Abstract
Purpose of Review
This review summarizes the pathophysiology of acute aortic dissection with recent findings and current strategies for diagnosis and treatment.
Recent Findings
The addition of clinical and laboratorial findings to radiologic classifications emerges as an approach to better stratify and treat patients with aortic dissection. Endovascular strategies grow in variety of treatment options, including for type A aortic dissection.
Summary
Aortic dissection is a wide spectrum syndrome, generally posing a life-threating risk. Early identification can be the difference between life and death. Better imaging diagnosis made clear that accurately classification is crucial for choosing the optimal treatment. Mortality rates are still high, with the involvement of the ascending aorta being the most lethal type, despite a current trend toward better outcomes. Promising new developments in ascending aorta and aortic arch endovascular treatments are appealing for high surgical risk patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute aortic dissection (AD) is one of the main causes of cardiovascular death around the world, with an incidence estimated to be 5 to 30 cases per 1,000,000 people per year. Similar signs and symptoms characterize this syndrome, being acute onset of thoracic pain the most common one, present around in 80% of the patients, regardless of its site [1,2,3,4]. The first well described case of AD occurred in 1760 after an autopsy of the King George II, of England [5].
It can be defined as a separation of the aortic wall’s layers which creates a false-lumen (FL) where blood can flow. Single or multiple intimal disruptions can appear as entry tear (ET), causing connection between the true and FL of the aorta. Pressurization of the FL can lead to obstruction of arterial branches and hypoperfusion syndromes. On the other hand, the inflammatory response to thrombus between layers can progress to necrosis of the aortic wall, resulting in aortic rupture [4, 6, 7].
Its incidence tends to get higher in older ages, being the majority of patients between 50 and 65 years old, and although it affects more men than women, it has poorer outcomes in woman mainly because late diagnosis and treatment due to atypical presentation [3, 4, 8].
The objective of this review is to help the understanding of the full spectrum of the disease and extension of its involvement in order to achieve the best treatment option possible, since the wide variety of acute AD can pose a challenge in the patient management.
Methods
This review summarizes the recent findings regarding acute AD. We conducted the search for relevant articles with the following terms: “Aorta,” “Aortic Dissection,” “Acute Aortic Dissection,” “Aortic Syndromes,” “Aortic Repair,” “Endovascular Aortic Repair”.
The following databases were used (until April 2020): MEDLINE; EMBASE; CENTRAL/CCTR (Cochrane Controlled Trials Register); ClinicalTrials.gov; SciELO (Scientific Electronic Library Online); LILACS (Literatura Latino Americana em Ciências da Saúde); Google Scholar; and reference lists of relevant articles. We gave preference to articles published in the past 5 years.
Pathophysiology
All mechanisms that weaken the media layers of the aorta or increases aortic wall’s shear stress can eventually results in intramural hemorrhage, AD, and/or aortic rupture [9]. Noteworthy, a considerable number of patients has intramural hematoma (IMH) before developing AD. It is hypothesized that the bleeding from the vasa vasorum into the media layer creates areas of increased shear stress in the intima, ultimately resulting in an increased risk for development of AD [7, 10,11,12], although some suggests that IMH is actually originated through micro intimal tears that does not appear in imaging exams, hence the similarities between these two entities [10, 13].
The most common risk factor for AD is poorly controlled hypertension, with prevalence ranging around 52–96% [1, 14]. One possible explanation to this high association is the role that shear stress can have in AD’s development, as demonstrated by Taguchi et al. They studied 98 patients with AD and shown that more frequently intimal tears appear in sites of high shear stress (78%), being the anterior site of ascending aorta and the greater curvature of distal aortic arch the most common place (40.2% and 29.5% respectively) [15].
It is still debatable if atherosclerosis plays a role in AD’s development. A necropsy report of 161 cases of AD suggests that a ruptured atheroma plaque can initiate the intimal tear leading to AD [16]. On the other hand, newer pathological studies have shown non-atherosclerotic plaques in intima nor media layers where the flap of AD is situated, and lower prevalence of hypercholesterolemia, obesity, and diabetes mellitus in patients with AD suggests that atherosclerosis may not be an important risk factor for AD [14, 15]. In addition, it is thought that the genetic effect that let patient’s aortic wall more susceptible to dilatation and dissection can protect them from systemic atherosclerosis [8].
Genetic aspects also play a role in the development of AD. The GenTAC Registry (National Registry of Genetically Triggered Thoracic Aortic Aneurysms and Cardiovascular Conditions) was composed by 1991 patients, 22% being carriers of Marfan syndrome. After a mean follow-up of 3.6 years, patients with Marfan syndrome had a sixfold incidence of acute AD [17]. Other familial syndromes such as Loeys-Dietz and Ehlers-Danlos are also associated with AD [18].
A minority of AD cases can be ascribed to iatrogenic causes, being the incidence after percutaneous coronary intervention around 0.062% during diagnostic or therapeutic procedures. In the majority of the cases, a lack of surgical preparation contributes to a high-risk surgery and high mortality rate of these events, when surgery is needed [19, 20]. Cardiac surgery in general also consists in a risk factor for development of AD. In the International Registry of Acute Aortic Dissection (IRAD) study, iatrogenic AD after cardiac surgery was reported as 2.2% of the patients. Shea and colleagues reported a review of 23,275 patients that underwent cardiac surgery at the New York-Presbyterian/Columbia University Aortic Center, identifying 15 patients that had iatrogenic AD. Most patients underwent aortic surgery, and the main site of dissection was the aortic cannulation [1, 21].
Recently, an unusual association between fluroquinolones and development of acute AD is being observed—especially in patients with aortic aneurysms. In one study, a 66% increase rate of AD or aortic aneurysm development within 60 days from start of treatment, corresponding to 82 cases per 1 million treatments, was reported. Possible explanation is related to matrix metalloproteinases, known to degrade critical components of the extracellular matrix, being upregulated after exposure to ciprofloxacin, that could lead to fragility in the aorta layer [22, 23•].
Other risk factors include smoking, pre-existing aortic diseases or aortic valve disease (such as bicuspid aortic valve), familial history of aortic diseases, male sex, age, history of cardiac surgery, direct blunt chest trauma, and use of intravenous drugs [1, 7, 10].
Classification
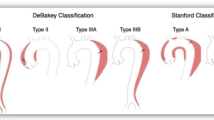
Classifying AD has an important prognostic value and is decisive to treatment. It can be classified as according to the site of the ET of the dissection, or based on involvement of the ascending aorta. The DeBakey classification divides the disease into 3 types: type I has the ET in the ascending aorta and progress distally, type II has also the ET in the ascending aorta but remains in it, not growing to the aortic arch, and type III has the ET in the descending aorta and may or not propagate proximally (IIIa) or distally (IIIb). The Stanford classification divides it into two types: type A involves the ascending aorta and type B does not, regardless the site of the ET (Fig. 1a) [24,25].
a Scheme of different aortic dissections’ classifications. b Computed-tomography angiography in axial view showing acute type A aortic dissection. Red arrow shows the entry tear. c Computed-tomography angiography in sagittal view showing distal progression of the aortic dissection. d Intra-operative photo of an opened ascending aorta. Blue arrow shows the aortic valve, green arrow shows the intima layer, and white arrow shows the adventitia layer. e Computed-tomography angiography control after a successful endovascular treatment of acute type A aortic dissection
Recently, a new subdivision type of Stanford classification emerged, trying to address the aortic arch dissections, called non-A/non-B type. Type non-A/non-B aortic dissection (TnABAD) can be divided further into two types: descending-entry type is when the ET is located distal to the left subclavian artery (LSA) and there is a retrograde involvement of the aortic arch without compromising the ascending aorta. Arch-entry type is when the ET is in the aortic arch, between the brachiocephalic trunk (BCT) and the LSA, and may progress distally (Fig. 1a) [26••,27].
Further merging of imaging, laboratory, and clinical signs gave rise to a new classification: TEM AD stratification. It combines the classical Stanford classification with the addition of the TnABAD, also adding site of ET and malperfusion as a way to better predict outcomes and to choose the best treatment option for patients. “T” refers to type (A, B or non-A/non-B), “E”refers to ET (E0 = no ET, E1 = in the ascending aorta, E2, in the aortic arch, E3 = in the descending aorta), and “M” refers to malperfusion (M0 = no signs of malperfusion, M1 = dissection of at least 1 main coronary artery with (M1+) or without (M1−) indicators of cardiac ischemia, M2 = dissection of at least 1 supra-aortic vessel or collapse of the aortic arch true lumen with (M2+) or without (M2) clinical symptoms of cerebral or upper extremity malperfusion, M3 = dissection, FL origin or closure due to aortic true lumen collapse of at least 1 visceral, renal or iliac artery with (M3+) or without (M3−) clinical symptoms of bowel, kidney or lower extremity) [28•].
Another division of the aorta is based on landing zones for endovascular devices. In this system, the aorta is divided in 10 parts (0 to 9): zone 0 corresponding to the ascending aorta, further divided into 0A (the aortic root), 0B (proximal half of the AA, from the coronary arteries to the level of the right main pulmonary artery), and 0C (distal half of the AA, from the right main pulmonary artery to the BCT); zone 1–2 the aortic arch; zones 3 to 5 the thoracic descending aorta; and zones 6 to 9 the abdominal descending aorta (Fig. 1a) [29••,30].
Type A (TAAD)
TAAD is the most common type of AD, accounting for 62% of patients, according to the IRAD [1], and carries a high mortality rate. In the past, virtually 100% of the patients with TAAD evolved to death due to the lack of capacity of early diagnosis and surgery [6]. Even with medical advances, mortality rate is still high, around 1–2% per hour in the first 48 h [1, 4, 6, 31].
It is a surgical emergency, meaning that surgical repair must be performed immediately after the diagnosis. Medical therapy alone is not indicated, and in-hospital mortality rate of patients treated medically only can get as high as 58% [1, 4]. When not surgically repaired, TAAD can progress to aortic rupture and cardiac tamponade or to coronary dissection and myocardial infarction (MI), being these as the most common causes of death [1, 10, 32].
Type B (TBAD)
According to its initial manifestation, TBAD can be classified as complicated or uncomplicated [1, 33, 34]. At admission, the majority of patients with acute TBAD do not have complications [34, 35]. In a single-center retrospective cohort, Reutersberg and colleagues found out that up to 37% of the initially uncomplicated TBAD developed late complications, in a median interval of 7 days after first onset of symptoms, resulting in a higher mortality risk [36].
Acute complicated TBAD are defined by the presence of at least one of the following signs: aortic rupture, refractory pain, rapid aortic expansion, uncontrollable hypertension (hypertension persisting despite use of 3 different classes of antihypertensive therapy), or organic malperfusion syndrome, which worsen the prognosis and increase mortality [34, 37]. The latter is the most common one and is caused by a dynamic obstruction (when an intimal flap tear blocks blood flow to one arterial branch) or by a static obstruction, in most cases caused by expansion of the FL into a branch vessel [38•, 39, 40].
For patients with non-complicated TBAD, optimal medical therapy alone must be adopted, achieving low 30-day mortality rate [4, 7, 39, 41].
Type Non-A/Non-B (TnABAD)
For AD that involves the aortic arch but not the ascending aorta, a new type of classification emerged in the past years, grouping them as TnABAD. Its incidence is still not well known, but in some studies, it accounts up for 11% of patients with acute AD [26••]. To understand this new classification is important to note that the aortic arch is a well-established anatomical region of the aorta, situated between the BCT and the LSA. These two vessels offer a natural barrier that can stop the extension of the dissection, creating new entities of AD that are neither type A nor type B [27].
The first one—arch entry type—happens when the intimal tear and the dissection are located in the aortic arch, and the second one—descending entry type—when a TBAD retrogradely involves more than only the LSA. Both have incidence around 50% [26••, 27]. AD that affects the ascending aorta and progress into the arch, or on the contrary, has the ET in the aortic arch but retrogradely involves the ascending aorta, sometimes being called arch A type [42], are established as TAAD with aortic arch involvement and should be treat accordingly [26••, 42, 43].
Clinical Manifestation
Initial presentation is often abrupt onset of pain, being anterior thoracic pain more associated with TAAD, while abdominal and back pain more related to TBAD [1, 2, 4, 9, 10, 44]. Hypertension is often found in patients with acute AD [1, 2, 4, 9, 10]. In the IRAD study, of 451 patients that had their blood pressure (BP) measured at the hospital admission, 35% has systolic BP > 150 mmHg for TAAD and 70% for TBAD [1]. However, in some cases, mainly in TAAD, systolic BP can be found below 100 mmHg, or even shock can be the first sign of the disease (often when complications are present) [1, 9]. Other signs and symptoms include aortic regurgitation, MI, syncope, stroke, pulse deficit, and mesenteric ischemia [1, 2, 9, 10].
Initial chest-Rx can show widened mediastinum, abnormal aortic contour, calcification of the aorta, or pleural effusion. Abnormal electrocardiogram findings range from left ventricular hypertrophy to altered ST segments and T wave. The absence of chest-Rx or electrocardiogram (ECG) finds is found in 21% and 31%, respectively [1, 9].
Imaging
For the diagnosis and treatment of patients with acute AD, imaging studies are fundamental. After clinically stabilization of acute patients, an image study must be performed in order to classify the AD, localize ET and total extension, asset severity, complications, and indicators of urgent/emergent treatment—i.e., involvement of ascending aorta, pericardial effusions, contained rupture, and large branches blockade [2, 39].
There are mainly three types of images studies currently well used, computed-tomography angiography (CTA), echocardiography—transesophageal and transthoracic (TEE and TTE respectively), and magnetic resonance imaging (MRI). All three are comparable with sensitivity and specificity over 98% and 95%, respectively [45], although important differences exist.
CTA can be found in most emergency room, is an easy to do exam, being performed rapidly, and with no need of additional preparation. It can clearly point out the local of the dissection and its extension, as well as complications such as branches compressions, pleural effusions (which can indicate aortic rupture), and association with aortic aneurysms (Fig. 1b, c) [2,39]. The non-contrasted images are useful to see intimal calcification, hemorrhage, hematomas, asset the status of the lungs, and check for fluid effusions. Final diagnosis must be done under contrasted-enhanced step [2, 39, 46]. Imaging exams protocol vary according to the available CTA and the patient’s characteristics; being crucial small cuts and a good timing in contrast agent bolus and first images. Motion artifacts that could mimic aortic flaps, causing false positives exams and overdiagnosis, can be overcome by using ECG-gated CTA [39, 46].
It is important to make the differentiation between true and FL of the aorta. It can modify the surgical or endovascular procedure [2, 47]. The FL usually have higher pressure than the true-lumen, causing it to expand. This expansion can later result in branches compression, anterograde or retrograde progression of the dissection, recommunication with the true lumen via new fenestrations, or rupture of the aortic wall [39, 46]. Important signs that helps to differentiate true and FL are the “beak sign” and the “cobweb sign.” The beak sign is a wedge of hematoma that creates space for propagation of the FL. The cobweb sign is made when the media layer of the aorta has been uncompleted sheared, creating slender linear areas of attenuation, specific of the FL. Also, at contrasted-enhanced CTA, the true lumen is in continuation with the undissected portion of the aorta, and the FL may be thrombosed at some points [48].
TTE and TEE can be useful tools in emergency room since they are portable and ready to use, especially for unstable patients that cannot stand transportation. Bedside TTE combined with TEE and Doppler examination can accurately show involvement of the ascending aorta, and/or the aortic valve, pericardial tamponade, and coronaries obstructions, thus indicating the need of emergency operation [2, 49,50,51]. For TTE, the “four S” approach is useful to see the aorta in shorter time. A long-axis parasternal view could show the aortic root; in a suprasternal view, the supra aortic branches can be seen and surveyed for dissection and or compression. By adjusting the window depth, the descending aorta can be seen in the parasternal view, and the abdominal aorta in the subxiphoid view [4, 50].
Although TEE needs esophageal intubation, the proximity of the aorta with the esophagus make easy to identify dissections, both in the ascending and descending aorta, with the addition of Doppler exam. In the majority of the cases, ET can be located this way [49, 52]. Echocardiography may be limited to several factors, including bad window due to lungs and ribbons; it is an operator depending exam, and not every hospital has transesophageal probes ready to use in the emergency room. Despite that, both TTE and TEE can achieve high rate of sensibility and specificity in diagnosing acute AD [49, 51, 53], although for TBAD, TTE is not that sensitive, detecting dissections in 31–55% of the cases [51, 54].
MRI is another great imaging modality in diagnosis and follow-up of patients with AD. For it possible not to use of ionized radiation or contrast, it is the primary choice for patients with allergies and the ones that need multiples follow-up images [2, 49]. Current advances in MRI scanners technology further increment the ability of diagnosis, follow-up, and assessment of additional blood-flow dynamics. Computer-based algorithms involving fluids and flow dynamics complement MRI and can quantify changes in total pressure and aortic wall shear stress and FL involvement. 4D MRI is another modality which integrates the three spatial dimensions with volumetric data; blood flow appears in the image as streamlines and is possible to see the direction of vectors. These new modalities can be used in the future as additional information to predict futures aortic related outcomes such as aneurysmal dilatation and FL later patency [55,56,57].
Clinical Management
Definitive treatment of AD depends of the affected segment of the aorta. For TAAD, surgical intervention is usually the recommended therapy due to the poor prognosis if left untreated; for TnABAD, open or endovascular intervention paired with debranching might be an option. TBAD have a significantly better prognosis and have a greater pool of treatment options. Either way, medical treatment is recommended for any type of AD [27, 42, 43, 58].
A fast diagnosis and referral of the patient to a specialized hospital with an aortic team can be the difference between life and death [59]. The creation of a dedicated fast pathway of referring patients, such as the “Aortentelefon” program, established in Germany, can reduce the time between onset of pain and treatment in up to 40% [60]. At the emergency care, the use of the AD detection risk score (ADDRS) has proven to be useful in helping physicians to properly diagnosis an acute AD [61•, 62].
The main goal of medical therapy in AD is to reduce shear stress on the diseased segment of the aorta, limiting propagation of the FL thus preventing compression of visceral trunks and also decreasing the pressure over the adventitia [38, 63]. These effects can be achieved by controlling BP and heart rate (HR) [4]. Initial management of HR with intravenous beta-blockers (such as labetolol, metoprolol, esmolol) is the cornerstone of the treatment [4, 38•, 63].
The second step is to control BP, initially with intravenous arterial dilatating drugs such as sodium nitroprusside, for being easily titratable and of rapid onset. Controlling HR first is desirable because these agents can induce reflex tachycardia, increasing left ventricle contraction force and leading to greater aortic wall stress [38•, 63]. Calcium channel blockers, such as diltiazem and verapamil, are suggested as an alternative for chronotropic control for people with contraindications or intolerance to beta-blockers, and can also be used to reduce BP. Morphine sulfate or buprenorphine are recommended for persistent pain control [4, 63, 64].
The main causes of shock in acute AD are cardiac tamponade, heart failure associated with severe aortic regurgitation, and hypovolemic shock due to aortic rupture [1]. Coronary involvement of the TAAD resulting in MI is a rare but devastating condition that occur in around 3% of the cases. The right coronary artery is most commonly affected [1, 65]. Due to its initial presentation, differentiating an isolated MI from AD (especially type A) is challenging. Initial ECG can show a wide variety of findings [1, 65, 66]. To rule out AD in those cases is crucial since MI medical therapy includes dual antiplatelet therapy and anticoagulation that can worsen AD surgical prognosis, with massive blood transfusion and its complications [66,67,68].
In TBAD cases, medical therapy must be the first approach. It is still controversial whether early endovascular repair can be beneficial in uncomplicated cases [38, 69]. The latest systematic review published about this subject, including 17 studies examining growth of the aorta, found that endovascular repair of AD (being it acute or chronic) did not alter the natural history of the disease in regard to late aneurysmal formation [70].
Surgical Management
Type A
Surgery is the treatment of choice in any TAAD, and it should be done as fast as possible, since mortality rate grows 1% per hour in these patients. Already in the first month, surgery can reduce mortality rates from 90 to 30% [1, 2, 10, 59, 71, 72, 73•, 74].
Although surgical mortality significantly increases in elderly population, being 70 or more age an independent risk factor for operative mortality, age per se should not be a factor to justify medical treatment alone. Even in octogenarians, mortality rate appears to be lower with surgical approach than conservative management [75].
The main goal of the surgery is, at the first, to diminish life-threatening aortic rupture, pericardial tamponade, and further progression of the dissection into coronary arteries or supra aortic branches resulting in obstructions [2, 10, 64]. Another initial goal is to relief the possible aortic valve involvement. Aortic valve regurgitation happens in 44% of the cases of TAAD, resulting of extension of the dissection into Valsalva sinuses or the valve itself, dilation of the aortic root leading to incomplete coaptation of the leaflets and prolapse of the dissection flap into the aortic valve [1, 4]. In most cases, the aortic valve is normal and can be preserved (Fig. 1d).
Techniques of valve sparing ascending aorta replacement are many and range in spectra from commissural resuspensions to Tirone’s valve-sparring aortic root replacement. If the valve cannot be preserved due to intrinsic valve pathology, extensive destruction, or root involvement, replacement of the root with a composite valve graft must be done [4, 6, 76].
Considering the increased risk in neurologic events during TAAD repair, major attention should be done in order to secure good brain protection [1, 2, 47, 59, 71]. Intraoperative neuromonitoring is fundamental to achieve good outcomes. The arsenal of options includes cerebral oximetry, bispectral index monitor, intraoperative electroencephalogram, and transcranial Doppler ultrasound. It can be useful during the hypothermic circulatory arrest period, giving insights about cerebral perfusion, and even providing evidence of an intraoperative stroke, which can be useful for more aggressive neuroprotective measures in the postoperative period [47, 77].
Up to 30% of the ET in TAAD is located in the aortic arch. Following the principle of tear-oriented surgery, definitive surgical treatment in those cases should count with an aortic arch intervention, either by total, hemiarch repair or hybrid approach [2, 6, 10, 72]. When the ET is located in the small curvature, an appeal to make the less aggressive approach exists, leaving an island of tissue for the supra aortic branches and repairing the small curvature. Although a less aggressive intervention can potentially reduce the mortality risk, it should be balanced by the fact that leaving diseased tissue raises the risk of late recurrence [72, 78••].
Unfortunately, the hemi arch approach cannot solve problems when the ET is located near the supra aortic branches. In these cases, total arch repair (TAR) or hybrid approach is the way to go [31]. Following 156 patients with acute DeBakey type 1 AD, and divided them in 4 groups (proximal aorta replacement only; concomitant TAR; concomitant TAR and stent grafting deployment in the descending aorta; and proximal aorta replacement with descending aorta stent grafting), Hsu et al. found that root reconstruction and TAR were significantly associated with in-hospital mortality, being TAR also associated with surgery-related stroke (OR 3.85; CI 95%: 1.22–12.15; P = 0.021 and OR 3.31; CI 95% 1.22–8.99; P = 0.019, respectively) [79].
Type B
The surgical approach to TBAD aims to close the ET in the aortic intima layer, resect or occlude any rupture in the aortic wall, preventing the spread of the FL. There are currently two types of surgical treatment for complicated TBAD: endovascular repair (TEVAR for thoracic and EVAR for abdominal) and open surgery repair (OSR) [2, 4, 34].
Indications that justify a more aggressive treatment in TBAD include the presence of complications. Although there is not yet a universally accepted definition for complicated TBAD [34, 39], malperfusion syndrome, hypotension, aortic rupture, refractory hypertension and refractory pain constitute a clearly indication for endovascular or surgical approach [34, 37, 41, 78••, 80].
The goal of OSR in patients with TBAD is to resect the segment of the aorta that the ET is situated, as well as any aortic aneurysm that may be present, replacing it with a Dacron tube, increasing blood flow to the true lumen thus improving distal ischemia. Usually, a left posterolateral thoracotomy is performed to expose the descending aorta, and cardiopulmonary bypass is achieved using the femoral artery and vein [34, 81, 82].
In the past years, medical communities are experiencing a shifting in indications for OSR. Past indications are now grade IA for endovascular repair. Part of this paradigm shifting is due to high mortality and morbidities rate related to OSR [39, 41]. In a large data analyses from 1529 patients with complicated TBAD submitted to OSR, early mortality rate was shown to range from 15.6 to 19.6% and 5-year survival rate ranged from 44 to 66.8%. Early stroke after surgery was 5.9% and spinal cord ischemia after was 3.3% [34, 39].
OSR is now being reserved for cases that cannot be repaired only with TEVAR. Causes of impossibilities include anatomically unsuitability for the endoprosthesis, malperfusion syndromes that cannot be addressed with fenestrated prosthesis and patients with connective tissue disorders [41, 80, 83].
Controversy regarding the use of TEVAR in patients with connective tissue disorders such as Marfan or Loeys-Dietz remains since the presence of these disease was an exclusion criteria in the clinical trials that evaluated the use of TEVAR in AD [83, 84]. The latest European guideline for TEVAR does not recommend its uses for patients with connective tissue diseases [80]. Indeed, in a small series of 16 Marfan patients treated with TEVAR for acute or chronic AD, Waterman and colleagues reported 44% of primary treatment failure with five patients who underwent open conversion. In a median follow-up of 9 months, mortality rate was 25%. Of note, according to the study, TEVAR in those patients is feasible, but questionable; and a more rational use of it is as a bridge to OSR [85, 86].
TEVAR works by deploying, via endovascular, an intra-aortic Dacron tube that stays in place using radial force, closing the ET. The blood flow is redirected into the true lumen, leading to improved distal perfusion. The absence of flow in the FL induces its thrombosis. Remodulation and stabilization of the dissected aorta can result in shrinkage of the aorta diameter and prevent late complications [2, 38, 70]. TEVAR offers the advantages of lower perioperative morbidity and mortality, shorter hospitalization, no need of cardiopulmonary bypass nor aortic cross clamping, lesser pain, and the obviously cosmetic aspect [41, 87].
Both TEVAR and OSR have generally similar risk of paraplegia due to perioperatory spinal cord ischemia (SCI), being the risk around 2–10%. Delayed paralysis/paresis (> 24 h after procedure) is more common after TEVAR and immediate paralysis to OSR [87,88,89]. The two main explanations to the pathophysiology behind SCI are inadequate remodeling of the collateral blood supply of the spinal cord and possible embolism of aortic plaques into supplying arteries of the spinal cord [87, 88].
SCI spectrum varies from transient paresis of one limb to full paraplegia (with 21% of risk in high-risk patients), with or without autonomic dysfunction—hypotension and bradycardia [87, 89]. Risk factors for SCI after TEVAR are related to a spinal cord blood supply stop and include coverage of the LSA, extensive coverage of long segments of the thoracic aorta, especially when T8-L1 are compromised, perioperative hypotension, and shaggy aorta [87, 88]. The LSA supplies the proximal portion of the spinal cord through the vertebral and internal thoracic arteries. When coverage of the LSA is intended, its revascularization should be made in order to lower the risk of SCI. Other strategies to prevent SCI include proper oxygenation, maintenance of periprocedural BP over 90 mmHg and cerebrospinal pressure < 10 mmHg, and avoidance of postoperative hypotension [4, 78, 87,88,89,90,91].
Cerebrospinal fluid drainage (CSF) is recommended for OSR. For TEVAR, there is no strong evidence to suggest its routine use. In a large systematic review with 4936 patients, Wong et al. were unable to stablish a prophylactic role in CSF drainage in TEVAR [4, 91]. Despite that, suggestions to use CSF drainage in high-risk patients exist [89].
PIS is a systemic inflammatory response observed after any interventional endovascular procedure with a prevalence of around 16% after TEVAR [92]. There are no universally accepted diagnostic criteria yet, but its signs and symptoms include fever and leukocytosis in the absence of site of infection, elevation in C-reactive protein, and back pain [92, 93•]. It seems to be triggered by exposition of the stent-graft material to the blood flow and manipulation of thrombus inside the aneurysmal sac [93•]. For treatment, first, infection should be ruled out as it is a catastrophic complication. After that, non-steroidal anti-inflammatory agents can be used to control the fever and pain [93•].
One of the most feared complications following TEVAR is retrograde type A aortic dissection (RTAAD). It is a deadly pathology, with mortality rate of 37.1% and incidence ranging between 1.33 and 17.9% [94••, 95,96,97]. RTAAD is associated with guide wire, catheters and delivery system mobilization, and balloon dilatation. The exact mechanism of its development is still controversial [94••, 95]. Despite that, some risk factors are known. In a recent meta-analysis with 50 studies totalizing 8969 patients, Chen et al. showed that acute AD (RR = 1.81 vs chronic AD), the use of proximal bare stent graft (RR = 2.06 vs non bare stent grafts), stent positioning in zone 0 (p < 0.0001) increased the risk of RTAAD. Less experienced centers also had more cases of RTAAD, as well as patients with Marfan syndrome [94••]. In another study, Canaud et al. found that excessive stent oversizing increased the risk of RTAAD, being each 1% above 9% increasing in 14% the risk of this complication [96].
The treatment of RTAAD requires replacement of the ascending aorta, supra aortic branches bypasses, and aortic arch correction. Hu and colleagues published 20 patients case report showing a modified technique to treat RTAAD, in which they inverted the Dacron graft inside the endoprosthesis, achieving better exposure and good distal suture view. This way, they found no need for retrieve the endoprosthesis or to use an elephant trunk in the descending aorta, avoiding reduction in aortic lumen and elephant trunk-stent graft complications [95].
Type Non-A/Non-B (TnABAD)
Regarding definitive therapy to the TnABAD, OSR or hybrid endovascular approach can be done, and the choice depends of the dissection’s anatomy. To choose the best option, carefully imaging exams should be done in order to check patency of the supra aortic arch branches, which segments of the aorta are compromised and where the ET is located, in order to properly close it [42].
Patients with descending ET type of TnABAD, and no signs of complications (aortic rupture, pleural or pericardial effusions, supra-aortic branches compressions, end-organ malperfusion), were classically initially managed conservatively as they had uncomplicated TBAD, although evidence suggest that the minority will remain treated this way and early intervention could be beneficial [26••, 43]. In a recent small study comparing patients treated conservatively versus OSR, poor mid- and long-term survival marked the conservative group, 3 out of 4 patients died in 28 months, being progression of the aortic dissection with malperfusion syndromes the main cause of death [27]. Also, the majority of these patients will require aortic intervention in emergency or urgency fashion, as demonstrated by Rylski et al. In a series of 43 patients with descending ET TnABAD, they reported emergency operation in 33% of the patients, reasons being aortic rupture, malperfusion syndromes, and TAAD suspicion; and within 2 weeks, 17 patients more needed urgency operation [26••].
The hybrid treatment of the aortic arch AD refers to a combination of supra aortic arch branches revascularization and TEVAR sealing of the aortic arch. In this way, depending of which supra aortic branches were involved, a wide variation of revascularization options can be performed. Common techniques include cervical bypass from the BCT to the left common carotid artery (LCCA) and then to the LSA—or just from the LCCA to the LSA if the BCT is healthy, and sternotomy followed by bypasses from the ascending aorta to those vessels. Anatomical transpositions can also be made, such as LSA disconnection from the aortic arch and anastomosed in the LCCA. In a second time or at the same procedure, using and hybrid operation room, an endoprosthesis can be deployed covering the aortic arch [78, 98]. In a large meta-analysis with a total of 956 patients with aortic arch pathologies (28.6% due to AD) which were submitted to total supra aortic arch debranching plus TEVAR, technical success, defined as complete debranching and successful stent-graft deployment, was estimated to be 92.8%. In-hospital mortality was 11.9% [98].
Limitations can happen when there is not an adequate diameter in the access vessel (commonly the femoral artery, < 7.0 mm), presence of anomalies in the aortic arch, or connective tissue syndromes [78, 99, 100]. Unsuitable landing zones can also impose a contra-indication, but even in an aneurysmal zone 0, a safety region can be made in order to land the endoprosthesis [101]. Complications include the ones mentioned in the TBAD section, such as access site complications, SCI, endoleak, PIS [78••, 92, 98].
Currently, recommendations for OSR with elephant trunk technique has become rare, being most of the time substituted for the frozen elephant trunk (FET) approach. Of note, when the true lumen is too thin and there is a risk for pseudocoarctation of the aorta, classical elephant trunk is preferable. In patients with high risk of RTAAD (ascending aorta > 38 mm, bicuspid aortic valve, lost sinotubular junction, and extended ascending aortic length), OSR should be also considered [78••].
FET refers to a combination of open aortic arch replacement and open deployment of an endoprosthesis in the descending aorta. It has the advantage of be held in place by circumferential suture, which eliminates the risk of proximal endoleak. This surgical proximal suture associated with endovascular sealing secures thrombosis of the FL and positive remodeling of the aorta [26••, 78••, 102]. It also can be used as a safety lading zone for a future planned intervention in the descending aorta [26••, 78••]. Disadvantages of the FET include the need of aortic cross clamping and cardiopulmonary bypass. Despite that, it can be indicated virtually in any situation [78••, 100].
Debates which kind of intervention is better remain. In a meta-analysis of four studies and 378 patients with aortic arch aneurysm, comparing OSR vs hybrid approach, Benedetto and colleagues found no significantly difference in early (OR 0.67; 95% CI 0.27–1.63; P = 0.92) and late (RR 1.73; 95% CI 0.9–3.3; P = 0.10) mortality between groups [103]. More recently, Trimarchi et al. analyzed data from the IRAD and found that in-hospital mortality in OSR of TnABAD—with no involvement of the ascending aorta—was higher than endovascular or medical treatment (30.8% vs 14.3% vs 13.9%, respectively). The major causes of deaths were neurological complications [42].
New Strategies
Since 2000, with the first described success case of human endovascular treatment of TAAD by Dorros and colleagues, new strategies have been pursued adopted [104, 105]. The ascending aorta, zone 0, was always the last frontier in percutaneous treatment of aortic aneurysms and dissections, due to its anatomical configuration and relationship with noble structures such as coronarian ostia, aortic valve, and left ventricle outflow tract. Little migration or mispositioning can provoke MI or aortic valve insufficiency (Fig. 1e) [104].
Li et al. proposed a set of criteria for elect deemed unsuitable for open surgery patients which zone 0 TEVAR could be beneficial, such as presence of previous sternotomy, important organic dysfunction (i.e., severe chronic obstructive pulmonary disease, renal or hepatic insufficiency, stroke), limiting comorbidities, and New York Heart Association functional class III or worse [106].
Early results are promising and show in-hospital mortality rates ranging from 0 to 14%. In a meta-analysis of 46 publications and accounting with 118 patients submitted to zone 0 TEVAR (50% due to TAAD), Muetterties et al. found 14.9% of mortality rate, with aorta-related mortality of 5%. Other complications included type I endoleak (18.2%), reintervention (9.3%), postoperative MI (3.4%), open conversion (3.4%), stroke (3.4%), and stent migration (1.7%) [107•]. In a CTA study of 167 TAAD patients, Kreibich and colleagues found that the ET was located in zone 0 in 131 patients, with 46% being in a potentially TEVAR covered zone and other 22% patients with the ET beyond the ascending aorta (zone 1 and 2) being also candidate to TEVAR treatment [100].
Limitations are due to anatomical requirements and devices models. Currently, there is an almost absence of standardized device in the industry. Adaptations exist, but the small length of the ascending aorta and its structures limit the use of usually long endoprosthesis. Also, some devices’ noses can damage the aortic valve during deployment, and some just do not have the length required in the delivery system to reach the ascending aorta [104]. Anatomical requirements for zone 0 TEVAR include: At least 2.0 cm of proximal landing zone, aortic diameter < 40 mm and sinutubular diameter < 38 mm, aortic angulation < 90°, minimum distance of ET and coronarian ostia > 20 mm, absence of bypass grafts in the proposed covered zone, and ET distal > 10 mm distal to sinutubular junction [104, 106, 108].
Recently, medical community witnessed the first in human “endo-Bentall” procedure, realized with a custom-made device that allowed stent graft implantation from the prosthesis to both coronary arteries [109].
Another option for AD is total endovascular aortic arch repair, which uses fenestration in the graft or branched stent grafts to promote blood flow to supra aortic branches. Both models are available only in custom-made orders. Anatomical requirements include diameter of landing zone < 38 mm and proximal landing zone length > 30 mm, absence of thrombus, or excess of calcification. Mortality rates ranges from 1.6 to 20% in the use of fenestrated devices and 0 to 6.7% in branched devices [78••, 110,111,112].
Conclusion
Acute AD is a large group of diseases that carries high mortality and morbidities to affected persons. Its wide spectrum of symptoms sometimes imposes a challenge in the precise diagnosis, which can delay the optimal treatment and consequently leads to worse outcomes.
With the past years, progress in physiopathological knowledge and advancement in imaging exams had made substantial the importance of a clear classification to better choose the definitive approach. New surgical and endovascular treatment options arisen and started to change the classical poor outcomes of some of the AD variants. In the future, we can hope for more advances in the medical technology to further reduce mortality and morbidity.
Abbreviations
- AD:
-
Aortic dissection
- FL:
-
False-lumen
- ET:
-
Entry tear
- IMH:
-
Intramural hematoma
- IRAD:
-
International Registry of Acute Aortic Dissection
- TnABAD:
-
Type non-A/non-B aortic dissection
- TAAD:
-
Type A aortic dissection
- TBAD:
-
Type B aortic dissection
- LSA:
-
Left subclavian artery
- BCT:
-
Brachiocephalic trunk
- LCCA:
-
Left common carotid artery
- ECG:
-
Electrocardiogram
- CTA:
-
Computed-Tomography Angiography
- TEE:
-
Transesophageal Echocardiography
- TTE:
-
Transthoracic Echocardiography
- MRI:
-
Magnetic Resonance Imaging
- BP:
-
Blood pressure
- HR:
-
Heart rate
- TAR:
-
Total arch repair
- TEVAR:
-
Thoracic endovascular aortic repair
- OSR:
-
Open surgery repair
- FET:
-
Frozen elephant trunk
- SCI:
-
Spinal cord ischemia
- CSF:
-
Cerebrospinal fluid drainage
- PIS:
-
Post-implantation syndrome
- RTAAD:
-
Retrograde type A aortic dissection
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. https://doi.org/10.1001/jama.283.7.897.
Fukui T. Management of acute aortic dissection and thoracic aortic rupture. J Intensive Care. 2018;6:15. https://doi.org/10.1186/s40560-018-0287-7.
Clouse WD, Hallett JW, Schaff HV, Spittell PC, Rowland CM, Ilstrup DM, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc. 2004;79(2):176–80. https://doi.org/10.4065/79.2.176.
Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of Aortic diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2873–926.
Nicholls F. Observations concerning the body of his late majesty, October 26, 1760. Philos Trans. 1761;52:265–75.
Chiu P, Miller DC. Evolution of surgical therapy for Stanford acute type A aortic dissection. Ann Cardiothorac Surg. 2016;5(4):275–95. https://doi.org/10.21037/acs.2016.05.05.
Criado FJ. Aortic dissection: a 250-year perspective. Tex Heart Inst J. 2011;38(6):694–700.
Achneck H, Modi B, Shaw C, Rizzo J, Albornoz G, Fusco D, et al. Ascending thoracic aneurysms are associated with decreased systemic atherosclerosis. Chest. 2005;128(3):1580–6. https://doi.org/10.1378/chest.128.3.1580.
Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management: part I: from etiology to diagnostic strategies. Circulation. 2003;108(5):628–35. https://doi.org/10.1161/01.CIR.0000087009.16755.E4.
Elsayed RS, Cohen RG, Fleischman F, Bowdish ME. Acute type A aortic dissection. Cardiol Clin. 2017;35(3):331–45. https://doi.org/10.1016/j.ccl.2017.03.004.
Harris KM, Braverman AC, Eagle KA, Woznicki EM, Pyeritz RE, Myrmel T, et al. Acute aortic intramural hematoma: an analysis from the International Registry of Acute Aortic Dissection. Circulation. 2012;126(11 Suppl 1):S91–6. https://doi.org/10.1161/CIRCULATIONAHA.111.084541.
Evangelista A, Mukherjee D, Mehta RH, O'Gara PT, Fattori R, Cooper JV, et al. Acute intramural hematoma of the aorta: a mystery in evolution. Circulation. 2005;111(8):1063–70. https://doi.org/10.1161/01.CIR.0000156444.26393.80.
Nakashima M, Kaji S, Murai R, et al. Detection of micro intimal tear at a very early stage in patients with acute aortic intramural hematoma. Circulation. 2016;134:A13989.
Wada H, Sakata N, Tashiro T. Clinicopathological study on penetrating atherosclerotic ulcers and aortic dissection: distinct pattern of development of initial event. Heart Vessel. 2016;31(11):1855–61. https://doi.org/10.1007/s00380-016-0813-2.
Taguchi E, Nishigami K, Miyamoto S, Sakamoto T, Nakao K. Impact of shear stress and atherosclerosis on entrancetear formation in patients with acute aortic syndromes. Heart Vessel. 2014;29:78–82. https://doi.org/10.1007/s00380-013-0328-z.
Larson EW, Edwards WD. Risk factors for aortic dissection: a necropsy study of 161 cases. Am J Cardiol. 1984;53:849–55. https://doi.org/10.1016/0002-9149(84)90418-1.
Weinsaft JW, Devereux RB, Preiss LR, Feher A, Roman MJ, Basson CT, et al. GENTAC Registry Investigators. Aortic Dissection in Patients With Genetically Mediated Aneurysms: Incidence and Predictors in the GenTAC Registry. J Am Coll Cardiol. 2016;67(23):2744–54. https://doi.org/10.1016/j.jacc.2016.03.570.
Isselbacher EM, Lino Cardenas CL, Lindsay ME. Hereditary influence in thoracic Aortic aneurysm and Dissection. Circulation. 2016;133(24):2516–28. https://doi.org/10.1161/CIRCULATIONAHA.116.009762.
Núñez-Gil IJ, Bautista D, Cerrato E, Salinas P, Varbella F, Omedè P, et al. Fernández-Ortiz a; Registry on Aortic Iatrogenic Dissection (RAID) Investigators. Incidence, management, and immediate- and long-term outcomes after iatrogenic aortic dissection during diagnostic or interventional coronary procedures. Circulation. 2015;131(24):2114–9. https://doi.org/10.1161/CIRCULATIONAHA.115.015334.
Li JC, Guan XL, Gong M, Zhang HJ. Iatrogenic aortic dissection during percutaneous coronary intervention: a case report and review of the literature. J Int Med Res. 2018;46(1):526–32. https://doi.org/10.1177/0300060517716342.
Shea N, Polanco AR, D'Angelo A, Bethancourt CN, Sanchez J, George I, et al. Improving outcomes of iatrogenic type A aortic dissection during cardiac surgery. Aorta (Stamford). 2019;7(4):115–20. https://doi.org/10.1055/s-0039-1695729.
Pasternak B, Inghammar M, Svanström H. Fluoroquinolone use and risk of aortic aneurysm and dissection: nationwide cohort study. BMJ. 2018;360:k678. https://doi.org/10.1136/bmj.k678.
• Rawla P, El Helou ML, Vellipuram AR. Fluoroquinolones and the risk of aortic aneurysm or aortic dissection: a systematic review and meta-analysis. Cardiovasc Hematol Agents Med Chem. 2019;17(1):3–10. https://doi.org/10.2174/1871525717666190402121958It provides new insights into the pathophysiology of AD.
DeBakey ME, Henly WS, Cooley DA, Morris GC Jr, Crawford ES, Beall AC Jr. Surgical management of dissecting aneurysms of the aorta. J Thorac Cardiovasc Surg. 1965;49:130–49.4.
Daily PO, Trueblood HW, Stinson EB, Wuerflein RD, Shumway NE. Management of acute aortic dissections. Ann Thorac Surg. 1970;10:237–47. https://doi.org/10.1016/s0003-4975(10)65594-4.
•• Rylski B, Pérez M, Beyersdorf F, Reser D, Kari FA, Siepe M, et al. Acute non-a non-B aortic dissection: incidence, treatment and outcome. Eur J Cardiothorac Surg. 2017;52:1111–7. https://doi.org/10.1093/ejcts/ezx142This article clarifies the importance of distinction between aortic arch dissections and classic type A or type B dissections.
Urbanski PP, Wagner M. Acute non-A-non-B aortic dissection: surgical or conservative approach? Eur J Cardiothorac Surg. 2016 Apr;49(4):1249–54. https://doi.org/10.1093/ejcts/ezv301.
• Sievers H-H, Rylski B, Czerny M, Baier ALM, Kreibich M, Siepe M, et al. Aortic dissection reconsidered: type, entry site, malperfusion classification adding clarity and enabling outcome prediction. Interact Cardiovasc Thorac Surg. 2019;30:451. https://doi.org/10.1093/icvts/ivz281A new way to better predict outcome following acute aortic dissections.
•• Roselli EE, Idrees JJ, Johnston DR, Eagleton MJ, Desai MY, Svensson LG. Zone zero TEVAR: a proposed modification to the classification of landing zones. J Thorac Cardiovasc Surg. 2017. https://doi.org/10.1016/j.jtcvs.2017.11.054A new classification for entry tear in the ascending aorta.
Fillinger MF, Greenberg RK, McKinsey JF, Chaikof EL. Reporting standards for thoracic endovascular aortic repair (TEVAR). J Vasc Surg. 2010;52(4):1022–1033.e5. https://doi.org/10.1016/j.jvs.2010.07.008.
Merkle J, Sabashnikov A, Deppe AC, Zeriouh M, Maier J, Weber C, et al. Impact of ascending aortic, hemiarch and arch repair on early and long-term outcomes in patients with Stanford A acute aortic dissection. Ther Adv Cardiovasc Dis. 2018;12(12):327–40. https://doi.org/10.1177/1753944718801568.
Mehta RH, Suzuki T, Hagan PG, Bossone E, Gilon D, Llovet A, et al. Predicting death in patients with acute type A aortic dissection. Circulation. 2002;105:200–6. https://doi.org/10.1161/hc0202.102246.
Dake MD, Thompson M, van Sambeek M, Vermassen F, Morales JP, Investigators DEFINE. DISSECT: a new mnemonic-based approach to the categorization of aortic dissection. Eur J Vasc Endovasc Surg. 2013;46:175–90. https://doi.org/10.1016/j.ejvs.2013.04.029.
Fattori R, Cao P, De Rango P, Czerny M, Evangelista A, Nienaber C, et al. Interdisciplinary expert consensus document on management of type B aortic dissection. J Am Coll Cardiol. 2013;61(16):1661–78. https://doi.org/10.1016/j.jacc.2012.11.072.
Afifi RO, Sandhu HK, Leake SS, Boutrous ML, Kumar V 3rd, Azizzadeh A, et al. Outcomes of patients with acute type B (DeBakey III) aortic dissection: a 13-year, single-center experience. Circulation. 2015;132(8):748–54. https://doi.org/10.1161/CIRCULATIONAHA.115.015302.
Reutersberg B, Trenner M, Haller B, Geisbüsch S, Reeps C, Eckstein H-H. The incidence of delayed complications in acute type B aortic dissections is underestimated. J Vasc Surg. 2018;68(2):356–63. https://doi.org/10.1016/j.jvs.2017.11.089.
Kaji S. Update on the therapeutic strategy of type B aortic dissection. J Atheroscler Thromb. 2018;25(3):203–12. https://doi.org/10.5551/jat.rv17017.
• Tadros RO, Tang GHL, Barnes HJ, Mousavi I, Kovacic JC, Faries P, et al. Optimal treatment of uncomplicated Type B Aortic Dissection: JACC review topic of the week. J Am Coll Cardiol. 2019;74(11):1494–504. https://doi.org/10.1016/j.jacc.2019.07.063A review of different options in type B aortic dissection treatment.
Nienaber CA, Clough RE. Management of acute aortic dissection. Lancet. 2015;385(9970):800–11. https://doi.org/10.1016/S0140-6736(14)61005-9.
Crawford TC, Beaulieu RJ, Ehlert BA, Ratchford EV, Black JH 3rd. Malperfusion syndromes in aortic dissections. Vasc Med. 2016;21:264–73. https://doi.org/10.1177/1358863X15625371.
Hogendoorn W, Hunink MG, Schlösser FJ, Moll FL, Sumpio BE, Muhs BE. Endovascular vs. open repair of complicated acute type B aortic dissections. J Endovasc Ther. 2014;21(4):503–14. https://doi.org/10.1583/14-4716R.1.
Trimarchi S, de Beaufort HWL, Tolenaar JL, Bavaria JE, Desai ND, Di Eusanio M, et al. Acute aortic dissections with entry tear in the arch: a report from the international Registry of acute Aortic Dissection. J Thorac Cardiovasc Surg. 2019 Jan;157(1):66–73. https://doi.org/10.1016/j.jtcvs.2018.07.101.
Leshnower BG. Non-a, non-B aortic dissections: unresolved issues. J Thorac Cardiovasc Surg. 2019;157(1):74. https://doi.org/10.1016/j.jtcvs.2018.08.016.
Manea MM, Dragos D, Antonescu F, Sirbu AG, Tiron AT, Dobri AM, et al. Aortic dissection: an easily missed diagnosis when pain doesn't hold the stage. Am J Case Rep. 2019;20:1788–92. https://doi.org/10.12659/AJCR.917179.
Shiga T, Wajima Z, Apfel CC, Inoue T, Ohe Y. Diagnostic accuracy of transesophageal echocardiography, helical computed tomography, and magnetic resonance imaging for suspected thoracic aortic dissection: systematic review and meta-analysis. Arch Intern Med. 2006;166:1350–6. https://doi.org/10.1001/archinte.166.13.1350.
Duran ES, Ahmad F, Elshikh M, et al. Computed tomography imaging findings of acute aortic pathologies. Cureus. 2019;11(8):e5534. https://doi.org/10.7759/cureus.5534.
Leshnower BG. Cannulation strategies, circulation management and neuroprotection for type A intramural hematoma: tips and tricks. Ann Cardiothorac Surg. 2019;8(5):561–6. https://doi.org/10.21037/acs.2019.08.08.
McMahon MA, Squirrell CA. Multidetector CT of aortic dissection: a pictorial review. Radiographics. 30(2):445–60. https://doi.org/10.1148/rg.302095104.
Nienaber CA. The role of imaging in acute aortic syndromes. Eur Heart J Cardiovasc Imaging. 2013;14(1):15–23. https://doi.org/10.1093/ehjci/jes215.
Nishigami K. Update on cardiovascular echo in aortic aneurysm and dissection. Ann Vasc Dis. 2018;11(4):437–42. https://doi.org/10.3400/avd.ra.18-00112.
Evangelista A, Maldonado G, Gruosso D, Gutiérrez L, Granato C, Villalva N, et al. The current role of echocardiography in acute aortic syndrome. Echo Res Pract. 2019;6(2):R53–63. https://doi.org/10.1530/ERP-18-0058.
Koschyk DH, Nienaber CA, Knap M, Hofmann T, Kodolitsch YV, Skriabina V, et al. How to guide stent-graft implantation in type B aortic dissection? Comparison of angiography, transesophageal echocardiography, and intravascular ultrasound. Circulation. 2005;112(9 Suppl):I260–4. https://doi.org/10.1161/CIRCULATIONAHA.104.525972.
Nienaber CA, Kische S, Skriabina V, Ince H. Noninvasive imaging approaches to evaluate the patient with known or suspected aortic disease. Circ Cardiovasc Imaging, 2009, vol. 2 (pg. 499-506). DOI: https://doi.org/10.1161/CIRCIMAGING.109.850206.
Evangelista A, Flachskampf FA, Erbel R, Antonini-Canterin F, Vlachopoulos C, Rocchi G, et al. Echocardiography in aortic diseases: EAE recommendations for clinical practice. Eur J Echocardiogr. 2010;11:645–58. https://doi.org/10.1093/ejechocard/jeq056.
Karmonik C, Müller-Eschner M, Partovi S, Geisbüsch P, Ganten MK, Bismuth J, et al. Computational fluid dynamics investigation of chronic aortic dissection hemodynamics versus normal aorta. Vasc Endovasc Surg. 2013;47(8):625–31. https://doi.org/10.1177/1538574413503561.
Sherrah AG, Grieve SM, Jeremy RW, Bannon PG, Vallely MP, Puranik R. MRI in chronic aortic dissection: a systematic review and future directions. Front Cardiovasc Med. 2015;2:5. Published 2015 Feb 19. https://doi.org/10.3389/fcvm.2015.00005.
François CJ, Markl M, Schiebler ML, Niespodzany E, Landgraf BR, Schlensak C, et al. Four-dimensional, flow-sensitive magnetic resonance imaging of blood flow patterns in thoracic aortic dissections. J Thorac Cardiovasc Surg. 2013;145(5):1359–66. https://doi.org/10.1016/j.jtcvs.2012.07.019.
LeMaire SA, Russell L. Epidemiology of thoracic aortic dissection. Nat Rev Cardiol. 2011;8(2):103–13. https://doi.org/10.1038/nrcardio.2010.187.
Pepper J. Differential aspects of the disease and treatment of thoracic acute aortic dissection (TAAD)—the European experience. Ann Cardiothorac Surg. 2016;5(4):360–7. https://doi.org/10.21037/acs.2016.06.05.
Zschaler S, Schmidt G, Kurz SD. Aortentelefon: the Berlin project aiming for shorter response times and sharper diagnostic accuracy in acute type A aortic dissection (ATAAD). Cardiovasc Diagn Ther. 2018;8:811. https://doi.org/10.21037/cdt.2018.09.19.
•• Zaschke L, Habazettl H, Thurau J, Matschilles C, Göhlich A, Montagner M, Falk V, Kurz SD. Acute type a aortic dissection: Aortic Dissection detection risk score in emergency care – surgical delay because of initial misdiagnosis. Eur Heart J Acute Cardiovasc Care 1-8. DOI: https://doi.org/10.1177/2048872620914931. The importance of early and correct diagnosis to successful treatment of acute aortic dissections.
Salmasi MY, Al-Saadi N, Hartley P, et al. The risk of misdiagnosis in acute thoracic aortic dissection: a review of current guidelines. Heart Published Online First: 13 March 2020. DOI: https://doi.org/10.1136/heartjnl-2019-316322.
Suzuki T, Eagle KA, Bossone E, Ballotta A, Froehlich JB, Isselbacher EM. Medical management in type B aortic dissection. Ann Cardiothorac Surg. 2014;3(4):413–7. https://doi.org/10.3978/j.issn.2225-319X.2014.07.01.
Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management: part II: therapeutic management and follow-up. Circulation. 200;108(6):772–8. https://doi.org/10.1161/01.CIR.0000087400.48663.19.
Wu BT, Li CY, Chen YT. Type A aortic dissection presenting with inferior ST-elevation myocardial infarction. Acta Cardiol Sin. 2014;30(3):248–52.
Lentini S, Perrotta S. Aortic dissection with concomitant acute myocardial infarction: from diagnosis to management. J Emerg Trauma Shock. 2011;4(2):273–8. https://doi.org/10.4103/0974-2700.82221.
Zschaler S, Schmidt G, Kukucka M, Syrmas G, Zaschke L, Kurz SD. How to prevent inadvertent emergency anticoagulation in acute type A aortic dissection: when in doubt, don't. Cardiovasc Diagn Ther. 2018;8(6):805–10. https://doi.org/10.21037/cdt.2018.10.13.
Cannesson M, Burckard E, Lefèvre M, Bastien O, Lehot JJ. Predictors of in-hospital mortality in the surgical management of acute type a aortic dissections: impact of anticoagulant therapies. Ann Fr Anesth Reanim. 2004;23:568–74. https://doi.org/10.1016/j.annfar.2004.03.009.
Nienaber CA, Kische S, Rousseau H, Eggebrecht H, Rehders TC, Kundt G, et al. Ince H; INSTEAD-XL trial. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv. 2013;6(4):407–16. https://doi.org/10.1161/CIRCINTERVENTIONS.113.000463.
Famularo M, Meyermann K, Lombardi JV. Aneurysmal degeneration of type B aortic dissections after thoracic endovascular aortic repair: a systematic review. J Vasc Surg. 2017;66(3):924–30. https://doi.org/10.1016/j.jvs.2017.06.067.
Chiappini B, Schepens M, Tan E, Amore AD', Morshuis W, Dossche K, et al. Early and late outcomes of acute type a aortic dissection: analysis of risk factors in 487 consecutive patients. Eur Heart J. 2005;26:180–6. https://doi.org/10.1093/eurheartj/ehi024.
Okita Y. Current surgical results of acute type A aortic dissection in Japan. Ann Cardiothorac Surg. 2016;5(4):368–76. https://doi.org/10.21037/acs.2016.06.02.
Kim JH, Choi JB, Kim TY, Kim KH, Kuh JH. Simplified surgical approach to improve surgical outcomes in the center with a small volume of acute type A aortic dissection surgery. Technol Health Care. 2018;26(4):675–85. https://doi.org/10.3233/THC-171169 Simplified surgery in small centers may be beneficial.
Trimarchi S, Nienaber CA, Rampoldi V, Myrmel T, Suzuki T, Mehta RH, et al. Contemporary results of surgery in acute type A aortic dissection: the International Registry of Acute Aortic Dissection experience. J Thorac Cardiovasc Surg. 2005;129:112–22. https://doi.org/10.1016/j.jtcvs.2004.09.005.
Trimarchi S, Eagle KA, Nienaber CA, Rampoldi V, Jonker FH, De Vincentiis C, et al. Role of age in acute type A aortic dissection outcome: report from the International Registry of Acute Aortic Dissection (IRAD). J Thorac Cardiovasc Surg. 2010;140:784–9. https://doi.org/10.1016/j.jtcvs.2009.11.014.
Aubin H, Akhyari P, Rellecke P, Pawlitza C, Petrov G, Lichtenberg A, et al. Valve-sparing aortic root replacement as first-choice strategy in acute Type a aortic dissection. Front Surg. 2019;6:46. https://doi.org/10.3389/fsurg.2019.00046.
Kowalczyk AK, Bachar BJ, Liu H. Neuromonitoring during adult cardiac surgery. J Biomed Res. 2016;30(3):171–3. https://doi.org/10.7555/JBR.30.20150159.
•• Czerny M, Schmidli J, Adler S, van den Berg JC, Bertoglio L, Carrel T, et al. Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus document of the European Association for Cardio-Thoracic surgery (EACTS) and the European Society for Vascular Surgery (ESVS). Eur J Cardiothorac Surg. 2018;55:133. https://doi.org/10.1093/ejcts/ezy313Updated version of recommendations for the treatment of aortic arch dissections.
Hsu CP, Huang CY, Wu FY. Relationship between the extent of aortic replacement and stent graft for acute DeBakey type I aortic dissection and outcomes: results from a medical center in Taiwan. PLoS One. 2019;14(1):e0210022. https://doi.org/10.1371/journal.pone.0210022.
Grabenwöger M, Alfonso F, Bachet J, Bonser R, Czerny M, Eggebrecht H, et al. Thoracic Endovascular Aortic Repair (TEVAR) for the treatment of aortic diseases: a position statement from the European Association for Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2012;33(13):1558–63. https://doi.org/10.1093/eurheartj/ehs074.
Fattori R, Tsai TT, Myrmel T, Evangelista A, Cooper JV, Trimarchi S, et al. Complicated acute type B dissection: is surgery still the best option? A report from the International Registry of Acute Aortic Dissection. JACC Cardiovasc Interv. 2008;1(4):395–402. https://doi.org/10.1016/j.jcin.2008.04.009.
Lansman SL, Hagl C, Fink D, Galla JD, Spielvogel D, Ergin MA, et al. Acute type B aortic dissection: surgical therapy. Ann Thorac Surg. 2002;74:S1833–5 discussionS1857-S1863.
Ray HM, Charlton-Ouw KM, Miller CC 3rd, Estrera AL, Safi HJ. Open repair of complicated acute type B aortic dissection. Ital J Vasc Endovasc. 2018;25(4):320–31. https://doi.org/10.23736/S1824-4777.18.01344-X.
Kilic A, Shah AS, Black JH 3rd, Whitman GJ, Yuh DD, Cameron DE, et al. Trends in repair of intact and ruptured descending thoracic aortic aneurysms in the United States: a population-based analysis. J Thorac Cardiovasc Surg. 2014;147(6):1855–60. https://doi.org/10.1016/j.jtcvs.2013.06.032.
Waterman AL, Feezor RJ, Lee WA, Hess PJ, Beaver TM, Martin TD, et al. Endovascular treatment of acute and chronic aortic pathology in patients with Marfan syndrome. J Vasc Surg. 2012;55(5):1234–40; disucssion 1240–1. https://doi.org/10.1016/j.jvs.2011.11.089.
Geisbüsch P, Kotelis D, von Tengg-Kobligk H, Hyhlik-Dürr A, Allenberg JR, Böckler D. Thoracic aortic endografting in patients with connective tissue diseases. J Endovasc Ther. 2008 Apr;15(2):144–9. https://doi.org/10.1583/07-2286.1.
Awad H, Ramadan ME, El Sayed HF, Tolpin DA, Tili E, Collard CD. Spinal cord injury after thoracic endovascular aortic aneurysm repair. Lésion de la moelle épinière après réparation endovasculaire d’un anévrisme de l’aorte thoracique. Can J Anaesth. 2017;64(12):1218–35. https://doi.org/10.1007/s12630-017-0974-1.
Uchida N. How to prevent spinal cord injury during endovascular repair of thoracic aortic disease. Gen Thorac Cardiovasc Surg. 2014;62(7):391–7. https://doi.org/10.1007/s11748-014-0395-9.
Dijkstra ML, Vainas T, Zeebregts CJ, Hooft L, van der Laan MJ. Editor's choice - spinal cord ischaemia in endovascular thoracic and thoraco-abdominal aortic repair: review of preventive strategies. Eur J Vasc Endovasc Surg. 2018;55(6):829–41. https://doi.org/10.1016/j.ejvs.2018.02.002.
Xue L, Luo S, Ding H, et al. Risk of spinal cord ischemia after thoracic endovascular aortic repair. J Thorac Dis. 2018;10(11):6088–96. https://doi.org/10.21037/jtd.2018.10.99.
Wong CS, Healy D, Canning C, Coffey JC, Boyle JR, Walsh SR. A systematic review of spinal cord injury and cerebrospinal fluid drainage after thoracic aortic endografting. J Vasc Surg. 2012;56(5):1438–47. https://doi.org/10.1016/j.jvs.2012.05.075.
Gorla R, Erbel R, Kahlert P, Tsagakis K, Jakob H, Mahabadi AA, et al. Clinical features and prognostic value of stent-graft-induced post-implantation syndrome after thoracic endovascular aortic repair in patients with type B acute aortic syndromes. Eur J Cardiothorac Surg. 2016;49(4):1239–47. https://doi.org/10.1093/ejcts/ezv355.
• Gorla R, Erbel R, Eagle KA, Bossone E. Systemic inflammatory response syndromes in the era of interventional cardiology. Vasc Pharmacol. 2018. https://doi.org/10.1016/j.vph.2018.04.003IIt is important to understand this, every day more common, entity.
•• Chen Y, Zhang S, Liu L, Lu Q, Zhang T, Jing Z. Retrograde Type A Aortic dissection after thoracic endovascular aortic repair: a systematic review and meta-analysis. J Am Heart Assoc. 2017;6(9). DOI: https://doi.org/10.1161/JAHA.116.004649. It draws attention to this catastrophic complication.
Hu W, Zhang Y, Guo L, Fan J, Lu Y, Ma L. A graft inversion technique for retrograde type A aortic dissection after thoracic endovascular repair for type B aortic dissection. J Cardiothorac Surg. 2019;14(1):29. https://doi.org/10.1186/s13019-019-0851-9.
Canaud L, Ozdemir BA, Patterson BO, Holt PJ, Loftus IM, Thompson MM. Retrograde aortic dissection after thoracic endovascular aortic repair. Ann Surg. 2014;260:389–95. https://doi.org/10.1097/SLA.0000000000000585.
Neuhauser B, Greiner A, Jaschke W, Chemelli A, Fraedrich G. Serious complications following endovascular thoracic aortic stent-graft repair for type B dissection. Eur J Cardiothorac Surg. 2008;33:58–63. https://doi.org/10.1016/j.ejcts.2007.10.010.
Moulakakis KG, Mylonas SN, Markatis F, Kotsis T, Kakisis J, Liapis CD. A systematic review and meta-analysis of hybrid aortic arch replacement. Ann Cardiothorac Surg. 2013;2:247–60. https://doi.org/10.3978/j.issn.2225-319X.2013.05.06.
Ince H, Rehders TC, Petzsch M, Kische S, Nienaber CA. Stent-grafts in patients with Marfan syndrome. J Endovasc Ther. 2005;12:82–8. https://doi.org/10.1583/04-1415MR.1.
Kreibich M, Berger T, Morlock J, Kondov S, Scheumann J, Kari FA, et al. The frozen elephant trunk technique for the treatment of acute complicated Type B aortic dissection. Eur J Cardiothorac Surg. 2018;53:525–30. https://doi.org/10.1093/ejcts/ezx281.
Soares AMMN, Sá MPBO, Escorel Neto AC, Cavalcanti LRP, Zhigalov K, Weymann A, et al. Wrapping of ascending aortic aneurysm with supra-aortic debranching and endovascular repair for aortic arch aneurysm and ruptured descending thoracic aortic aneurysm. J Card Surg. 2020;35(2):503–6. https://doi.org/10.1111/jocs.14406.
Dohle D-S, Tsagakis K, Janosi RA, Benedik J, Kühl H, Penkova L, et al. Aortic remodelling in aortic dissection after frozen elephant trunk. Eur J Cardiothorac Surg. 2016;49:111–7. https://doi.org/10.1093/ejcts/ezv045.
Benedetto U, Melina G, Angeloni E, Codispoti M, Sinatra R. Current results of open total arch replacement versus hybrid thoracic endovascular aortic repair for aortic arch aneurysm: a meta-analysis of comparative studies. J Thorac Cardiovasc Surg. 2013;145(1):305–6. https://doi.org/10.1016/j.jtcvs.2012.09.011.
Saadi EK, Tagliari AP, Almeida RMS. Endovascular treatment of the ascending aorta: is this the last frontier in aortic surgery? Braz J Cardiovasc Surg. 2020;34(6):759–64. Published 2020 Jan 1. https://doi.org/10.21470/1678-9741-2019-0317.
Dorros G, Dorros AM, Planton S, O'Hair D, Zayed M. Transseptal guidewire stabilization facilitates stent-graft deployment for persistent proximal ascending aortic dissection. J Endovasc Ther. 2000;7(6):506–12. https://doi.org/10.1177/152660280000700612.
Li Z, Lu Q, Feng R, Zhou J, Zhao Z, Bao J, et al. Outcomes of endovascular repair of ascending aortic dissection in patients unsuitable for direct surgical repair. J Am Coll Cardiol. 2016;68(18):1944–54. https://doi.org/10.1016/j.jacc.2016.08.031.
• Muetterties CE, Menon R, Wheatley GH 3rd. A systematic review of primary endovascular repair of the ascending aorta. J Vasc Surg. 2018;67(1):332–42. https://doi.org/10.1016/j.jvs.2017.06.099TEVAR in zone 0 as a new option to treat type A aortic dissection in selected patients.
Moon MC, Greenberg RK, Morales JP, Martin Z, Lu Q, Dowdall JF, et al. Computed tomography-based anatomic characterization of proximal aortic dissection with consideration for endovascular candidacy. J Vasc Surg. 2011;53(4):942–9. https://doi.org/10.1016/j.jvs.2010.10.067.
Gaia DF, Bernal O, Castilho E, Ferreira CBND, Dvir D, Simonato M, et al. First-in-human endo-bentall procedure for simultaneous treatment of the ascending aorta and aortic valve. J Am Coll Cardiol Case Rep. 2(3):480–5. https://doi.org/10.1016/j.jaccas.2019.11.071.
Yokoi Y, Azuma T, Yamazaki K. Advantage of a precurved fenestrated endograft for aortic arch disease: simplified arch aneurysm treatment in Japan 2010 and 2011. J Thorac Cardiovasc Surg. 2013;145:S103–9. https://doi.org/10.1016/j.jtcvs.2012.11.058.
Tsilimparis N, Debus ES, von Kodolitsch Y, Wipper S, Rohlffs F, Detter C, et al. Branched versus fenestrated endografts for endovascular repair of aortic arch lesions. J Vasc Surg. 2016;64(3):592–9. https://doi.org/10.1016/j.jvs.2016.03.410.
Czerny M, Rylski B, Morlock J, Schröfel H, Beyersdorf F, Saint Lebes B, et al. Orthotopic branched endovascular aortic arch repair in patients who cannot undergo classical surgery. Eur J Cardiothorac Surg. 2018;53(5):1007–12. https://doi.org/10.1093/ejcts/ezx493.
Funding
The present contribution did not receive any external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Cardiovascular Care
Rights and permissions
About this article
Cite this article
Cavalcanti, L.R.P., Sá, M.P.B.O., Campos, J.C.S. et al. Acute Aortic Dissection: an Update. Curr Emerg Hosp Med Rep 8, 90–102 (2020). https://doi.org/10.1007/s40138-020-00216-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40138-020-00216-3