Abstract
Acute aortic dissection is a life-threatening condition associated with high morbidity and mortality rates and a long history of challenges to both diagnose and manage this condition successfully. The International Registry of Acute Aortic Dissection (IRAD) was established in 1996 with the mission to raise awareness of this condition and provide insights to improve and guide diagnosis and treatment. Since then, more than 6500 cases have been included in more than 40 sites located in 12 countries. Although presenting symptoms and physical findings have not changed significantly over two decades, the use of computed tomography in the diagnosis has increased. Moreover, more patients are managed with interventional procedures: surgery in type A and endovascular therapy in type B, with these changes in diagnostic testing and care, there has been significant decrease in overall in-hospital mortality in type A but not in type B. Herein, we summarise the key lessons learnt from this IRAD over the past 20 years.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Acute aortic dissection (AAD) is a life-threatening condition with a population-based incidence of ~30 per 100,000 person-year in the elderly (>65 years) population. The International Registry of Acute Aortic Dissection (IRAD) was established in 1996 with the idea to create a new understanding of this old disease including demographics, presenting history, physical examination, imaging studies, and management.
The conception and implementation of the IRAD database under the direction of Drs. Eric Isselbacher, Christoph Nienaber, and Kim Eagle represented a practical resource has contributed immensely to the advancement in the management of aortic diseases with more than 80 peer-reviewed publications. Since its inception, IRAD has expanded to 43 active sites in 12 countries in the USA, Europe, and Asia. A recent trend analysis has shown that clinical presentation of acute aortic dissection had not changed. However, the use of computed tomography (CT) as a diagnostic modality increased; the role of chest X-rays in diagnosis of acute dissection diminished; surgical results for type A and type B aortic dissection improved; endovascular therapies were applied increasingly for type B dissection; beta blockers, angiotensin receptor blockers (ARBs), diuretics, and statins were prescribed more frequently at hospital discharge following acute aortic dissection [1]; and overall hospital mortality had improved for type A aortic dissection but less so for type B aortic dissection (Table 20.1).
IRAD was inaugurated with the mission to improve the care of dissection patients worldwide, in the expectation that information from a large registry will influence diagnostic and therapeutic management in the years to come.
However, there are drawbacks as IRAD data are collected retrospectively, there is no core laboratory for image analysis, and the tertiary referral nature of the IRAD centres impairs its ability to be “representative of all patients with acute aortic dissection”. IRAD information comes from a referral hospital basis rather than a community population basis, with subsequently some inherent potential for misleading statistics [2]. It would be helpful to have population-based information in addition to knowledge of non-consecutive dissected patients only. Nevertheless, IRAD has shed tremendous light on the “silent killer” and “great masquerader” or aortic dissection.
2 Demographics at the Start of the Twenty-First Century
In the IRAD, 67% of patients presented with type A dissection and 33% with type B; two-thirds of patients were men, with an average age of 63 years. Many risk factors were related to aortic dissection, with hypertension (76.6%) being the most common [1, 3]. Twice as many hypertensive women than men older than 70 years experienced an acute aortic syndrome [4]. Iatrogenic dissection occurred in 3.3% of patients [5], cocaine use was recorded in 1.8% [6, 7], and 17% of patients with type A dissection had previous cardiac surgery [8]. Black patients who were younger (mean age 55 years) suffered more frequently (52.4%) from type B dissection and more likely to have a history of cocaine abuse (12%), hypertension (89.7%), and diabetes (13.2%) [9]. Young patients <40 years of age were less likely to have a history of hypertension (34%) or atherosclerosis (1%) but more likely of Marfan’s syndrome or bicuspid aortic valve (59%) [10]. Although dilation of the ascending aorta is a well-established risk factor for dissection, the maximum ascending aortic diameters in acute phase averaged at about 5.3 cm, and 60% of patients had aortic diameters <5.5 cm and 40% even less than 5.0 cm. Thus, current surgical guidelines for thoracic aortic aneurysm repair (<5.5 cm) would miss the majority of type A dissection occurring in this cohort [11]. Similarly, only 18.4% of patients with type B aortic dissection had an aortic diameter ≥ 5.5 cm [12], and in 21% the maximum aortic diameter was <3.5 cm [13]. The occurrence of dissection showed a circadian variation, with a peak in the morning and also higher incidence during cold winter months [14,15,16].
3 Clinical Symptomatology
A sudden onset of severe chest or back pain continues to be the most frequent symptom. Chest pain is more common in patients with type A dissections (79 versus 63% of type B dissections), whereas back pain is more common in type B dissections (43%) [3]. However, ripping and migrating pain was a common feature in all cases. Patients who presented primarily with acute abdominal pain (4.6%) tended to be diagnosed later and had a higher mortality than those with more typical symptoms [16, 17]. Similarly, those with painless dissection (6.3%) presented often with syncope, congestive heart failure or stroke and a higher mortality risk [17, 18]. Syncope was reported in 13%, 19% in type A dissection, often indicating complications such as cardiac tamponade, obstruction of cerebral vessels, or stroke [18, 19]. Hypertension at presentation was more frequent in type B than in type A dissection (70 vs. 36%) [1, 3]. A diastolic murmur of aortic insufficiency was present at the time of presentation in 40% of type A dissections [1, 3], hypotension in >25% [19, 20], and congestive heart failure in 6% [20, 21]. A pulse differential is proven to be an important diagnostic finding [21, 22] and should raise the suspicions of acute aortic dissection.
4 Diagnostic Pathways
In view of a high early mortality, swift diagnostic work-up for dissection is essential for successful outcomes. Nevertheless, it is known that the relative infrequency of acute aortic syndrome with symptoms mimicking more common problems, such as angina pectoris, myocardial infarction, vascular embolisation, and abdominal conditions, can impede prompt diagnosis [23,24,25]. Therefore, a high degree of suspicion is warranted in patients with risk factors or suggestive symptoms and signs at presentation.
In the setting of acute dissection, an ischemic ECG is seen in more than 25% of patients, resulting either from hypotension, compromise of coronary ostia, or pre-existing coronary disease [3]. In IRAD an ECG with non-specific ST and T-wave changes is seen in 42%, ischaemic changes in 15% and evidence of an acute myocardial infarction in 5% of proximal dissection. Not surprisingly, an abnormal ECG leads to delays in diagnosis [26] and possibly to useless and dangerous invasive coronary angiography, rather than undelayed CT imaging.
Similarly, a normal chest X-ray can actually delay proper diagnosis by CT which should be used rather early than late in patients with chest and/or back pain. Although typical radiographic findings of type A dissection include widening of the mediastinum, in >20% of patients with confirmed proximal dissection, a normal mediastinum or aortic contour is present on chest pain or even CT [3, 26].
4.1 Imaging
Initial diagnostic imaging, today, is performed by CT in 69% of cases, echocardiography in 25%, magnetic resonance imaging (MRI) in 4%, and aortography in 2–3% [26]. Highly prolific availability and faster scanning may account for the greater utilisation of CT [27]. The use of transoesophageal echocardiography (TEE) as diagnostic imaging study has decreased over time [1]. Diagnosis of type A aortic dissection was substantially delayed in North Americans compared with Europeans; however, no significant differences in early mortality rates were observed between the two groups [28]. Preoperative coronary arteriography was rarely performed in patients with aortic dissection (11%) except, on occasions, in those at high risk for or with confirmed myocardial ischaemia [29]. Some imaging findings also have prognostic value and are useful for improving management strategy, such as pericardial effusion (p = 0.04), tamponade (p < 0.01), periaortic haematoma (p = 0.02), and patent false lumen (p = 0.08) all more frequent in non-survivors [30].
4.2 Symptoms and Biomarkers
Plasma biomarkers, except for D-dimer are not yet available in emergency rooms [31]. The widely used D-dimer cutoff level of 500 ng/mL for ruling out pulmonary embolism can also reliably rule out aortic dissection [32]. Dissection was more rapidly diagnosed when CT or echocardiography formed part of the early diagnostic work-up. Delays in aortic dissection diagnosis occurred more often in female patients, in those with atypical symptoms that were not sudden or did not include pain [4, 18], those with previous cardiac surgery [7], or those who initially attended a nontertiary care hospital (all P < 0.05) [26]. Critically ill patients with hypotension, tamponade, signs of lower limb ischaemia (as evidenced by pulse deficits), coma, or altered consciousness were diagnosed earlier [26]. Thus, high-risk, presenting symptoms and physical finding validated the high sensitivity (>95%) of the clinical risk score [33]. Moreover, when survival curves were constructed, hyperacute (symptom onset to 24 h), acute (2–7 days), subacute (8–30 days), and chronic (>30 days) could be separated (Fig. 20.1) [34]. Overall mortality for AAD is 27.4%, but mortality differed between type A or type B and on medical or surgical/interventional treatment.
5 Management
5.1 Proximal Aortic Dissection
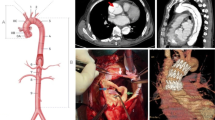
Patients presenting with proximal aortic dissection are usually managed surgically (86%), with about 90% today [1] (Fig. 20.2). The in-hospital mortality of patients with type A aortic dissection came down from 31 to 22%, as a result of better survival after surgery from 25 to 18% (Fig. 20.3). The interval from diagnosis to surgical intervention was 4.3 h (Q1–Q3, 2.4–14 h) [26]. Not surprisingly, the period was shorter in unstable than in stable patients and major predictors of death, such as tamponade and shock, also force the surgeon to operate sooner on the most moribund patients. Standard supra-coronary ascending aorta replacement was performed in 59% of patients, while extensive aortic resections involving aortic root replacement were performed in 34% and total arch replacement in 12% of patients. An open procedure with hypothermic circulatory arrest was used in 92% patients, with antegrade cerebral perfusion in half of all [35]. More recently, some high-risk patients can be treated endovascularly (Fig. 20.4).
From top to bottom: two-dimensional transverse and coronal sections of a proximal (type A) aortic dissection before (left) and after stent grafting (right), demonstrating thoracic endovascular aortic repair reconstruction and remodelling of the aorta. The bottom panels demonstrate the successful intervention in three-dimensional reconstruction. Reproduced with permission of Nienaber CA, J Thorac Cardiovasc Surg. 2017 Feb;153(2):S3–S11
5.2 Distal Aortic Dissection
Overall in-hospital mortality of distal dissection is 13%, with most deaths in the first week of medical management (63%). Over the last two decades, the concept of endovascular management has been developed, and its use was enhanced from 7% to the staggering 31% of cases and now replaces open surgery as the preferred treatment modality for most complications of distal dissection [1]. One-third of patients with acute type B dissection present with complications such as malperfusion syndromes, imminent rupture, early expansion, or haemodynamic instability; two independent predictors of surgical mortality are age > 70 years (OR 4.3) and preoperative shock/hypotension (OR 6.0) [35]. Mortality is significantly higher after open surgery (33.9%) than after endovascular treatment (10.6%, p = 0.002) [36], supporting the currently increasing use of endovascular strategies.
The aortic arch is involved in 16% of distal dissection (Fig. 20.5), with one-third of them being managed by surgery or endovascular treatment; mortality at 3 years is similar to the remaining type B patients [37, 38]. Isolated abdominal dissection is rare (1.3%) and has a similar profile than the rest of distal aortic dissection (Fig. 20.6) [39]. The most ominous findings on physical examination is hypotension (systolic blood pressure < 90 mmHg), presented in >25% of patients and often associated with neurological deficits, altered mental status, myocardial and mesenteric ischaemia, limb ischaemia, or death in 55% of patients [19]. The shock was seen in 15% of patients and associated with increased hospital mortality (30.2 vs. 23.9%, P = 0.007) [40]. Recurrent pain and refractory hypertension appeared associated with increased in-hospital mortality in patients with type B dissection (17.4 vs. 4%), particularly when managed medically [41]. Pericardial tamponade is rare in distal dissection but seen in 18% of proximal dissection [42]. Similarly, the periaortic haematoma is an independent predictor of mortality in patients with aortic dissections (OR 1.71, p = 0.007) [43]. Visceral and peripheral ischaemia may result from severe true lumen compression or branch vessel involvement or from both. Patients with renal insufficiency (18%) seem to be at risk for drug-resistant hypertension due to renal artery compromise (OR 5.2) [44]. In-hospital mortality of patients with mesenteric malperfusion was 95% when treated medically, 73% with endovascular stenting, and 42% with hybrid open/endovascular management, respectively (P < 0.001) [45]. Visceral ischaemia was indeed an independent predictor of mortality (OR 5.91), as was a pulse deficit heralding early mortality and complications [22]. Limb ischaemia was reported in 9.7% [46] and often associated with acute renal failure (OR 2.78), acute mesenteric ischaemia/infarction (OR 6.9), or death (OR 3.5), prompting endovascular but not surgical therapy (31 vs. 10%, P = 0.004). Brain injury at the onset of dissection was a complication in 10% of type A dissection and arch vessel involvement was more frequent among patients with stroke (68%) [47]. Even with a stroke, surgery trended towards decrease overall hospital mortality (OR, 0.058; P < 0.001). Conversely, medical therapy was associated with dismal outcomes in patients with coma with 100% mortality and 76.2% mortality in those with cerebrovascular accident. Furthermore, the return of brain function was documented in 84.3 and 78.8% of patients presenting initially in a comatose state. Therefore aortic dissection patients with brain injury should always be considered for intervention, especially in the absence of major stroke [48].
(a) Axial view on computed tomography of the same acute retrograde aortic dissection, showing the entry tear (*), a patent false lumen in the descending aorta (white arrow), and a completely thrombosed false lumen in the ascending aorta (black arrow). (b) Axial view showing the dissection extension in the arch. (c) Two months following thoracic endovascular aortic repair demonstrates complete thrombosis of the descending aortic false lumen (black arrow). (d) Coronal view demonstrating the implanted endograft
Patients classified as hemodynamically unstable were associated with higher in-hospital mortality of 31% than stable patients with 17% (P < 0.001) (Table 20.2) [46]. Independent predictors of mortality were age > 70 years, previous cardiac surgery [49], hypotension or shock at presentation, migrating pain, cardiac tamponade, any pulse deficit, and an electrocardiogram with findings of myocardial ischaemia or myocardial infarction, as mentioned before. More extensive root replacement was not associated with increased hospital mortality [35], supporting such an approach in younger patients, especially those with connective tissue diseases or bicuspid aortic valves [50]. The choice of repair should thus be dependent on anatomical characteristics as well as the surgical experience of the operating surgeon [51].
With regard to outcomes, hypotension/shock, the absence of chest/back pain on presentation, and branch vessel involvement are highly predictive [52]. Painless dissection occurred in 4% of patients and was associated with increased in-hospital mortality [53]. Mortality in patients with complications (shock, periaortic haematoma, visceral or peripheral ischaemia, acute renal failure, recurrent pain, refractory pain, or refractory hypertension) was 20.0% compared to 6.1% in absence of complications [54]. Mesenteric ischaemia (OR, 9.03), hypotension/shock (OR, 6.43), descending diameter ≥ 5.5 cm (OR, 6.04), renal failure (OR, 3.61), periaortic haematoma (OR, 3.06), acute and limb ischaemia (OR, 3.02), and age (OR, 1.03) [55] were all important predictors.
5.3 Long-Term Outcomes
Patients who survived open surgery for type A aortic dissection had a 96.1 and 90.5% survival rate at 1 and 3 years, respectively. Independent predictors of survival do not appear to be influenced by in-hospital risks but rather by pre-existing comorbidities [56]. Conversely, in patients with distal dissection, 3-year survival was only 78%; mortality in type B dissection was best explained by complications, such as hypotension/shock (HR, 12.5), renal failure (HR, 2.5), pleural effusion on chest X-ray (HR, 2.56), and clinical factors [57]. A subgroup of acute type B dissection patients treated by endovascular repair reported a lower death rate (16 vs. 29%, p = 0.02) at 5 years compared with patients with medical therapy alone, despite the initially higher risk profile due to the complicated nature of the dissection cases subjected to this therapy [58].
Imaging variables of aortic dissection such as false lumen flow have been linked to false lumen expansion and mortality. Partial false lumen thrombosis is present in 33.8% of acute distal dissection and is an independent predictor of death at 3 years (RR, 2.7) [59]. Overall the impact of medical management on outcomes of aortic dissection remains poorly understood. Multivariate models showed the use of β-blockers was associated with improved survival in patients with type A dissection undergoing surgery (odds ratio 0.5; p = 0.02), while calcium channel blockers were linked to improved survival in patients with type B dissection (odds ratio 0.6; p = 0.01) [60]. In addition, the use of calcium-channel blockers after type B dissection may reduce the expansion rate of dissected aorta [61]. Alterations in lifestyle are usually recommended in survivors of aortic dissection. Some restrictions may indeed reduce functional capacity and quality of life in some post-dissection patients [62]. Clinicians should assess any psychological burden after dissection that may lessen the quality of life of survivors [63] and offer appropriate counselling [64].
Interestingly, in the first year after surviving dissection, women with aortic dissection die more frequently than men (OR, 1.4, P = 0.04). Type A dissection in women was associated with higher surgical mortality of 32 versus 22% in men (P = 0.013) despite the similar delay, surgical technique, and haemodynamics. Almost one-third of patients presenting with acute aortic dissection are over 70 years of age. With a type A dissection, fewer elderly patients were managed surgically compared to younger patients (71 vs. 89%, p < 0.0001) [65], and surgical mortality was 21% in patients <70 years and 31% in ≥70 years (p < 0.005). Yet, swift surgical management was associated with lower mortality up to an age of 80 [66]. Similarly, older age was an independent predictor of death in type B dissection. In complicated type B dissection, in-hospital mortality rates for patients under 70 were 10.1 versus 30.0% in patients over 70 when subjected to endovascular treatment (p = 0.001) in 17.2 versus 34.2% for surgical treatment (p = 0.027) and 14.2 versus 32.2% for medical treatment (p = 0.001) [67].
Despite their younger age at presentation, patients with Marfan’s syndrome had a higher mortality with any dissection but dissected of a large diameter of the aorta than non-Marfan patients [68].
Patients diagnosed in the stage of intramural haematoma (IMH) were older but presented with similar symptoms than classic dissection. Like classic dissection, IMH is a fatal condition when it involves the ascending aorta with similar mortality (26.6 vs. 26.5%, respectively) than classic dissection. While the in-hospital mortality of IMH ranges around 4.4%, 16% of patients showed an evolution to full dissection on serial imaging (Table 20.3).
6 Outlook
In the two decades of the inception of IRAD, the overall mortality in patients with proximal aortic dissection has declined. Notably, surgical/interventional mortality in patients with type A and type B dissection has decreased significantly. The improvement outcomes after aortic dissection are likely the result of an array of factors including timely clinical detection, improved diagnostic imaging, and advances in surgical and endovascular techniques followed by improved perioperative and long-term management [69].
The IRAD registry will continue to recruit patients with any kind of aortic dissection and specialise by branching out to various focus groups with particular interest in imaging technique, biomarkers, surgical and endovascular management strategies, or genetic profiling. Moreover, IRAD will always be instrumented as a global platform to offer interested surgeons and physicians to derive substantial new insights into an old disease with a low overall incidence but high personal impact.
References
Pape LA, Awais M, Woznicki EM, et al. Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the international registry of acute aortic dissection. J Am Coll Cardiol. 2015;66:350–8.
Nienaber CA, Cheshire N. Prospective community screening for aortic conditions—true incidence or just a better guess? J Am Heart Assoc. 2015;4:e001686.
Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903.
Nienaber CA, Fattori R, Mehta RH, Richartz BM, Evangelista A, Petzsch M, et al., International Registry of Acute Aortic Dissection. Gender-related differences in acute aortic dissection. Circulation. 2004;109:3014–21.
Januzzi JL, Sabatine MS, Eagle KA, Evangelista A, Bruckman D, Fattori R, et al., International Registry of Aortic Dissection Investigators. Iatrogenic aortic dissection. Am J Cardiol. 2002;89:623–6.
Eagle KA, Isselbacher EM, DeSanctis RW. Cocaine-related aortic dissection in perspective. Circulation. 2002;105:1529–30.
Dean JH, Woznicki EM, O’Gara P, Montgomery DG, Trimarchi S, Myrmel T, et al. Cocaine-related aortic dissection: lessons from the international registry of acute aortic dissection. Am J Med. 2014;127:878–85.
Collins JS, Evangelista A, Nienaber CA, Bossone E, Fang J, Cooper JV, et al., International Registry of Acute Aortic Dissection (IRAD). Differences in clinical presentation, management, and outcomes of acute type A aortic dissection in patients with and without previous cardiac surgery. Circulation. 2004;110:II237–42.
Bossone E, Pyeritz RE, O’Gara P, Harris KM, Braverman AC, Pape L, et al. Acute aortic dissection in blacks: insights from the international registry of acute aortic dissection. Am J Med. 2013;126:909–15.
Januzzi JL, Isselbacher EM, Fattori R, Cooper JV, Smith DE, Fang J, Eagle KA, Mehta RH, Nienaber CA, Pape LA, International Registry of Aortic Dissection (IRAD). Characterising the young patient with aortic dissection: results from the International Registry of Aortic Dissection (IRAD). J Am Coll Cardiol. 2004;43:665–9.
Pape LA, Tsai TT, Isselbacher EM, Oh JK, O’gara PT, Evangelista A, et al., International Registry of Acute Aortic Dissection (IRAD) Investigators. Aortic diameter >or = 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic. Dissection (IRAD). Circulation. 2007;116:1120–7.
Trimarchi S, Jonker FH, Hutchison S, Isselbacher EM, Pape LA, Patel HJ, et al. Descending aortic diameter of 5.5 cm or greater is not an accurate predictor of acute type B aortic dissection. J Thorac Cardiovasc Surg. 2011;142:101–7.
Trimarchi S, Jonker FH, Froehlich JB, Upchurch GR, Moll FL, Muhs BE, Rampoldi V, Patel HJ, Eagle KA, International Registry of Acute Aortic Dissection (IRAD) investigators. Acute type B aortic dissection in the absence of aortic dilatation. J Vasc Surg. 2012;56:311–6.
Mehta RH, Manfredini R, Bossone E, Hutchison S, Evangelista A, Boari B, et al. Does circadian and seasonal variation in occurrence of acute aortic dissection influence in-hospital outcomes? Chronobiol Int. 2005;22:343–51.
Mehta RH, Manfredini R, Bossone E, Fattori R, Evagelista A, Boari B, et al. The winter peak in the occurrence of acute aortic dissection is independent of climate. Chronobiology Int. 2005;22:723–9.
Mehta RH, Manfredini R, Hassan F, Sechtem U, Bossone E, Oh JK, et al., International Registry of Acute Aortic Dissection (IRAD) Investigators. Chronobiological patterns of acute aortic dissection. Circulation. 2002;106:1110–5.
Upchurch GR, Nienaber CA, Fattori R, Evangelista A, Oh JK, Cooper JV, Isselbacher EM, Suzuki T, Eagle KA. Acute aortic dissection presenting with primarily abdominal pain: a rare manifestation of deadly disease. Ann Vasc Surg. 2005;19:367–73.
Park SW, Hutchison S, Mehta RH, Isselbacher EM, Cooper JV, Fang J, et al. Association of painless acute aortic dissection with increased mortality. Mayo Clin Proc. 2004;79:1252–7.
Nallamothu BK, Metha RH, Saaint S, Llovet A, Bossone E, Sechtem U, et al. Syncope in acute aortic dissection: diagnostic, prognostic and clinical implications. Am J Med. 2002;113:468–71.
Tsai TT, Bossone E, Isselbacher EM, Nienaber CA, Evangelista A, Fang J, et al. Clinical characteristics of hypotension in patients with acute aortic dissection. Am J Cardiol. 2005;95:48–52.
Januzzi JL, Eagle KA, Cooper JV, Fang J, Sechtem U, Myrmel T, et al. Acute aortic dissection presenting with congestive heart failure: results from the international registry of acute aortic dissection. J Am Coll Cardiol. 2005;46:733–5.
Bossone E, Rampoldi V, Nienaber CA, Trimarchi S, Ballotta A, Cooper JV, Smith DE, Eagle KA, Mehta RH. Usefulness of pulse deficit to predict in-hospital complications and mortality in patients with acute type a aortic dissection. Am J Cardiol. 2002;89:851–5.
Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management. Part I: from etiology to diagnostic strategies. Circulation. 2003;108:628–35.
Ramanath VS, Oh JK, Sundt TM III, Eagle KA. Acute aortic syndromes and thoracic aortic aneurysm. Mayo Clin Proc. 2009;84:465–81.
Tsai TT, Trimarchi S, Nienaber CA. Acute aortic dissection: perspectives from the international registry of acute aortic dissection (IRAD). Eur J V Endovasc Surg. 2009;37:149–59.
Harris KM, Strauss CE, Eagle KA, Hirsch AT, Isselbacher EM, Tsai TT, et al., International Registry of Acute Aortic Dissection (IRAD) Investigators. Correlates of delayed recognition and treatment of acute type A aortic dissection: the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2011;124:1911–8.
Moore AG, Eagle KA, Bruckman D, Moon BS, Malouf JF, Fattori R, et al. Choice of computed tomography, transesophageal echocardiography, magnetic resonance imaging, and aortography in acute aortic dissection: international registry of acute aortic dissection (IRAD). Am J Cardiol. 2002;89:1235–8.
Raghupathy A, Nienaber CA, Harris KM, Myrmel T, Fattori R, Sechtem U, et al., International Registry of Acute Aortic Dissection (IRAD) Investigators. Geographic differences in clinical presentation, treatment, and outcomes in type A acute aortic dissection (from the International Registry of Acute Aortic Dissection). Am J Cardiol. 2008;102:1562–6.
Ramanath VS, Eagle KA, Nienaber CA, Isselbacher EM, Froehlich JB, Montgomery DG, et al. The role of preoperative coronary angiography in the setting of type A acute aortic dissection: insights from the international registry of acute aortic dissection. Am Heart J. 2011;161:790–6.
Bossone E, Evangelista A, Isselbacher E, Trimarchi S, Hutchison S, Gilon D, et al., International Registry of Acute Aortic Dissection Investigators Prognostic role of transesophageal echocardiography in acute type A aortic dissection. Am Heart J. 2007;153:1013–20.
Suzuki T, Distante A, Zizza A, Trimarchi S, Villani M, Salerno Uriarte JA, et al., International Registry of Acute Aortic Dissection Substudy on Biomarkers (IRAD-Bio) Investigators. Preliminary experience with the smooth muscle troponin-like protein, calponin, as a novel biomarker for diagnosing acute aortic dissection. Eur Heart J. 2008;29:1439–45.
Suzuki T, Distante A, Zizza A, Trimarchi S, Villani M, Salerno Uriarte JA, et al, IRAD-Bio Investigators. Diagnosis of acute aortic dissection by D-dimer: the International Registry of Acute Aortic Dissection Substudy on Biomarkers (IRAD-Bio) experience. Circulation. 2009;26;119:2702–7.
Rogers AM, Hermann LK, Booher AM, Nienaber CA, Williams DM, Kazerooni EA, et al., On behalf of the IRAD Investigators. The sensitivity of the aortic dissection detection risk score, a novel guideline-based tool for identification of acute aortic dissection at initial presentation. Results from the International registry of acute aortic dissection. Circulation. 2011;123:2213–8.
Booher AM, Isselbacher EM, Nienaber CA, Trimarchi S, Evangelista A, Montgomery DG, et al. The IRAD classification system for characterizing survival after aortic dissection. Am J Med. 2013;730:e19–24.
Rampoldi V, Trimarchi S, Eagle KA, Nienaber CA, Oh JK, Bossone E, Myrmel T, et al., International Registry of Acute Aortic Dissection (IRAD) Investigators. Simple risk models to predict surgical mortality in acute type A aortic dissection: the International registry of acute aortic dissection score. Ann Thorac Surg. 2007;83:55–61.
Trimarchi S, Nienaber CA, Rampoldi V, Myrmel T, Suzuki T, Bossone E, et al., IRAD Investigators. Role and results of surgery in acute type B aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2006;114: I357–64.
Fattori R, Tsai TT, Myrmel T, Evangelista A, Cooper JV, Trimarchi S, et al. Complicated acute type B dissection: is surgery still the best option?: a report from the international registry of acute aortic dissection. JACC Cardiovasc Interv. 2008;1:395–402.
Tsai TT, Isselbacher EM, Trimarchi S, Bossone E, Pape L, Januzzi JL, et al.., International Registry of Acute Aortic Dissection. Acute type B aortic dissection: does aortic arch involvement affect management and outcomes? Insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2007;116:I150–6.
Nauta FJ, Tolenaar JL, Patel HJ, Appoo JJ, Tsai TT, Desai ND, et al. Impact of retrograde arch extension in acute type B aortic dissection on management and outcomes. Ann Thorac Surg. 2016;102:2036–43.
Trimarchi S, Tsai T, Eagle KA, Isselbacher EM, Froehlich J, Cooper JV, Rampoldi V, Upchurch GR Jr, International Registry of Acute Aortic Dissection (IRAD) investigators. Acute abdominal aortic dissection: insight from the International Registry of Acute Aortic Dissection (IRAD). J Vasc Surg. 2007;46:913–9.
Bossone E, Pyeritz RE, Braverman AC, Peterson MD, Ehrlich M, O’Gara P, et al. Shock complicating type A acute aortic dissection: clinical correlates, management, and outcomes. Am Heart J. 2016;176:93–9.
Trimarchi S, Eagle KA, Nienaber CA, Pyeritz RE, Jonker FH, Suzuki T, et al., International Registry of Acute Aortic Dissection (IRAD) Investigators. The importance of refractory pain and hypertension in acute type B aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2010;122:1283–9.
Gilon D, Mehta RH, Oh JK, Januzzi JL Jr, Bossone E, Cooper JV, et al., International Registry of Acute Aortic Dissection Group Characteristics and in-hospital outcomes of patients with cardiac tamponade complicating type A acute aortic dissection. Am J Cardiol. 2009;103:1029–31.
Mukherjee D, Evangelista A, Nienaber CA, Sechtem U, Suzuki T, Trimarchi S, et al. Implications of periaortic hematoma in patients with acute aortic dissection (from the International Registry of Acute Aortic Dissection). Am J Cardiol. 2005;96:1734–8.
Beckman JA, Mehta RH, Isselbacher EM, Bossone E, Cooper JV, Smith DE, et al. Branch vessel complications are increased in aortic dissection patients with renal insufficiency. Vasc Med. 2004;9:267–70.
Trimarchi S, Nienaber CA, Rampoldi V, Myrmel T, Suzuki T, Mehta RH, et al., International Registry of Acute Aortic Dissection Investigators Contemporary results of surgery in acute type A aortic dissection: The International Registry of Acute Aortic Dissection experience. J Thorac Cardiovasc Surg. 2005;129:112–22.
Henke PK, Williams DM, Upchurch GR Jr, Proctor M, Cooper JV, Fang J, et al. Acute limb ischemia associated with type B aortic dissection: clinical relevance and therapy. Surgery. 2006;140:532–9.
Bossone E, Corteville DC, Harris KM, Suzuki T, Fattori R, Hutchison S, et al. Stroke and outcomes in patients with acute type A aortic dissection. Circulation. 2013;128:S175–9.
Teman NR, Peterson MD, Russo MJ, Ehrlich MP, Myrmel T, Upchurch GR Jr, et al. Outcomes of patients presenting with acute type A aortic dissection in the setting of prior cardiac surgery: an analysis from the International Registry of Acute Aortic Dissection. Circulation. 2013;128:S180–5.
Di Eusanio M, Trimarchi S, Peterson MD, Myrmel T, Hughes GC, Korach A, Sundt TM, et al. Root replacement surgery versus more conservative management during type A acute aortic dissection repair. Ann Thorac Surg. 2014;98:2078–84.
Berretta P, Patel H, Gleason TG, Sundt TM, Myrmel T, Desai M, et al. IRAD experience on surgical type A acute dissection patients: results and predictors of mortality. Ann Cardiothorac Surg. 2016;5:346–35.
Suzuki T, Mehta RH, Ince H, Nagai R, Sakomura Y, Weber F, et al., International Registry of Aortic Dissection. Clinical profiles and outcomes of acute type B aortic dissection in the current era: lessons from the International Registry of Aortic Dissection (IRAD). Circulation. 2003;108:II312–7.
Tolenaar JL, Hutchison S, Montgomery DG, O’Gara P, Fattori R, Pyeritz RE, et al. Painless type B aortic dissection: insights from the International Registry of Acute Aortic Dissection. Aorta. 2013;1:96–102.
Trimarchi S, Tolenaar JL, Tsai TT, Froehlich J, Pegorer M, Upchurch GR Jr, et al. Influence of clinical presentation on the outcome of acute B aortic dissection: evidences from IRAD. J Cardiovasc Surg. 2012;53:161–8.
Tolenaar JL, Froehlich W, Jonker FH, Upchurch GR Jr, Rampoldi V, Tsai TT, et al. Predicting in-hospital mortality in acute type B aortic dissection: evidence from International Registry of Acute Aortic Dissection. Circulation. 2014;130:S45–50.
Tsai TT, Evangelista A, Nienaber CA, Trimarchi S, Sechtem U, Fattori R, et al., International Registry of Acute Aortic Dissection (IRAD). Long-term survival in patients presenting with type A acute aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2006;114:I350–6.
Tsai TT, Fattori R, Trimarchi S, Isselbacher E, Myrmel T, Evangelista A, et al., International Registry of Acute Aortic Dissection Long-term survival in patients presenting with type B acute aortic dissection: insights from the International Registry of Acute Aortic Dissection. Circulation. 2006;114:2226–31.
Fattori R, Montgomery DG, Lovato L, Kische S, Di Eusanio M, Ince H, Eagle KA, Isselbacher EM, Nienaber CA. Survival after endovascular therapy in patients with type B aortic dissection: a report from the International Registry of Acute Aortic Dissection (IRAD). JACC Intervention. 2013;6:876–82.
Tsai TT, Evangelista A, Nienaber CA, Myrmel T, Meinhardt G, Cooper JV, et al., International Registry of Acute Aortic Dissection. Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med. 2007;357:349–59.
Suzuki T, Isselbacher EM, Nienaber CA, Pyeritz RE, Eagle KA, Tsai TT, et al., IRAD Investigators. Type-selective benefits of medications in treatment of acute aortic dissection (from the International Registry of Acute Aortic Dissection [IRAD]). Am J Cardiol. 2012;109:122–7.
Jonker FH, Trimarchi S, Rampoldi V, Patel HJ, O’Gara P, Peterson MD, et al., International Registry of Acute Aortic Dissection (IRAD) Investigators. Aortic expansion after acute type B aortic dissection. Ann Thorac Surg. 2012;94:1223–9.
Chaddha A, Eagle KA, Braverman AC, Kline-Rogers E, Hirsch AT, Brook R, et al. Physical activity exercise and physical activity for the post-aortic dissection patient: the Clinician’s Conundrum. Clin Cardiol. 2015;38:647–51.
Chaddha A, Kline-Rogers E, Braverman AC, Erickson SR, Jackson EA, Franklin BA, et al. Survivors of aortic dissection: activity, mental health, and sexual function. Clin Cardiol. 2015;38:652–9.
Chaddha A, Kline-Rogers E, Woznicki EM, Brook R, Housholder-Hughes S, Braverman AC, Pitler L, Hirsch AT, Eagle KA. Activity recommendations for postaortic dissection patients. Circulation. 2014;130:e140–2.
Mehta RH, O’Gara PT, Bossone E, Nienaber CA, Myrmel T, Cooper JV, et al. Acute type A aortic dissection in the elderly: clinical characteristics, management and outcomes in the current era. J Am Coll Cardiol. 2002;40:685–92.
Trimarchi S, Eagle KA, Nienaber CA, Rampoldi V, Jonker FH, De Vincentiis C, et al., International Registry of Acute Aortic Dissection Investigators. The role of age in acute type A aortic dissection outcome: report from the International Registry of Acute Aortic Dissection (IRAD). J Thorac Cardiovasc Surg. 2010;140:784–9.
Jonker FH, Trimarchi S, Muhs BE, Rampoldi V, Montgomery DG, Froehlich JB, et al., IRAD Investigators. The role of age in complicated acute type B aortic dissection. Ann Thorac Surg. 2013;96:2129–34.
Januzzi JL, Marayati F, Mehta RH, Cooper JV, O’Gara PT, Sechtem U, et al. Comparison of aortic dissection in patients with and without Marfan’s syndrome (results from the International Registry of Aortic Dissection). Am J Cardiol. 2004;94:400–2.
Mehta RH, Suzuki T, Hagan PG, et al. Predicting death in patients with acute type A aortic dissection. Circulation. 2002;105:200–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer-Verlag GmbH Austria, part of Springer Nature
About this chapter
Cite this chapter
Yuan, X., Nienaber, C.A. (2019). Lessons Learnt from the International Registry of Acute Aortic Dissection (IRAD). In: Stanger, O., Pepper, J., Svensson, L. (eds) Surgical Management of Aortic Pathology. Springer, Vienna. https://doi.org/10.1007/978-3-7091-4874-7_20
Download citation
DOI: https://doi.org/10.1007/978-3-7091-4874-7_20
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-4872-3
Online ISBN: 978-3-7091-4874-7
eBook Packages: MedicineMedicine (R0)