Abstract
Oral gabapentin enacarbil is approved in adult patients for the treatment of moderate to severe primary restless legs syndrome (RLS) [featured indication] and the management of postherpetic neuralgia. In the 12-week Patient Improvements in Vital Outcomes following Treatment (PIVOT) RLS I and II trials in adult patients with moderate to severe primary RLS (n > 500 total evaluable), once-daily gabapentin enacarbil 600 or 1,200 mg significantly improved mean International Restless Legs Scale (IRLS) total scores compared with placebo, with significantly higher investigator-rated Clinical Global Impression-Improvement (CGI-I) responder rates in gabapentin enacarbil groups than in placebo groups. Improvements in other sleep outcomes (assessed using various scales) also generally favoured gabapentin enacarbil treatment. These data are supported by results from a polysomnography, crossover (two 4-week treatment periods) trial (n > 100 evaluable). Improvements in RLS symptoms with gabapentin enacarbil were maintained in a 52-week extension study of clinical trials, including PIVOT RLS I and II. The longer-term efficacy of gabapentin enacarbil in patients with moderate to severe RLS was also demonstrated in the 36-week PIVOT RLS Maintenance study and a 52-week noncomparative study conducted in Japan. Gabapentin enacarbil was generally well tolerated in adult patients with RLS participating in short- and longer-term clinical trials. The most common treatment-emergent adverse events were somnolence/sedation and dizziness. Most adverse events were of mild to moderate severity, with relatively few patients discontinuing treatment because of an adverse event.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Featured indication | |
Treatment of moderate to severe primary restless legs syndrome (RLS) in adults Mechanism of action | |
Prodrug of gabapentin; unknown mechanism of action in RLS and postherpetic neuralgia; it is structurally related to GABA but has no effect on GABA binding, uptake or degradation; no affinity for a number of other common receptors, ion channels or transporter proteins Dosage and administration in RLS | |
Dose | 600 mg |
Route of administration | Oral |
Frequency of administration | Once daily taken with food at ≈1700 h |
Steady-state pharmacokinetic profile of gabapentin in patients with RLS receiving gabapentin enacarbil 600 mg once daily for 12 weeks (mean values) | |
Maximum plasma concentration (Cmax) | 4.2 μg/mL |
Time to Cmax | 7.0 h |
Area under plasma concentration-time curve from time 0 to 24 h | 51.4 μg · h/mL |
Apparent elimination half-life | 6.3 h |
Most common adverse events in patients with RLS (incidence ≥5 % and ≥2 times the rate of placebo in 12-week clinical trials) | |
Somnolence/sedation, dizziness | |
1 Introduction
Restless legs syndrome (RLS) is a sleep-related, movement disorder that is characterized by an irresistible urge to move, which usually involves the legs but may also involve other parts of the body [1, 2]. The four key criteria defining RLS are an urge to move limbs with or without sensations, a worsening of symptoms at rest, an improvement in symptoms with activity and a worsening of symptoms in the evening or night (i.e. a strong circadian rhythm) [1–3]. Most patients have primary (idiopathic) RLS, with symptomatic or secondary RLS potentially caused by various factors including iron deficiency, Parkinson’s disease, pregnancy and some medications (e.g. antidepressants, antihistamines) [2]. RLS is twice as common in women as in men and, although it may affect individuals of any age (including children; affects ≈2 % of paediatric population), it is more common in older adults than younger adults [1, 4]. It occurs with a lower prevalence in African, Middle Eastern, Asian, Hispanic or south eastern European populations than in Northern European and North American populations [1]. Although reported prevalence rates vary and its exact prevalence is controversial, in Western countries estimated prevalence rates range from 4 % to 15 % [1–4] and, in the USA, it is estimated that approximately 2–3 % of adults are affected by moderate to severe RLS [5].
Patients with mild symptoms do not require treatment; several treatment options are available for patients with more severe symptoms [2]. One such option is oral gabapentin enacarbil (Horizant® [USA]; Regnite® [Japan]), which is an extended-release formulation of gabapentin enacarbil. Gabapentin enacarbil, a prodrug of gabapentin with novel absorption via high-capacity nutrient transporters, was designed to provide dose-proportional and sustained exposure to gabapentin. This article reviews the pharmacological properties of oral gabapentin enacarbil and its clinical profile in adult patients with RLS. Discussion of its use in adult patients with postherpetic neuralgia is beyond the scope of this review.
2 Data selection
Sources:
Medical literature (including published and unpublished data) on ‘gabapentin enacarbil’ was identified by searching databases (including MEDLINE and EMBASE) for articles published since 1996, bibliographies from published literature, clinical trial registries/databases and websites (including those of regional regulatory agencies and the manufacturer). Additional information (including contributory unpublished data) was also requested from the company developing the drug.
Search strategy:
MEDLINE and EMBASE search terms were ‘gabapentin enacarbil’ and ‘restless legs syndrome’. Searches were last updated 2 November 2012.
Selection:
Studies in patients with primary restless legs syndrome who received gabapentin enacarbil. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms:
Gabapentin enacarbil, restless legs syndrome, therapeutic use.
3 Pharmacodynamic Profile
Gabapentin enacarbil is an actively transported prodrug of gabapentin (see also Sect. 3) [6], with its therapeutic effects attributable to gabapentin [7]. The precise mechanism(s) of action of gabapentin in RLS and postherpetic neuralgia is unknown [7]. Although it is a structural analogue of GABA, it has no effect on GABA binding, uptake or degradation. Based on radioligand binding assays, gabapentin enacarbil and gabapentin have no affinity for a number of other common receptors, ion channels or transporter proteins [7].
Gabapentin has been shown to bind with high affinity to the α2δ subunit of voltage-activated calcium channels in in vitro studies, although the relationship of this binding to the therapeutic effects of gabapentin is unknown [7].
Based on a population pharmacodynamic/pharmacokinetic model that used data from 12 clinical trials, a dose response/exposure relationship was shown for investigator-rated Clinical Global Impression-Improvement (CGI-I) scores, but not for the mean change in International Restless Legs Scale (IRLS) total scores, with gabapentin enacarbil dosages of 600–2,400 mg/day [8].
In 54 healthy adult volunteers, single oral doses of gabapentin enacarbil 1,200 or 6,000 mg were not associated with any clinically relevant QT interval prolongation in a double-blind, placebo- and active comparator-controlled, ‘Thorough’ QT/corrected QT (QTc) study [9]. These data are supported by another study in 32 healthy adult volunteers, in which no clinically relevant changes in QTc interval occurred after single doses of gabapentin enacarbil 2,400–6,000 mg (given as multiple 600 mg tablets) [10].
4 Pharmacokinetic Profile
The pharmacokinetics of oral gabapentin enacarbil have been evaluated in healthy volunteers, patients with RLS, patients with postherpetic neuralgia (not specifically discussed) and patients with varying degrees of renal impairment; where available, discussion focuses on data in patients with RLS. Additional data are derived from in vitro [6] and animal [11] studies and from the manufacturer’s prescribing information [7].
The steady-state pharmacokinetic properties of gabapentin in patients with RLS after 12 weeks of gabapentin enacarbil (at dosages used in clinical trials discussed in Sect. 4) are summarized in Table 1 [12]. Steady-state plasma concentrations of gabapentin are attained after 2 days with once-daily gabapentin enacarbil [7].
Gabapentin enacarbil is associated with approximately dose-proportional exposure to gabapentin over an extended period across a dose range of 300–6,000 mg (single or multiple doses) in healthy volunteers [10, 13] and across a dosage range of 600–2,400 mg/day in patients with RLS [12]. Gabapentin enacarbil (prodrug formulation of gabapentin) and the immediate-release formulation of gabapentin are not interchangeable because the same daily dose of each results in different plasma concentrations of gabapentin [7].
Gabapentin enacarbil is rapidly absorbed throughout the intestine via two high-capacity nutrient transporters (sodium-dependent multivitamin transporters and monocarboxylate transporter type-1 [MCT-1]) and subsequently undergoes extensive first-pass hydrolysis by nonspecific carboxylesterases (primarily in enterocytes and to a lesser extent in the liver) to gabapentin, carbon dioxide, acetaldehyde and isobutyric acid [6, 7, 11].
Concentrations of gabapentin enacarbil in the blood are low (≤2 % of corresponding plasma concentrations of gabapentin) and transient [7].
The mean bioavailability of the drug was estimated to be approximately 75 % in the fed state and 42–65 % in the fasted state, as assessed by recovery of gabapentin in the urine [7]. Gabapentin shows minimal binding to plasma proteins (<3 %). The apparent volume of distribution of gabapentin is 76 L [7].
In healthy adult volunteers, exposure to gabapentin after a single oral dose of gabapentin enacarbil 1,200 mg/day was increased in the fed versus the fasted state, with values for the area under the plasma concentration-time curve from time 0 to infinity increasing relative to the fed state by 23 %, 31 % and 40 %, respectively, after a low-, medium- or high-fat meal (i.e. ≈6 %, 30 % and 50 % of kilocalories derived from fat) [14]. There was no statistically significant delay in the median time to attain maximum plasma concentrations between the fed and fasted state [14].
After conversion from the prodrug gabapentin enacarbil, gabapentin is primarily eliminated renally as unchanged drug [15]. In healthy adult volunteers, 94.1 % of radioactivity was recovered in the urine and 5.2 % in the faeces after a single radiolabelled 600 mg dose of gabapentin enacarbil, with 85.9 % of the dose recovered in the urine within the first 24 h [15]. Renal excretion of gabapentin is thought to involve a component of active secretion via an organic cation transporter (OCT2) [7].
After a low-, medium- or high-fat meal, respective apparent oral clearance (CL/F) values of gabapentin after a single dose of gabapentin enacarbil 1,200 mg were 7.7, 8.1 and 8.9 L/h in healthy adult volunteers [14]. The apparent elimination half-life (t½β) in patients with RLS receiving gabapentin enacarbil 600 or 1,200 mg/day for 12 weeks was 6.3 and 6.6 h (Table 1) [12]. There was no effect of food or dose [14], or whether single or multiple doses were administered [7], on t½β values.
In the population pharmacokinetic study, most patients (94 %) receiving gabapentin enacarbil were of Caucasian ethnicity; thus, the effect of race on the pharmacokinetics of gabapentin could not be studied [7]. There are no clinically relevant effects on the pharmacokinetics of gabapentin enacarbil based on gender or age (≥65 vs. 18 to <65 years). However, the pharmacokinetics of the drug may be affected by age-related decline in renal function [7].
The renal clearance (CLR) of gabapentin following administration of gabapentin enacarbil is proportional to creatinine clearance (CLCR) [16]. There was a decrease in mean CL/F values in patients with moderate (i.e. CLCR 30–59 mL/min) and severe (CLCR <30 mL/min) renal impairment compared with individuals without renal impairment (4.2 and 1.7 vs. 6–9.3 L/h) [7]. Corresponding CLR values in these three groups were 3, 1 and 5–7 L/h. Dosage adjustments of gabapentin enacarbil are required in patients with renal impairment (Sect. 6). Gabapentin is removed from the plasma by haemodialysis and thus, the drug is not recommended for patients with RLS on haemodialysis [7].
In in vitro studies, gabapentin enacarbil and gabapentin were not substrates for, or inhibitors or inducers of, cytochrome P450 (CYP) isoenzymes CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2D6, CYP2E1 and CYP3A4 [7]. Gabapentin enacarbil was also not a substrate for or inhibitor of P-glycoprotein in vitro [7].
There were no clinically relevant effects on the pharmacokinetics of gabapentin enacarbil when it was coadministered with naproxen (absorption includes active transport via MCT-1) or cimetidine (eliminated via OCT2 pathway) and vice versa [17].
5 Therapeutic Efficacy
5.1 In Short-Term Trials
The efficacy of oral gabapentin enacarbil in patients with primary moderate to severe RLS was investigated in large (n > 100), randomized, double-blind, placebo-controlled, multicentre, phase III trials [18–20], including the key 12-week Patient Improvements in Vital Outcomes following Treatment (PIVOT) RLS I [18] and II [19] trials. In both the PIVOT RLS I and the PIVOT RLS II trials, approximately 40 % of patients were male, the mean age of patients was approximately 50 years, the mean duration of RLS was approximately 14–15 years and approximately two-thirds of patients had received no prior treatment for RLS [18, 19]. Pooled analyses of these clinical trials are also briefly discussed [21, 22]. In the other study (hereafter termed the polysomnography study) [20], which had a crossover design, patients had a mean age of 52 years, 58 % of patients were female and 42 % were treatment naive; the mean duration of RLS was not specified. Within each trial, there were no significant differences between groups in terms of baseline characteristics. These studies are supported by a 12-week, dose-ranging (600–2,400 mg/day), double-blind, placebo-controlled, multicentre study (n = 217; 38–48/group), which is not discussed further [12].
Unless stated otherwise, efficacy analyses were conducted in the modified intent-to-treat (mITT) population using the last observation carried forward (LOCF) for imputing missing data. See Table 2 for further design details of these phase III trials. All study drugs were taken orally at about 1700 h with food.
5.1.1 PIVOT RLS I and II Trials
After 12 weeks of treatment, reductions from baseline in mean IRLS total scores were significantly greater with gabapentin enacarbil 1,200 mg once daily (co-primary endpoint) than with placebo in PIVOT RLS I and II, with significant improvements relative to placebo also observed with gabapentin enacarbil 600 mg once daily in PIVOT RLS II (secondary endpoint) [Table 3] [18, 19].
Using an analysis of covariance adjusted for baseline values [18, 19], pooled site [18, 19], treatment [19] and/or treatment by pooled-site interaction [19], adjusted mean treatment differences (AMTD) for mean changes in IRLS total scores between gabapentin enacarbil 1,200 mg/day and placebo were −4 (95 % CI −6.2, −1.9; p = 0.0003) in PIVOT RLS I [18] and −3.5 (95 % CI −5.6, −1.3; p = 0.0015) in PIVOT RLS II [19]; where evaluated, the AMTD for this endpoint with gabapentin enacarbil 600 mg/day was −4.3 (95 % CI −6.4, −2.3; p < 0.0001) [19]. Moreover, improvements in mean IRLS total scores favouring treatment with gabapentin enacarbil 1,200 mg/day and, where evaluated gabapentin enacarbil 600 mg/day [19], were evident from week 1 onwards (earliest on-treatment assessment timepoint) [all p < 0.01 vs. placebo] [18].
At 12 weeks, CGI-I responder rates were significantly higher in the gabapentin enacarbil 1,200 mg/day (co-primary endpoint) [18, 19] or, in one study [19], 600 mg/day groups than in placebo groups (Table 3). The responder rate adjusted odds ratio (OR) for the gabapentin enacarbil 1,200 mg/day group versus the placebo group in PIVOT RLS I was 5.1 (95 % CI 2.8, 9.2; p < 0.0001) [18] and, in PIVOT RLS II [19], it was 4.3 (95 % CI 2.34, 7.86; p < 0.0001). At all evaluated timepoints from week 1 through to week 12 in both trials, significantly (p < 0.01) higher proportions of gabapentin enacarbil recipients than placebo recipients were rated by investigators as responders [18, 19].
A retrospective, integrated analysis of the mITT populations in these two trials (available as an abstract presentation) indicated that gabapentin enacarbil 1,200 mg/day significantly (p < 0.01) improved mean IRLS total scores relative to placebo at 12 weeks, irrespective of whether patients had severe/very severe sleep disturbance (n = 187 evaluable; AMTD −3.9) or moderate to no sleep disturbance (n = 240; AMTD −3.3) at baseline (severity defined according to item 4 of the IRLS) [21]. Respective CGI-I responder rates in these two subgroups of patients were also significantly (p < 0.0001) higher than placebo at 12 weeks, with adjusted ORs of 3.7 and 5.4.
In a post hoc analysis in the PIVOT RLS I trial, the numbers needed-to-treat to gain one response benefit were 6 (95 % CI 3.1, 14.3), 3 (95 % CI 2.0, 4.0) and 4 (95 % CI 2.4, 6.5), respectively, for IRLS total scores, investigator-rated responder rates and when both primary endpoints were considered together [18].
Secondary sleep endpoints also generally favoured gabapentin enacarbil treatment over placebo at study end, including the mean change from baseline in the average wake time after sleep onset (WASO) [Table 3], improvements in all Medical Outcomes Study (MOS)-sleep scale domain scores and all Post-Sleep Questionnaire (PSQ) sleep outcomes [18, 19]. The latter scale is a new assessment tool for evaluating sleep dysfunction, with the validity of the scale demonstrated using pooled data from the PIVOT RLS I and II trials; significant (p ≤ 0.007) correlations were observed between baseline PSQ item scores and baseline total scores for IRLS, Profile Mood States, RLS-Quality of Life and MOS-sleep scales [23].
At 12 weeks, there were no between-group differences for changes in mean daily total sleep time (TST) [Table 3] [18, 19]. However, the mean daily TST was significantly (p < 0.05) increased with gabapentin enacarbil 1,200 mg/day compared with placebo at weeks 2 and 4 in both trials [18, 19]. There were no significant between-group differences at any timepoint for changes in mean TST with gabapentin enacarbil 600 mg/day compared with placebo in PIVOT RLS II [19].
At 12 weeks, gabapentin enacarbil 600 or 1,200 mg/day increased the estimated time to median onset of RLS symptoms approximately 2-fold (using Kaplan-Meier methods and 24-h patient diaries) and the percentage of patients who were RLS symptom free [18, 19]. For example, in PIVOT RLS II, the median time to onset of RLS symptoms increased from 5.5 h (total population; 95 % CI 4.0, 7.5) to 13.5 h (95 % CI 12.5, 16.5), 13.8 h (95 % CI 11.5, 17.0) and 12.8 h (95 % CI 9.5, 15.0) in the gabapentin enacarbil 1,200 mg/day, gabapentin enacarbil 600 mg/day and placebo groups, respectively [19]. At 12 weeks, 35.3 %, 37 % and 23 % of patients in each of these groups were free from symptoms at the end of a 24-h assessment period compared with 2 % of patients at baseline [19].
At study end, there were significant (p < 0.01) reductions from baseline in mean pain scores with gabapentin enacarbil 600 or 1,200 mg/day versus placebo (assessed using the 11-point RLS pain scale; 0 = no pain and 10 = worst pain) [18, 24]. For example, in the PIVOT RLS II trial, gabapentin enacarbil 600 or 1,200 mg/day significantly (p ≤ 0.02) reduced mean RLS pain scores compared with placebo in patients with a baseline pain score of ≥ 4 and in those with a baseline score of >0 (abstract presentation) [24].
An integrated post hoc analysis [22] of three 12-week, randomized trials [12, 18, 19] supported the efficacy of gabapentin enacarbil 600 mg/day and showed similar beneficial effects relative to placebo to those observed with higher dosages of gabapentin enacarbil. Relative to placebo (n = 244), the mean change from baseline to week 12 in IRLS total score was significantly greater with gabapentin enacarbil 600 mg/day (n = 161; AMTD −4.3; 95 % CI −6.01, −2.52; p < 0.0001), 1,200 mg/day (n = 266; AMTD −3.9; 95 % CI −5.30, −2.46; p < 0.0001), 1,800 mg/day (n = 37; AMTD −4.4; 95 % CI −7.51, −1.25; p < 0.01) and 2,400 mg/day (n = 44; AMTD −3.2; 95 % CI −6.15, −0.26; p < 0.05) [22]. Investigator-rated CGI-I responder rates were also significantly (p ≤ 0.001) higher in the gabapentin enacarbil 600 mg (70.2 %), 1,200 mg (75.3 %), 1,800 mg (73.0 %) and 2,400 mg (81.8 %) groups than in the placebo group (42.2 %).
5.1.2 Polysomnography Trial
The phase III, polysomnography study consisted of two 4-week, crossover treatment periods (gabapentin enacarbil 1,200 once daily or placebo), with a 2-week period between each treatment period (1-week taper period during which patients received gabapentin enacarbil 600 mg once daily or placebo and then a 1-week wash-out period) [20]. Patients received gabapentin enacarbil or placebo for the first treatment period and then switched study treatment for the second treatment period (n = 131 patients in the mITT population). See Table 2 for further design details.
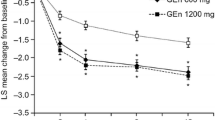
Gabapentin enacarbil 1,200 mg/day significantly improved sleep outcomes compared with placebo after 4 weeks of treatment, including wake time during sleep (primary endpoint) [Fig. 1], periodic limb movement associated with arousal per h of sleep (AMTD −3.07; 95 % CI −5.04, −1.10; p = 0.002; baseline 13.2) [key secondary endpoint], stage N3 sleep time (Fig. 1), the number of awakenings (AMTD −2.49; 95 % CI −3.33, −1.65; p < 0.0001; baseline 11.47) and periodic limb movement associated with awakening per h of sleep (AMTD −0.14; 95 % CI −0.21, −0.06; p < 0.001; baseline 0.51). Other polysomnography-assessed sleep outcomes also showed marked improvements from baseline after 4 weeks of gabapentin enacarbil treatment (Fig. 1), as did Periodic Limb Movement Index scores (AMTD −8.05; 95 % CI −12.38, −3.72; baseline 46.7; no adjustments made for multiple comparisons) [20].
Efficacy of oral gabapentin enacarbil in adult patients with moderate to severe primary restless legs syndrome. Sleep outcomes after 4 weeks of treatment (i.e. at weeks 4 or 10) in a double-blind, crossover, multicentre trial (n = 121–127 evaluable) [20]. The primary endpoint was the mean change in WTDS from baseline. Baseline values (n = 131) for WTDS, stage N3 sleep time, WASO, TST, SOL and LPS were 82.3, 44.5, 89.7, 373.0, 17.7 and 28.3 min, respectively. The numbers above/below the bars are the adjusted mean treatment difference (95 % CI); no adjustments for multiple comparisons were made for WASO, TST, SOL and LPS. GEn gabapentin enacarbil, LPS latency to persistent sleep, SOL sleep-onset latency, TST total sleep time, WASO wake time after sleep onset, WTDS wake time during sleep, *p < 0.0001 vs. placebo
Subjective patient-assessed measures of sleep quality also showed significantly (p < 0.0001) greater improvements with gabapentin enacarbil than with placebo after 4 weeks of treatment, including the percentage of responders based on Patient Global Impression of Therapy and Subjective Post-Sleep Diary scores for the domains of sleep quality and for rested upon awakening [20].
Adjusted mean improvements in IRLS total scores (AMTD −6.57; 95 % CI −8.58, −4.57; p < 0.0001) and CGI-I responder rates (74 % vs. 36 %; no adjustments made for multiple comparisons) were also greater with gabapentin enacarbil than with placebo after 4 weeks’ treatment (secondary endpoints) [20]. The adjusted OR for CGI-I responder rates was 5.8 (95 % CI 3.07, 10.77) at this timepoint.
5.2 In Longer-Term Studies
The longer-term efficacy of gabapentin enacarbil in adult patients with RLS was evaluated in the 36-week PIVOT RLS Maintenance study [25], a 52-week extension study [26] that enrolled participants who completed one of four [12, 18, 19] short-term trials (one of which [XP083] is not currently published) and a 52-week, noncomparative, multicentre, Japanese study [27]. Participants typically received gabapentin enacarbil 1,200 mg once daily at 1700 h with food [25–27]. In the 52-week extension study [26], dosage increases to 1,800 mg/day and decreases to 600 mg/day were permitted based on efficacy and tolerability and, in the Japanese study [27], dosage adjustments of 900–1,500 mg/day were permitted on the same basis.
The multicentre, PIVOT RLS Maintenance study consisted of a 24-week, single-blind, active-treatment phase (gabapentin enacarbil 1,200 mg/day; 221 patients completed this phase) followed by a 12-week, randomized, double-blind phase during which patients received the same dosage of gabapentin enacarbil (n = 96 evaluable) or placebo (n = 97 evaluable) [25]. Patients randomized to placebo in the double-blind phase underwent a taper period of 2 weeks (week 24–26; gabapentin enacarbil 600 mg plus placebo); thereafter patients received placebo. Inclusion and exclusion criteria were generally similar to those for the PIVOT RLS I and II studies. The primary endpoint was the proportion of patients who relapsed during the double-blind phase (i.e. had a worsening of RLS symptoms) or withdrew because of a lack of efficacy. A worsening of RLS symptoms was defined as an increase of ≥6 points in IRLS total score from the double-blind baseline value to a total score of ≥15 and a rating of ‘much worse’ or ‘very much worse’ on the investigator-rated CGI-Change (CGI-C) scale on two consecutive visits at least 1 week apart. The mITT population consisted of all patients who received ≥1 dose of study drug during the double-blind period and had at least one post-randomization IRLS and CGI-C assessment [25].
Relapse rates at the end of the double-blind phase (i.e. week 36) were significantly lower in the gabapentin enacarbil group than in the placebo group (9 % vs. 23 %; OR 0.35; 95 % CI 0.2, 0.8; p = 0.02) [primary endpoint] [25]. In addition, the time to relapse was significantly (p < 0.05) longer in the gabapentin enacarbil group (assessed using Kaplan-Meier methods), regardless of whether or not the taper period was included. The median time to relapse could not be estimated in either treatment group as <50 % of patients experienced a relapse. At 36 weeks, the change in mean IRLS total score was significantly lower in the gabapentin enacarbil group than in the placebo group (mean change in IRLS total score 1.9 vs. 3.9; AMTD −2.1; p = 0.03; baseline values at double-blind randomization 5.1 and 5.3) [25].
In a 52-week extension study of short-term clinical trials, improvements in mean IRLS total scores and CGI-I responder rates were maintained in gabapentin enacarbil-naive (n = 197) and -experienced patients (n = 376), based on the intent-to-treat population using the LOCF to impute missing data [26]. The mean change in IRLS total score from parent study baseline (23.2) to 52 weeks was −15.2, with 84.8 % of patients rated by investigators as CGI-I responders.
In the Japanese study, mean IRLS total scores were significantly (p < 0.001) reduced from baseline at all timepoints evaluated throughout the 1-year study (n = 181 in the full analysis set; i.e. all patients who received one dose of gabapentin enacarbil and had a post-baseline efficacy assessment) [27]. At week 52, the mean IRLS total score was reduced from a baseline of 24.4 to 6.3 (p < 0.001), with an IRLS responder rate of 80.3 % (IRLS responders were defined as patients who achieved an IRLS total score of ≤10 points). Investigator- and patient-rated CGI-I responder rates were both 87.1 %. At study end, most patients (83.4 %) were receiving gabapentin enacarbil 1,200 mg/day [27].
6 Tolerability
Oral gabapentin enacarbil was generally well tolerated in adult patients with moderate to severe RLS participating in short- and longer-term clinical trials (up to 52 weeks’ treatment) discussed in Sect. 4. Discussion focuses on a pooled analysis of three 12-week, placebo-controlled trials in patients with RLS, as reported in the US prescribing information [7]. In the overall clinical development programme for RLS, exposure to gabapentin enacarbil in 1,201 patients with RLS (aged 18–82 years) participating in clinical trials (primarily in placebo-controlled trials [n = 642] and long-term follow-up studies) included 613 patients exposed for at least 6 months and 371 exposed for at least 1 year.
Eleven patients (7 %) receiving the recommended dosage (i.e. 600 mg/day; n = 163 evaluable) of gabapentin enacarbil discontinued treatment because of an adverse event compared with 4 % of 245 patients receiving placebo [7].
The most common (i.e. occurring with an incidence of ≥5 % and at least 2 times the rate of placebo) treatment-emergent adverse events occurring in 12-week trials in patients with RLS were somnolence/sedation and dizziness (Fig. 2) [7]; these data are supported by another pooled analysis of short-term clinical trials [22]. Most treatment-emergent adverse were of mild to moderate severity.
Tolerability profile of oral gabapentin enacarbil in adult patients with restless legs syndrome. Pooled analysis of treatment-emergent adverse events occurring in ≥5 % of patients in any treatment group in three 12-week, placebo-controlled trials, as reported in the manufacturer’s prescribing information [7]. GEn gabapentin enacarbil
The most common (i.e. occurring with an incidence of ≥5 % and at least 2 times the rate of placebo) treatment-emergent adverse events occurring in 12-week trials in patients with RLS were somnolence/sedation and dizziness (Fig. 2) [7]; these data are supported by another pooled analysis of short-term clinical trials [22]. Most treatment-emergent adverse were of mild to moderate severity.
In patients receiving gabapentin enacarbil 600 mg/day during controlled clinical trials who reported somnolence/sedation (Fig. 2), somnolence persisted in approximately 30 % of patients, with symptoms resolving within 3–4 weeks in the remaining patients [7]. In patients who reported dizziness (Fig. 2), it persisted in approximately 20 % of patients receiving gabapentin enacarbil 600 mg/day. Somnolence/sedation and dizziness led to treatment withdrawal in 2 % and 1 % of patients receiving gabapentin enacarbil 600 mg/day. These adverse events occurred with a numerically higher incidence in the 1,200 mg/day group (Fig. 2) [7].
Other treatment-emergent adverse events that were considered dose related were headache (Fig. 2), feeling drunk (1 % in gabapentin enacarbil 600 mg/day group vs. 3 % in 1,200 mg/day group), libido decreased (< 1 % vs. 2 %), depression (< 1 % vs. 3 %), peripheral oedema (<1 % vs. 3 %) and vertigo (1 % vs. 3 %) [7].
As with all anti-epileptic drugs (AEDs), gabapentin potentially increases the risk of suicidal thoughts or behaviour [7]. Patients treated with AEDs for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behaviour and/or any unusual changes in mood or behaviour [7].
In a pooled analysis of four placebo-controlled clinical trials in patients with RLS, serious adverse events occurred in five patients; none of these were considered treatment related and all resolved with or without sequelae [22]. Two of these five events occurred in gabapentin enacarbil 600 mg/day recipients (one patient had cellulitis and the other had an intervertebral disc protrusion), one in a recipient of gabapentin enacarbil 2,400 mg/day (rotator cuff syndrome) and two in placebo recipients (one patient had cholelithiasis and the other appendicitis).
The nature of adverse events in longer-term studies appeared to be similar to that reported in short-term studies [25–27], For example, in the 52-week extension study [26], 38.8 % of gabapentin enacarbil-experienced patients (n = 376 evaluable) and 22.8 % of gabapentin enacarbil-naive patients (n = 197) had ongoing adverse events at study entry, with 80.1 % of patients experiencing at least one adverse event during treatment with gabapentin enacarbil. Overall, 11.2 % of patients withdrew from the study because of a treatment-emergent adverse event, with 1.4 % of patients discontinuing treatment because of an adverse event that started in the parent study [26]. Where reported [27], no incidence of investigator-reported augmentation of RLS symptoms occurred.
In this extension study, most adverse events were of mild to moderate intensity, with the most common being somnolence (19.7 % of 573 patients) and dizziness (11.5 %) [26]. Somnolence (27.4 % vs. 15.7 %) and dizziness (19.8 % vs. 7.2 %) occurred with a numerically higher incidence in gabapentin enacarbil-naive patients than in gabapentin enacarbil-experienced patients and were more common during the first 4 weeks of treatment. None of the adverse events reported during the taper or follow-up period were considered indicative of potential withdrawal syndrome [26].
Cases of Drug Reaction with Eosinophilia and Systemic Symptoms (also termed multi-organ hypersensitivity), including fatal or life-threatening occurrences, have been reported in patients receiving AEDs, including gabapentin [7]. If early manifestations of hypersensitivity, such as fever or lymphadenopathy with or without rash, are present, the patient should be evaluated immediately and gabapentin enacarbil treatment discontinued if an alternative aetiology for signs and symptoms cannot be established.
Oral gabapentin and gabapentin enacarbil increased the incidence of pancreatic acinar cell adenoma and carcinoma in rats, although the clinical significance of this finding is unknown [7]. In clinical trials in patients with epilepsy (2,085 patient-years of exposure to gabapentin in patients aged >12 years), 10 patients reported new tumours and pre-existing tumours worsened in 11 patients during or up to 2 years following adjunctive gabapentin therapy, although it is not possible to establish whether the incidence reported in this cohort is or is not affected by treatment [7]. Epidemiological data derived from two electronic databases of medical records (a US database [12 years’ follow-up] and a UK database [15 years’ follow-up]) do not indicate a carcinogenic effect with gabapentin use, although the wide variability in some confidence intervals means that a carcinogenic effect cannot be confidently excluded [28].
7 Dosage and Administration
In the USA, oral gabapentin enacarbil is approved in adults for the treatment of moderate to severe primary RLS (featured indication) and the management of postherpetic neuralgia [7]. It is also approved in Japan for the treatment of adult patients with moderate to severe primary RLS [29].
In the USA, the recommended dosage of gabapentin enacarbil is 600 mg once daily taken with food at about 1700 h [7]. If the dose is not taken at the recommended time, the next dose should be taken the following day as prescribed. A daily dose of 1,200 mg provided no additional benefit compared with the 600 mg dose (Sect. 4), but caused an increase in adverse events (Sect. 5). The dosage of gabapentin enacarbil should be adjusted in accordance with renal function, as assessed by CLCR (Table 4). When discontinuing gabapentin enacarbil, patients receiving 600 mg/day or less may discontinue treatment without tapering. If the recommended dosage is exceeded, the dosage should be reduced to 600 mg/day for 1 week prior to discontinuation to minimize the potential of withdrawal seizure. Gabapentin enacarbil is not interchangeable with other formulations of gabapentin because of differing pharmacokinetic profiles. There are no contraindications to the use of gabapentin enacarbil [7].
Local prescribing information should be consulted for detailed information, including other precautions, use in special patient populations and potential drug interactions.
8 Gabapentin Enacarbil: Current Status in Restless Legs Syndrome
Oral gabapentin enacarbil is approved in adult patients for the treatment of moderate to severe primary RLS (focus of this review) and the management of postherpetic neuralgia.
The 2012 American Academy of Sleep Medicine Clinical Practice Guidelines recommend pramipexole and ropinirole as standard therapy for the treatment of RLS; drugs that were given a ‘guideline’ level of recommendation for treating RLS include gabapentin enacarbil, opioids and levodopa with a dopa decarboxylase inhibitor [30]. The International RLS Study Group (IRLSSG) Task Force recommend a dopamine-receptor agonist (e.g. cabergoline) or α2δ ligand (e.g. gabapentin enacarbil) as first-line therapy, with the choice of therapy based on individual clinical features [31]. The European Federation of Neurological Societies guidelines give a Level A recommendation to ropinirole, cabergoline, levodopa and gabapentin enacarbil for the treatment of primary RLS [32].
Long-term treatment with levodopa or a dopamine-receptor agonist (e.g. ropinirole or pramipexole), but not with an α2δ ligand such as gabapentin enacarbil, may be associated with augmentation of RLS, including severe exacerbation of RLS symptoms, with augmentation being one of the main causes of treatment failure in this patient population [31]. The risk of augmentation of RLS increases with increasing treatment duration and with higher doses of drugs. The IRLSSG Task Force recommend that in patients with severe or progressive augmentation of RLS symptoms, dopamine-receptor agonist treatment is discontinued and patients are switched to an α2δ ligand, an opioid, or possibly another dopamine-receptor agonist (e.g. switching from a shorter-acting dopamine-receptor agonist such as ropinirole to a longer-acting agonist such as cabergoline, as the latter agents may be associated with a lower incidence of augmentation) [31].
In the 12-week PIVOT RLS I and II trials in adult patients with moderate to severe primary RLS (n > 500 evaluable), once-daily gabapentin enacarbil 600 or 1,200 mg significantly improved mean IRLS total scores compared with placebo, with significantly higher investigator-rated CGI-I responder rates in gabapentin enacarbil groups than in placebo groups. Improvements in other sleep outcomes (assessed using various scales) also generally favoured gabapentin enacarbil treatment in these trials. These data are supported by results from a polysomnography, crossover (two 4-week treatment periods) trial (n > 100 evaluable). Improvements in RLS symptoms with gabapentin enacarbil treatment were maintained in a 52-week extension study in patients enrolled in clinical trials, including participants in the PIVOT RLS trials. The longer-term efficacy of gabapentin enacarbil in patients with moderate to severe RLS was also demonstrated in the 36-week PIVOT RLS Maintenance study and a 52-week noncomparative study conducted in Japan.
Gabapentin enacarbil was generally well tolerated in adult patients with moderate to severe RLS participating in short- and longer-term clinical trials, with somnolence/sedation and dizziness being the most common treatment-emergent adverse events. Most treatment-emergent adverse events were of mild to moderate severity and relatively few patients discontinued treatment because of an adverse event.
References
Leschziner G, Gringras P. Restless legs syndrome. BMJ. 2012;344:e3056.
Yaltho TC, Ondo WG. The use of gabapentin enacarbil in the treatment of restless legs syndrome. Ther Adv Neurol Disord. 2010;3(5):269–75.
Satija P, Ondo WG. Restless legs syndrome: pathophysiology, diagnosis and treatment. CNS Drugs. 2008;22(6):497–518.
Sivam S, Yee BJ. Role of gabapentin enacarbil XR in restless legs syndrome. Ther Clin Risk Manag. 2012;8:201–8.
National Institute of Neurological Disorders and Stroke. Restless legs syndrome fact sheet [online]. Available from URL: http://www.ninds.nih.gov/disorders/restless_legs/detail_restless_legs.htm. Accessed 15 Oct 2012.
Cundy KC, Branch R, Chernov-Rogan T, et al. XP13512 [(±)-1-([(alpha-isobutanoyloxyethoxy)carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: I. Design, synthesis, enzymatic conversion and transport by intestinal solute transporters. J Pharmacol Exp Ther. 2004;311(1):315–23.
GlaxoSmithKline, XenoPort Inc. Horizant (gabapentin enacarbil) extended-release tablets for oral use: US prescribing information [online]. Available from URL: http://us.gsk.com/products/assets/us_horizant.pdf. Accessed 23 Aug 2012.
Lal R, Sukbuntherng J, Luo W, et al. Population pharmacokinetics and pharmacodynamics of gabapentin after administration of gabapentin enacarbil. J Clin Pharmacol. 2012. doi:10.1177/0091270012439209.
Chen D, Lal R, Zomorodi K, et al. Evaluation of gabapentin enacarbil on cardiac repolarization: a randomized, double-blind, placebo- and active-controlled, crossover thorough QT/QTc study in healthy adults. Clin Ther. 2012;34(351–62):e3.
Lal R, Sukbuntherng J, Luo W, et al. Pharmacokinetics and tolerability of single escalating doses of gabapentin enacarbil: a randomized-sequence, double-blind, placebo-controlled crossover study in healthy volunteers. Clin Ther. 2009;31(8):1776–86.
Cundy KC, Annamalai T, Bu L, et al. XP13512 [(±)-1-([(alpha-isobutanoyloxyethoxy)carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: II. Improved oral bioavailability, dose proportionality, and colonic absorption compared with gabapentin in rats and monkeys. J Pharmacol Exp Ther. 2004;311(1):324–33.
Lal R, Ellenbogen A, Chen D, et al. A randomized, double-blind, placebo-controlled, dose-response study to assess the pharmacokinetics, efficacy, and safety of gabapentin enacarbil in subjects with restless legs syndrome. Clin Neuropharmacol. 2012;35(4):165–73.
Cundy KC, Sastry S, Luo WD, et al. Clinical pharmacokinetics of XP13512, a novel transported prodrug of gabapentin. J Clin Pharmacol. 2008;48(12):1378–88.
Lal R, Sukbuntherng J, Luo W, et al. The effect of food with varying fat content on the clinical pharmacokinetics of gabapentin after oral administration of gabapentin enacarbil. Int J Clin Pharmacol Ther. 2010;48(2):120–8.
Lal R, Sukbuntherng J, Ho J, et al. A phase I, single-dose study of the disposition of 14C-radiolabeled gabapentin enacarbil in healthy male volunteers. Int J Clin Pharmacol Ther. 2011;49(2):109–15.
Lal R, Sukbuntherng J, Luo W, et al. Clinical pharmacokinetics of gabapentin after administration of gabapentin enacarbil extended-release tablets in patients with varying degrees of renal function using data from an open-label, single-dose pharmacokinetic study. Clin Ther. 2012;34(1):201–13.
Lal R, Sukbuntherng J, Luo W, et al. Clinical pharmacokinetic drug interaction studies of gabapentin enacarbil, a novel transported prodrug of gabapentin, with naproxen and cimetidine. Br J Clin Pharmacol. 2010;69(5):498–507.
Kushida CA, Becker PM, Ellenbogen AL, et al. Randomized, double-blind, placebo-controlled study of XP13512/GSK1838262 in patients with RLS. Neurology. 2009;72(5):439–46.
Lee DO, Ziman RB, Perkins AT, et al. A randomized, double-blind, placebo-controlled study to assess the efficacy and tolerability of gabapentin enacarbil in subjects with restless legs syndrome. J Clin Sleep Med. 2011;7(3):282–92.
Winkelman JW, Bogan RK, Schmidt MH, et al. Randomized polysomnography study of gabapentin enacarbil in subjects with restless legs syndrome. Mov Disord. 2011;26(11):2065–72.
Bogan R, Ellenbogen AL, Becker PM, et al. Gabapentin enacarbil improves RLS symptoms and subjective measures of sleep in subjects with primary restless legs syndrome with and without severe sleep disturbance: secondary analyses from two studies [abstract no. P02.283]. In: 62nd Annual Meeting of the American Academy of Neurology; 2010 April 10–17; Toronto.
VanMeter SA, Kavanagh ST, Warren S, et al. Dose response of gabapentin enacarbil versus placebo in subjects with moderate-to-severe primary restless legs syndrome: an integrated analysis of three 12-week studies. CNS Drugs. 2012;26(9):773–80.
Canafax DM, Bhanegaonkar A, Bharmal M, et al. Validation of the post sleep questionnaire for assessing subjects with restless legs syndrome: results from two double-blind, multicenter, placebo-controlled clinical trials. BMC Neurol. 2011;11:48.
Lee D, Ziman R, Perkins A, et al. Gabapentin enacarbil improves pain associated with restless legs syndrome (RLS) [abstract no. 311]. J Pain. 2010;11(4 Suppl. 1):S53.
Bogan RK, Bornemann MAC, Kushida CA, et al. Long-term maintenance treatment of restless legs syndrome with gabapentin enacarbil: a randomized controlled study. [Erratum appears in Mayo Clin Proc. 2010, 85(7), pp. 693–4]. Mayo Clin Proc. 2010;85(6):512–21.
Ellenbogen AL, Thein SG, Winslow DH, et al. A 52-week study of gabapentin enacarbil in restless legs syndrome. Clin Neuropharmacol. 2011;34(1):8–16.
Inoue Y, Uchimura N, Kuroda K, et al. Long-term efficacy and safety of gabapentin enacarbil in Japanese restless legs syndrome patients. Prog Neuro Psychopharmacol Biol Psychiatry. 2012;36(2):251–7.
Irizarry MC, Webb DJ, Boudiaf N, et al. Risk of cancer in patients exposed to gabapentin in two electronic medical record systems. Pharmacoepidem Drug Safety. 2012;21:214–25.
Astellas Pharma Inc., XenoPort Inc. Astellas and XenoPort announce the launch of Regnite® tablets for restless legs syndrome in Japan [online]. Available from URL: http://www.astellas.co/en/corporate/news/pdf/120709.eg.pdf Accessed 23 Aug 2012.
Aurora RN, Kristo DA, Bista SR, et al. The treatment of restless legs syndrome and periodic limb movement disorders in adults: an update for 2012. Practice parameters with an evidence-based systematic review and meta-analyses: an American Academy of Sleep Medicine Clinical Practice Guideline. Sleep. 2012;35(8):1039–62.
International Restless Legs Syndrome Study Group Task Force. Summary of recommendations for the long-term treatment of RLS/WED from an IRLSSG task force [online]. Available from URL: http://irlssg.org/wp-content/uploads/2012/07/Summary-of-RLS-treatment-recommendations-FINAL.pdf. Accessed 15 Oct 2012.
Vignatelli L, Billiard M, Clarenbach P, et al. EFNS guidelines on management of restless legs syndrome and periodic limb movement disorder in sleep. Eur J Neurol. 2006;13(10):1049–65.
Disclosure
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the author on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Additional information
The manuscript was reviewed by: J. Chen, Lorna Linda University Movement Disorders Center, Schools of Medicine and Pharmacy, Lorna Linda, California, USA; C. Guilleminault, Stanford University Sleep Medicine Division, Redwood, California, USA; H.B. Lee, John Hopkins University School of Medicine, Department of Psychiatry and Behavioral Sciences, Baltimore, Maryland, USA.
Rights and permissions
About this article
Cite this article
Scott, L.J. Gabapentin Enacarbil. CNS Drugs 26, 1073–1083 (2012). https://doi.org/10.1007/s40263-012-0020-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-012-0020-3