Abstract
Objective
The aim was to assess gabapentin enacarbil (GEn) treatment effects on quality of life (QOL) and mood in adults with moderate-to-severe primary restless legs syndrome (RLS).
Methods
Data were pooled from three placebo-controlled, randomized, double-blind, 12-week trials for adults receiving GEn (600 mg or 1200 mg) or placebo once daily. QOL was assessed with the RLS QOL questionnaire in two studies. Mood was examined with the Profile of Mood States Brief Form (POMS-B), and as an exploratory analysis with International Restless Legs Scale (IRLS) item 9 (daily affairs) and item 10 (mood disturbance) across all three studies. Mood and QOL were secondary endpoints in the individual clinical trials. No adjustments for multiplicity were applied.
Results
The QOL analysis modified intent-to-treat (MITT) population included 541 adults (placebo, n = 204; GEn 600 mg, n = 114; GEn 1200 mg, n = 223). Both GEn doses significantly improved QOL versus placebo (week 12; p < 0.01). The mood analysis MITT population included 671 adults (placebo, n = 244; GEn 600 mg, n = 161; GEn 1200 mg, n = 266). GEn 600 mg significantly improved POMS vigor-activity versus placebo (week 12; p < 0.05); other POMS criteria were not significantly affected. GEn 1200 mg significantly improved POMS scores for total mood disturbance, depression-dejection, fatigue-inertia, vigor-activity, and confusion-bewilderment versus placebo at week 12 (p < 0.05); tension-anxiety and anger-hostility were not significantly affected. Both GEn doses significantly improved IRLS item 9 and item 10 versus placebo at week 12 (p < 0.05). The most frequent treatment-emergent adverse events with GEn were somnolence and dizziness.
Conclusions
GEn (600 mg and 1200 mg) once daily significantly improved QOL in adults with moderate-to-severe primary RLS at all time points examined. While the only POMS item significantly improved by GEn 600 mg versus placebo at week 12 was vigor-activity, GEn 1200 mg significantly improved total mood disturbance and several other POMS items versus placebo at week 12. Both QOL and mood improvements were numerically greater with GEn 1200 mg versus 600 mg.
Trial Registrations
Clinicaltrials.gov identifiers NCT00298623, NCT00365352, NCT01332305.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In this pooled analysis of adults with moderate-to-severe primary restless legs syndrome, gabapentin enacarbil (GEn) 600 mg and 1200 mg once daily significantly improved quality of life (QOL) and some mood outcomes versus placebo. |
QOL and mood improvements were numerically greater with GEn 1200 mg versus 600 mg once daily. |
The most frequent treatment-emergent adverse events were somnolence and dizziness. |
1 Introduction
Restless legs syndrome (RLS), also known as Willis-Ekbom disease, is a sleep-related neurological disorder that affects approximately 5–10 % of adults [1, 2]. The five essential criteria used for diagnosing RLS are an urge to move the legs with or without abnormal sensations, worsening of symptoms at night, temporary relief of sensations by movement, worsening of sensations during rest, and symptoms that are not solely due to another medical or behavioral condition [1, 3, 4]. RLS occurs twice as often in women as in men, and is more common in North American and Northern European populations than in African or Asian populations [2, 5–7].
Patients with RLS frequently report impairments in sleep and daytime functioning, mood disturbances, and painful dysesthesias, all of which can contribute to reduced quality of life (QOL) [2, 8–10]. Patients with RLS experience deficits in a number of QOL-related items compared with the general population, including physical functioning, pain, vitality, and social functioning [9, 10]. The reduced QOL for individuals with RLS is comparable to that of patients with serious chronic medical conditions, such as hypertension and diabetes [9, 10]. Several population-based studies have found that patients with RLS are more likely to experience depression, anxiety, and panic disorder compared with controls [8, 11, 12]. The severity of these mood disorders is significantly correlated with RLS symptom severity [8]. Additionally, while current pharmacotherapy for RLS often improves symptoms, some treatment-related side effects, such as hypotension, impulsive/compulsive disorders, augmentation, or nausea, may also negatively impact QOL [13].
Treatments for moderate-to-severe primary RLS that are currently approved by the US Food and Drug Administration (FDA) include gabapentin enacarbil (GEn), a member of the alpha-2-delta calcium-channel ligand class, as well as the dopamine agonists (DAs) ropinirole, rotigotine, and pramipexole [3]. The use of either a DA or an alpha-2-delta calcium-channel ligand is recommended as the first-line treatment for most patients with RLS [3]. DAs have been shown to effectively treat RLS symptoms, as well as RLS-related impairments in sleep, mood, and QOL [14–16]; however, they have also been associated with adverse side effects such as impulse control disorders and augmentation [17–20]. Augmentation involves a paradoxical worsening and earlier phase shift of RLS symptoms during treatment, as well as a shorter latency to symptom onset, shorter relief period after administration of medication, and geographical spread of symptoms to previously unaffected areas [21]. Several RLS treatment guidelines advise that patients who experience intolerable side effects from DAs can benefit from non-DA treatments, including alpha-2-delta ligands such as GEn [3, 22, 23]. Augmentation was not observed with GEn in three pivotal clinical trials (XP052, XP053, XP081) [24–26], although the 12-week duration of these trials may not have been sufficient to observe any cases of augmentation.
GEn is the only non-dopaminergic medication approved by the FDA for the treatment of moderate-to-severe primary RLS syndrome in adults [27]. GEn is an actively transported prodrug of gabapentin that is absorbed by high-capacity nutrient transporters located throughout the large and small intestines. GEn provides dose-proportional exposure to gabapentin, has low interpatient variability, and delays time to peak plasma concentration [28, 29]. Compared with placebo, GEn significantly improved RLS symptoms in adults with moderate-to-severe primary RLS, as assessed by the International Restless Legs Scale (IRLS) and the investigator-rated Clinical Global Impression-Improvement (CGI-I) scale [24, 25, 30, 31]. QOL and mood outcomes, which were evaluated using the RLS QOL questionnaire and Profile of Mood States (POMS), respectively, also improved with GEn treatment compared with placebo [24, 26]. A pooled analysis of XP052, XP053, and XP081 found that patients with moderate-to-severe primary RLS with either no-to-moderate or severe-to-very severe sleep disturbance benefited from GEn treatment. GEn 600 mg and 1200 mg once daily significantly improved RLS symptoms, pain, and mood compared with placebo in both sleep subgroups in this study [32]. Subjective sleep parameters, including sleep quality, nighttime awakenings, and number of hours awake per night, also improved with GEn treatment regardless of baseline sleep disturbance level in a pooled analysis of XP052 and XP053 [33]. In another pooled analysis of the same studies (XP052, XP053, XP081), RLS-associated pain was significantly improved with GEn 600 mg and 1200 mg, and there were moderate to strong correlations between RLS symptoms and pain [34]. The most common treatment-emergent adverse events (TEAEs) in these studies were somnolence and dizziness.
We conducted the present analysis to investigate the effects of GEn (600 mg or 1200 mg) once-daily treatment compared with placebo on QOL and mood outcomes using data from three clinical trials of adults with moderate-to-severe primary RLS. Safety outcomes were also evaluated in both the QOL and mood populations. Whereas previous analyses by Bogan et al. [32] investigated sleep-related endpoints of interest, including mood, in patients with different levels of baseline sleep disturbance, the current analysis examined both mood and QOL in the entire pooled patient population from these three studies. GEn 600 mg once daily is currently the only approved dosage for the treatment of moderate-to-severe primary RLS in adults [27]. Compared with GEn 600 mg once daily, GEn 1200 mg once daily has not demonstrated a clinically meaningful improvement in efficacy, as measured by the co-primary endpoints of IRLS total score and investigator-rated CGI-I [25, 26, 31]. GEn 1200 mg has also been associated with a greater degree of somnolence and dizziness compared with GEn 600 mg. However, we believe clinicians may benefit from examination of all data related to the efficacy and safety of GEn 1200 mg. The totality of data, includes additional secondary efficacy endpoints such as QOL and mood in this analysis and other previously published analyses, as well as information on the higher GEn dose. These data may enable clinicians to understand the risks and benefits of the higher GEn dose and thereby facilitate more patient-specific treatment and dosing decisions, particularly for patients with RLS who might not respond to the approved GEn dose.
2 Materials and Methods
2.1 Patients and Study Design
Our investigation is based on data from the XP052, XP053, and XP081 trials, and the designs of these studies have been previously published (ClinicalTrials.gov NCT00298623, NCT00365352, and NCT01332305) [24–26]. Briefly, each study was a placebo-controlled, 12-week, double-blind, randomized trial that enrolled adults with moderate-to-severe primary RLS, as defined by the International Restless Legs Syndrome Study Group diagnostic criteria [1].
Adult patients ≥18 years of age were eligible if they experienced RLS symptoms for ≥15 days during the month before screening (or, if on treatment, similar symptom frequency before the start of treatment), had an IRLS total score of ≥15, had documented RLS symptoms for four or more of the seven consecutive evenings during the baseline period, and discontinued other RLS treatments ≥2 weeks prior to baseline. Exclusion criteria included a body mass index of ≥34 kg/m2, evidence of secondary RLS, pregnancy, a neurological or movement disorder (e.g., diabetic neuropathy, Parkinson’s disease, multiple sclerosis, dyskinesias, and dystonias), or moderate-to-severe depression.
The three studies investigated doses of GEn ranging from 600 mg to 2400 mg, as follows: GEn 1200 mg in XP052; GEn 600 mg and 1200 mg in XP053; and GEn 600 mg, 1200 mg, 1800 mg, and 2400 mg in XP081. The co-primary endpoints for all three trials were mean change from baseline to week 12 in IRLS total score, and the proportion of responders (rated “very much” or “much” improved) on the investigator-rated CGI-I scale at week 12. For the present analysis, data were pooled for the placebo (XP052, XP053, XP081), GEn 600 mg (XP053, XP081), and GEn 1200 mg (XP052, XP053, XP081) groups.
2.2 Efficacy and Tolerability Outcomes
The assessment of QOL was a secondary objective in the XP052 and XP053 studies. QOL was assessed at weeks 4, 8, and 12 using the validated RLS QOL questionnaire, an 18-item questionnaire that examines the effect of RLS on daily life, emotional well-being, social life, and work life [35]. Scores on the RLS QOL questionnaire range from 0 to 100, and higher scores indicate greater improvements in QOL. This is the only QOL scale designated by the Movement Disorder Society Task Force as “recommended” for use in cross-sectional assessments and treatment-related changes in RLS QOL [36].
Mood was assessed as a secondary objective of the XP052, XP053, and XP081 studies at weeks 4, 8, and 12. In our analysis, mood was assessed with the POMS Brief Form (POMS-B) questionnaire at weeks 4, 8, and 12 and with IRLS items 9 and 10 at weeks 1, 2, 3, 4, 6, 8, 10, and 12. The POMS-B is a shortened version of the standard validated psychological 65-item POMS, and consists of 30 adjectives describing moods and feelings that the respondent may have experienced during the past week [37]. These items comprise six identifiable mood states: tension-anxiety, depression-dejection, anger-hostility, fatigue-inertia, vigor-activity, and confusion-bewilderment. The total mood disturbance score is calculated by summing the tension-anxiety, depression-dejection, anger-hostility, fatigue-inertia, and confusion-bewilderment scores, then subtracting the vigor-activity score [37]. Lower scores for all POMS items indicate improved mood outcomes, with the exception of vigor-activity, for which a higher score indicates a better outcome.
Two individual items of the IRLS—IRLS item 9 (severity of impact of RLS symptoms on daily affairs) and IRLS item 10 (severity of mood disturbance due to RLS symptoms)—were also investigated to measure the extent to which RLS impacts mood. These individual IRLS items are part of an exploratory analysis, and were secondary to the POMS analysis for the evaluation of mood outcomes. In the primary studies, mean change from baseline to week 12 in IRLS total score was one of the co-primary endpoints [24–26]. In our analysis, IRLS item 9 and item 10 were evaluated because they are directly related to QOL and mood. The IRLS is a validated scale for evaluating RLS symptoms, and consists of ten questions related to the intensity, frequency, and consequences of RLS [38]. Individual IRLS item scores range from 0 to 4 points, with the maximum total IRLS score being 40 points. Lower IRLS scores indicate improvements in RLS symptoms and their related outcomes. Safety outcomes were evaluated in the pooled populations on the basis of TEAEs, serious TEAEs, and discontinuations due to TEAEs.
2.3 Statistical Analyses
Data were pooled by treatment group for GEn (600 mg or 1200 mg) or placebo. The mood analysis included data from all three studies. For the QOL analysis, data were pooled for studies XP052 and XP053, as QOL was not evaluated in XP081. The safety populations for both the QOL and mood analyses included all adult patients with moderate-to-severe primary RLS in the placebo, GEn 600 mg, and GEn 1200 mg groups who received at least one dose (or portion of a dose) of study medication. The modified intent-to-treat (MITT) population, which was used for the present analysis, included adult patients in the safety population who completed the IRLS at baseline and at least once during the treatment period.
To measure the change from baseline for the endpoints according to the RLS QOL, IRLS, and POMS, treatment effects were analyzed using mixed models for repeated measures (MMRM) with unstructured covariance matrices. These analyses included the baseline score as a covariate and treatment group, week (categorical), and the treatment-by-week interaction as effects. Least squares (LS) means were used to calculate the differences between treatment arms. The MMRM approach utilizes all observed data over all visits in the model, and no imputation is performed for a subject. Data from earlier time points are used to estimate the LS means at later time points when some data may be missing. As the MMRM approach is a standard analysis in this type of study, the assumptions were not formally assessed. However, the data were not highly skewed and the sample size was relatively large. Study was initially included in the model as an effect to test for differences between studies; however, only one model had a significant study effect (POMS: anger-hostility; p = 0.024). Because the studies were all very similar and the results with or without study included in the model were also similar, study effect was dropped from all models. Safety was evaluated within both the QOL and mood analysis populations. Safety data were summarized using descriptive statistics for the safety populations.
3 Results
3.1 Patients
The MITT population for the QOL analysis included a total of 541 adult patients with moderate-to-severe primary RLS from the XP052 and XP053 studies (placebo, n = 204; GEn 600 mg, n = 114; GEn 1200 mg, n = 223) (Fig. 1). For the mood analysis, the MITT population included a total of 671 adult patients with moderate-to-severe primary RLS from the XP052, XP053, and XP081 studies (placebo, n = 244; GEn 600 mg, n = 161; GEn 1200 mg, n = 266). Baseline and demographic characteristics were similar across treatment groups within the patient cohorts analyzed for either QOL or mood outcomes (Table 1).
Patient disposition. GEn gabapentin enacarbil, MITT modified intent-to-treat, QOL quality of life. a In XP081, GEn 1800 mg and GEn 2400 mg were also investigated, but these doses were not included in the present analysis. b Included all adults who received at least 1 dose (or portion of a dose) of study medication. c Included all adults in the safety population who completed the International Restless Legs Scale at baseline and at least once during the treatment period. d In the QOL analysis, 83 % (169/204) of patients in the placebo group, 91 % (104/114) of patients in the GEn 600 mg group, and 89 % (198/223) of patients in the GEn 1200 mg group completed their respective study. e QOL results were not reported for XP081. f In the mood analysis, 82 % (200/244) of patients in the placebo group, 86 % (138/161) of patients in the GEn 600 mg group, and 86 % (229/266) of patients in the GEn 1200 mg group completed their respective study
3.2 Efficacy: QOL Outcomes
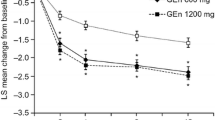
At week 12, both GEn 600 mg and GEn 1200 mg once daily significantly improved QOL scores compared with placebo (Fig. 2). Improvements were also significant at weeks 4 and 8. LS mean treatment differences from placebo in RLS QOL score were significant at all time points examined for both GEn 600 mg and GEn 1200 mg (Fig. 3).
LS mean changes from baseline in RLS QOL scores by visit in adults with moderate-to-severe primary RLS. Error bars represent standard error. *p < 0.001 vs. placebo, **p < 0.01 vs. placebo. p values are for pairwise comparisons of the differences in treatments at each time point (GEn 600 mg vs. placebo and GEn 1200 mg vs. placebo). GEn gabapentin enacarbil, LS least squares, QOL quality of life, RLS restless legs syndrome
Treatment differences between GEn and placebo in the change from baseline RLS QOL score by visit in adults with moderate-to-severe primary RLS. Error bars represent standard error. Treatment differences were calculated using LS means. *p < 0.01 (asterisks indicate comparisons between GEn and placebo). GEn gabapentin enacarbil, LS least squares, QOL quality of life, RLS restless legs syndrome
Although this analysis was not powered to detect statistical differences between the GEn doses, RLS QOL scores were numerically higher for GEn 1200 mg versus GEn 600 mg at all time points (Fig. 2). Treatment differences were also numerically greater with GEn 1200 mg versus placebo than with GEn 600 mg versus placebo at all time points (Fig. 3).
3.3 Efficacy: Mood Outcomes
Compared with placebo, GEn 600 mg once daily significantly improved the POMS assessment criterion of total mood disturbance at weeks 4 and 8, but not at week 12. GEn 1200 mg once daily significantly improved total mood disturbance at weeks 4, 8, and 12 (Fig. 4).
LS mean changes from baseline in POMS subscale scores by visit in adults with moderate-to-severe primary RLS: depression-dejection (a); fatigue-inertia (b); total mood disturbance (c); vigor-activity (d); anger-hostility (e); confusion-bewilderment (f); tension-anxiety (g). Error bars represent standard error. Lower scores for all POMS items correspond to improved mood outcomes, with the exception of vigor-activity, for which a higher score corresponds to a better outcome. *p < 0.05, **p < 0.01, ***p < 0.001 vs. placebo. p values are for pairwise comparisons at each time point (GEn 600 mg vs. placebo and GEn 1200 mg vs. placebo). GEn gabapentin enacarbil, LS least squares, POMS Profile of Mood States, RLS restless legs syndrome
Assessment of the other POMS subscale items demonstrated that GEn 600 mg significantly improved the POMS assessment criterion of vigor-activity at week 12 (Fig. 4). Other POMS items did not show significant differences with GEn 600 mg compared with placebo at week 12. At weeks 4 and 8, there were significant improvements with GEn 600 mg compared with placebo for depression-dejection, anger-hostility, and confusion-bewilderment. The POMS criterion of vigor-activity also significantly improved with GEn 600 mg compared with placebo at week 4. GEn 1200 mg significantly improved depression-dejection, fatigue-inertia, vigor-activity, and confusion-bewilderment compared with placebo at week 12 (Figs. 4, 5). Significant improvements were also observed for all POMS subscale items with GEn 1200 mg versus placebo at weeks 4 and 8. LS mean treatment differences in POMS subscale scores at week 12 are shown in Fig. 5. Compared with placebo, GEn 1200 mg improved most POMS subscale scores to a greater extent than GEn 600 mg compared with placebo at weeks 4, 8, and 12 (Fig. 4).
Treatment differences between GEn and placebo in the change from baseline POMS subscale scores at week 12. Error bars represent standard error. Lower scores for all POMS items correspond to improved mood outcomes, with the exception of vigor-activity, for which a higher score corresponds to a better outcome. *p < 0.05 (asterisks indicate comparison between GEn and placebo). GEn gabapentin enacarbil, LS least squares, POMS Profile of Mood States
For the exploratory IRLS items, GEn 600 mg and 1200 mg once daily significantly improved scores for IRLS item 9 (impact on daily affairs) and item 10 (mood disturbance) compared with placebo at week 12 (Fig. 6). Improvements were observed as early as week 1 and were sustained throughout the 12-week clinical trial period. At week 12, treatment differences from placebo for IRLS items 9 and 10, respectively, were as follows: GEn 600 mg, −0.3 (standard error, 0.07) and −0.2 (0.07); GEn 1200 mg, −0.3 (0.07) and −0.3 (0.06) (all p < 0.001). Both GEn doses also significantly improved scores for IRLS items 9 and 10 at most other time points examined compared with placebo. Treatment differences between GEn and placebo were similar for the GEn 600 mg and 1200 mg groups.
LS mean changes from baseline for individual IRLS item scores by visit. IRLS item 9: impact of RLS symptoms on daily affairs (a); IRLS item 10: severity of mood disturbance (b). Error bars represent standard error. *p < 0.05 vs. placebo. p values are for pairwise comparisons at each time point (GEn 600 mg vs. placebo and GEn 1200 mg vs. placebo). GEn gabapentin enacarbil, IRLS International Restless Legs Scale, LS least squares, RLS restless legs syndrome
3.4 Tolerability
For the patients assessed for either QOL or mood outcomes, the proportions of patients experiencing TEAEs and discontinuation due to a TEAE are shown in Table 2. For both the QOL and mood analyses, the most common TEAEs (≥5 % and at least two times the rate of placebo) for GEn 600 mg and GEn 1200 mg were somnolence and dizziness. The TEAEs occurring in ≥5 % of patients in at least one of the treatment arms included somnolence, dizziness, headache, nasopharyngitis, nausea, fatigue, upper respiratory tract infection, and back pain.
Within the populations analyzed for QOL or mood, a numerically greater percentage of patients in the GEn 600 mg and 1200 mg treatment groups experienced any TEAE compared with patients in the placebo groups. For patients analyzed for QOL outcomes, discontinuations due to TEAEs occurred more frequently in the GEn 600 mg group than in the GEn 1200 mg or placebo groups. For the mood analysis population, discontinuations due to TEAEs occurred more often in the GEn 1200 mg group than in the GEn 600 mg or placebo groups.
4 Discussion
In this pooled analysis of adult patients with moderate-to-severe-primary RLS, GEn 600 mg and GEn 1200 mg once daily significantly improved QOL and some mood outcomes compared with placebo. Regarding mood outcomes, GEn 600 mg significantly improved the POMS criterion of vigor-activity and IRLS items 9 and 10 at week 12 compared with placebo. GEn 1200 mg significantly improved a wider range of POMS criteria, including total mood disturbance, depression-dejection, fatigue-inertia, vigor-activity, and confusion-bewilderment, as well as IRLS items 9 and 10, at week 12 compared with placebo. The improvements in QOL and mood with both doses occurred at the earliest time points examined, and continued through the 12 weeks of treatment. These improvements are clinically meaningful, as they are comparable to those observed in previously published RLS clinical trials that have utilized the RLS QOL and/or POMS questionnaire, including trials that investigated DA treatment of RLS [14, 32, 39–42]. In our analysis, both QOL and mood improvements were numerically higher for GEn 1200 mg versus GEn 600 mg. As previously shown in the primary analyses, somnolence and dizziness were the most commonly reported TEAEs, which is consistent with the overall safety profile of GEn [24–27].
Patients with RLS often report sleep loss and extreme discomfort due to their RLS symptoms, resulting in problems with daily performance and concentration [1]. Functioning in sedentary situations, as well as in the evening when symptoms are exacerbated, poses significant challenges. Patients with RLS report significant deficits in QOL compared with the general population as a result of these difficulties [9, 10, 43]. The reduction in QOL among RLS sufferers is similar to that of patients with serious chronic medical conditions, including diabetes and hypertension. In a study utilizing the Short Form 36 (SF-36), a general QOL assessment tool, patients with RLS had significantly lower scores (worse QOL) on all eight scales of the SF-36 compared with patients with hypertension (p < 0.01). The RLS group also had lower scores on seven of eight scales compared with patients with diabetes [10]. In the RLS Epidemiology, Symptoms, and Treatment (REST) primary care study, 36 % of RLS sufferers reported a high negative impact of RLS symptoms on their overall QOL [2]. In the present analysis, we found that adult patients with moderate-to-severe primary RLS had impaired QOL as evidenced by baseline QOL scores of 66.8–68.3 points, and that treatment with both GEn doses improved QOL at all time points examined.
RLS is also associated with a negative impact on mood, largely due to sleep impairments as well as uncomfortable and sometimes painful dysesthesias [8, 11]. Patients with RLS are at an increased risk of developing anxiety or depressive disorders over their lifetime compared with the general population. These associations are particularly strong for panic disorder, major depression, and generalized anxiety disorder [44]. In our analysis, treatment with GEn compared with placebo significantly improved some mood outcomes in patients with moderate-to-severe primary RLS based on the POMS and IRLS items 9 and 10.
Although GEn 1200 mg is not currently approved for the treatment of moderate-to-severe primary RLS in adults, clinical trials have described numerically similar or superior effects of GEn 1200 mg compared with GEn 600 mg in RLS symptom treatment [24–26]. In our analysis, QOL and mood improvements compared with placebo were numerically greater with GEn 1200 mg than with GEn 600 mg. Direct comparisons between the doses also showed numerical improvements with GEn 1200 mg versus GEn 600 mg for both QOL and mood scores. Thus, treatment with GEn 1200 mg could be a viable option for some adult patients with moderate-to-severe primary RLS if the benefits outweigh the risks. It is interesting to note that, within the QOL population, there were fewer discontinuations due to TEAEs with GEn 1200 mg versus GEn 600 mg, whereas the opposite trend was observed within the mood population. Whether this suggests a difference in how patients interpret benefit and tolerate treatment based on their perception of QOL versus mood remains an interesting speculation as well as an area of future research.
Our analysis is limited to the population studied in these clinical trials. In our analysis, demographic and baseline characteristics were generally similar for GEn treatment groups and placebo within the QOL and mood analyses. Most patients in both pooled data sets were female and/or white, reflecting the epidemiology of RLS in the general population [9, 35]. This was not a formal meta-analysis, so additional covariates such as site effect and study effect were not assessed. P values were also unadjusted for multiple comparisons, and the endpoints discussed were secondary outcomes in the primary studies. This analysis was not powered to compare the GEn 600 mg and 1200 mg doses, and was limited to the GEn doses assessed in the XP052, XP053, and XP081 studies. In addition, the 12-week trial period may be too short to assess other potential late-onset complications impairing long-term QOL or mood. Whereas the IRLS total score is a validated measure of RLS symptoms, the individual IRLS items have not been validated. Therefore, our analysis of the single items of the IRLS was exploratory in nature, and was secondary to the POMS analysis for the evaluation of the impact of RLS on mood. Nevertheless, these data provide compelling evidence of the positive effects of GEn on QOL and mood outcomes in adults with moderate-to-severe primary RLS.
5 Conclusion
In conclusion, GEn (600 mg or 1200 mg) administered once daily significantly improved QOL and some mood outcomes compared with placebo in adult patients with moderate-to-severe primary RLS. The most common TEAEs were somnolence and dizziness, which are consistent with the primary studies, as well as the overall safety profile of GEn [21, 24, 26, 28]. For both the QOL and mood analyses, treatment effects compared with placebo were generally numerically greater for GEn 1200 mg than for GEn 600 mg. However, dosage should be carefully considered on a case-by-case basis during clinical practice, as GEn 600 mg is the only FDA-approved dose for moderate-to-severe primary RLS. The data presented here underscore the relevance of GEn as a treatment option for patients with RLS, particularly those whose mental health and overall QOL have been adversely affected by their disease. Additional future studies, including long-term studies, are recommended to further explore the benefits of GEn in patients with moderate-to-severe primary RLS who experience impairments in QOL and mood.
References
Allen RP, Picchietti D, Hening WA, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epide-miology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19.
Hening W, Walters AS, Allen RP, et al. Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: the REST (RLS epidemiology, symptoms, and treatment) primary care study. Sleep Med. 2004;5:237–46.
Garcia-Borreguero D, Kohnen R, Silber MH, et al. The long-term treatment of restless legs syndrome/Willis-Ekbom disease: evidence based guidelines and clinical consensus best practice guidance: a report from the international restless legs syndrome study group. Sleep Med. 2013;14:675–84.
Allen RP, Picchietti DL, Garcia-Borreguero D, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria—history, rationale, description, and significance. Sleep Med. 2014;15:860–73.
Berger K, Luedemann J, Trenkwalder C, et al. Sex and the risk of restless legs syndrome in the general population. Arch Intern Med. 2004;164:196–202.
Ohayon MM, O’Hara R, Vitiello MV. Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med Rev. 2012;16:283–95.
Innes KE, Selfe TK, Agarwal P. Prevalence of restless legs syndrome in North American and Western European populations: a systematic review. Sleep Med. 2011;12:623–34.
Sevim S, Dogu O, Kaleagasi H, Aral M, Metin O, Camdeviren H. Correlation of anxiety and depression symptoms in patients with restless legs syndrome: a population based survey. J Neurol Neurosurg Psychiatry. 2004;75:226–30.
Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165:1286–92.
Abetz L, Allen R, Follet A, et al. Evaluating the quality of life of patients with restless legs syndrome. Clin Ther. 2004;26:925–35.
Scholz H, Benes H, Happe S, et al. Psychological distress of patients suffering from restless legs syndrome: a cross-sectional study. Health Qual Life Outcomes. 2011;9:73.
Cho SJ, Hong JP, Hahm BJ, et al. Restless legs syndrome in a community sample of Korean adults: prevalence, impact on quality of life, and association with DSM-IV psychiatric disorder. Sleep. 2009;32:1069–76.
Kalloo A, Garnaldo CE, Kwan AB, et al. The impact of restless legs syndrome/Willis-Ekbom Disorder on quality of life. Eur Neurol Rev. 2013;8:97–104.
Walters AS, Ondo WG, Dreykluft T, et al. Ropinirole is effective in the treatment of restless legs syndrome. TREAT RLS 2: a 12-week, double-blind, randomized, parallel-group, placebo-controlled study. Mov Disord. 2004;19:1414–23.
Montagna P, Hornyak M, Ulfberg J, et al. Randomized trial of pramipexole for patients with restless legs syndrome (RLS) and RLS-related impairment of mood. Sleep Med. 2011;12:34–40.
Scholz H, Trenkwalder C, Kohnen R, et al. Dopamine agonists for restless legs syndrome. Cochrane Database Syst Rev. 2011;16:CD006009.
Buchfuhrer MJ. Strategies for the treatment of restless legs syndrome. Neurotherapeutics. 2012;9:776–90.
Ondo W, Romanyshyn J, Vuong KD, et al. Long-term treatment of restless legs syndrome with dopamine agonists. Arch Neurol. 2004;61:1393–7.
Winkelman JW, Johnston L. Augmentation and tolerance with long-term prami-pexole treatment of restless legs syndrome (RLS). Sleep Med. 2004;5:9–14.
Cornelius JR, Tippmann-Peikert M, Slocumb NL, et al. Impulse control disorders with the use of dopaminergic agents in restless legs syndrome: a case-control study. Sleep. 2010;33:81–7.
Trenkwalder C, Winkelmann J, Inoue Y, et al. Restless legs syndrome-current therapies and management of augmentation. Nat Rev Neurol. 2015;11:434–5.
Garcia-Borreguero D, Stillman P, Benes H, et al. Algorithms for the diagnosis and treatment of restless legs syndrome in primary care. BMC Neurol. 2011;11:28.
Silber MH, Becker PM, Earley C, et al. Willis-Ekbom Disease Foundation revised consensus statement on the management of restless legs syndrome. Mayo Clin Proc. 2013;88:977–86.
Kushida CA, Becker PM, Ellenbogen AL, et al. Randomized, double-blind, placebo-controlled study of XP13512/GSK1838262 in patients with RLS. Neurology. 2009;72:439–46.
Lee DO, Ziman RB, Perkins AT, et al. A randomized, double-blind, placebo-controlled study to assess the efficacy and tolerability of gabapentin enacarbil in subjects with restless legs syndrome. J Clin Sleep Med. 2011;7:282–92.
Lal R, Ellenbogen A, Chen D, et al. A randomized, double-blind, placebo-controlled, dose-response study to assess the pharmacokinetics, efficacy, and safety of gabapentin enacarbil in subjects with restless legs syndrome. Clin Neuropharmacol. 2012;35:165–73.
HORIZANT® Prescribing Information and Medications Guides 2013, XenoPort, Inc., Santa Clara, CA. http://www.horizant.com. Accessed June 2015.
Cundy KC, Annamalai T, Bu L, et al. XP13512 [(±)-1-([(alpha-isobutanoyloxyethoxy)carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: II. Improved oral bioavailability, dose proportionality, and colonic absorption compared with gabapentin in rats and monkeys. J Pharmacol Exp Ther. 2004;311:324–33.
Cundy KC, Sastry S, Luo W, et al. Clinical pharmacokinetics of XP13512, a novel transported prodrug of gabapentin. J Clin Pharmacol. 2008;48:1378–88.
Kushida CA, Walters AS, Becker P, et al. A randomized, double-blind, placebo-controlled, crossover study of XP13512/GSK1838262 in the treatment of patients with primary restless legs syndrome. Sleep. 2009;32:159–68.
Inoue Y, Hirata K, Uchimura N, et al. Gabapentin enacarbil in Japanese patients with restless legs syndrome: a 12-week, randomized, double-blind, placebo-controlled, parallel-group study. Curr Med Res Opin. 2013;29:13–21.
Bogan RK, Lee DO, Buchfuhrer MJ, et al. Treatment response to sleep, pain, and mood disturbance and their correlation with sleep disturbance in adult patients with moderate-to-severe primary restless legs syndrome: pooled analyses from 3 trials of gabapentin enacarbil. Ann Med. 2015;47:269–77.
Bogan RK, Ellenbogen A, Becker PM. Gabapentin enacarbil in subjects with moderate to severe primary restless legs syndrome with and without severe sleep disturbance: an integrated analysis of subjective and novel sleep endpoints from two studies. J Parkinsonism Restl Legs Syndr. 2013;3:31–40.
Hermanowicz N, Ellenbogen A, Irving G, et al. The effect of gabapentin enacarbil on pain associated with moderate-to-severe primary restless legs syndrome in adults: pooled analyses from 3 randomized controlled trials. CNS Drugs. 2016 (in press).
Abetz L, Vallow SM, Kirsch J, et al. Validation of the restless legs syndrome quality of life questionnaire. Value Health. 2005;8:157–67.
Walters AS, Frauscher B, Allen R, et al. Review of quality of life instruments for the restless legs syndrome/Willis-Ekbom Disease (RLS/WED): critique and recommendations. J Clin Sleep Med. 2014;10:1351–7.
McNair DM, Loor M, Droppleman LF. Manual for the profile of mood states. San Diego: Educational and Industrial Testing Service; 1992.
Walters AS, LeBrocq C, Dhar A, et al. Validation of the international restless legs syndrome study group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–32.
Innes KE, Selfe TK. The effects of a gentle yoga program on sleep, mood, and blood pressure in older women with restless legs syndrome (RLS): a preliminary randomized controlled trial. Evid Based Complement Alternat Med. 2012;2012:294058.
Trenkwalder C, Garcia-Borreguero D, Montagna P, et al. Ropinirole in the treatment of restless legs syndrome: results from the TREAT RLS 1 study, a 12 week, randomised, placebo-controlled study in 10 European countries. J Neurol Neurosurg Psychiatry. 2004;75:92–7.
Bogan RK, Fry JM, Schmidt MH, et al. Ropinirole in the treatment of patients with restless legs syndrome: a US-based randomized, double-blind, placebo-controlled clinical trial. Mayo Clin Proc. 2006;81:17–27.
Ferini-Strambi L, Aarskog D, Partinen M, et al. Effect of pramipexole on RLS symptoms and sleep: a randomized, double-blind, placebo-controlled trial. Sleep Med. 2008;9:874–81.
Abetz L, Arbuckle R, Allen RP, et al. The reliability, validity and responsiveness of the restless legs syndrome quality of life questionnaire (RLSQoL) in a trial population. Health Qual Life Outcomes. 2005;3:79.
Winkelmann J, Prager M, Lieb R, et al. “Anxietas tibiarum”. Depression and anxiety disorders in patients with restless legs syndrome. J Neurol. 2005;252:67–71.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Alon Avidan serves on the speakers bureaus for Merck and XenoPort, Inc., has received consulting fees or honorarium from Merck, XenoPort, Inc., and Sunovion, and has developed educational presentations for the American Academy of Neurology. Daniel Lee serves on the speakers bureaus for Lundbeck and XenoPort, Inc. Margaret Park is a consultant on the advisory board to XenoPort, Inc. Mark J. Jaros serves as a consultant for XenoPort, Inc. Gwendoline Shang was employed by and owned stock in XenoPort, Inc. at the time of manuscript development. Richard Kim is employed by and owns stock in XenoPort, Inc.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. These studies were managed according to the Medical Research Council’s Good Clinical Practice Guidelines and received appropriate multicenter and local ethics and research committee approval. Informed consent was obtained from all individual participants included in the study.
Funding
These studies and this analysis were conducted by XenoPort, Inc., Santa Clara, CA. Medical writing support was provided by CodonMedical, a division of Ashfield Healthcare Communications (a UDG Healthcare plc company), and was funded by XenoPort, Inc.
Rights and permissions
About this article
Cite this article
Avidan, A.Y., Lee, D., Park, M. et al. The Effect of Gabapentin Enacarbil on Quality of Life and Mood Outcomes in a Pooled Population of Adult Patients with Moderate-to-Severe Primary Restless Legs Syndrome. CNS Drugs 30, 305–316 (2016). https://doi.org/10.1007/s40263-016-0329-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-016-0329-4