck, USWorld Meds, and Merz, as well as personal fees from Avanir, TEVA, UCBPharma, and Xenoport, outside the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by the author.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Sleep Disorders
Rights and permissions
About this article
Cite this article
Ondo, W.G. Restless Legs Syndrome: Pathophysiology and Treatment. Curr Treat Options Neurol 16, 317 (2014). https://doi.org/10.1007/s11940-014-0317-2
Published:
DOI: https://doi.org/10.1007/s11940-014-0317-2