Abstract
Purpose of Review
The following describes the recent advancement in the diagnosis, imaging, and treatment of type B aortic dissections. We will review the recent updates of aortic dissection classifications, and potential impact on clinical management.
Recent Findings
Type B aortic dissections can be classified anatomically and temporally using the recent Society for Vascular Surgery and Society of Thoracic Surgeons reporting standards. A number of high-risk features have been correlated with poor prognoses with medical management alone, leading to the expansion of indications for thoracic endovascular aortic repair (TEVAR). Emerging data suggest that timing of intervention may play a role in patient outcomes. Special attention to endovascular technique regarding landing zones and device selection can also significantly impact patient outcomes.
Summary
Anti-impulse therapy should promptly be initiated for all dissections. Type A dissections continue to depend largely on emergent open surgical intervention, whereas TEVAR remains first line for complicated type b aortic dissections. Uncomplicated type b aortic dissections are historically managed conservatively; however, data continue to emerge suggesting benefits of early endovascular intervention to prevent risk of late aortic degeneration or rupture. These approaches continue to be challenged as growing registry data and progressive endovascular technology develop. Regardless of initial management strategy, continued surveillance is crucial. Widespread utilization of standardized classification systems can aid in understanding the natural history and outcomes of aortic dissections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute aortic dissection, initially thought to be rare, has become known as the most common aortic emergency, demonstrating an overall high mortality of up to 30% on presentation [1]. This prompted continued scrutiny of the diagnosis and management of aortic dissections, resulting in the rapid evolution of approaches encompassing medical management, to open and novel endovascular techniques. In this article, we review the classifications, presentation, management, postoperative care, potential complications, and future directions, with a focus on type B aortic dissections.
Classification
Anatomic
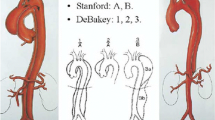
Aortic dissections have historically been classified based on anatomic extent and timing of presentation. Anatomically, dissections were described based on location of the primary intimal entry tear and extent of aortic involvement. This was first developed by DeBakey in 1965, who stratified dissections as Type I–III (Fig. 1). In this classification system, DeBakey type I dissections have the entry tear in the ascending aorta, and involve the aortic arch through the descending and/or abdominal aorta, whereas Type II dissections have the entry tear and extent confined to the ascending aorta, and Type III dissections have the entry tear and extent confined to the descending and/or abdominal aorta [2]. This was later simplified into the now popular Stanford A, B classification (Fig. 1), which accounts for the location of the entry tear, with type A including those with tears in the ascending aorta, and type B those with tears originating distal to the left subclavian artery [3].
DeBakey and Stanford classifications for aortic dissections. (Lombardi JV, Hughes GC, Appoo JJ, Bavaria JE, Beck AW, Cambria RP, Charlton-Ouw K, Eslami MH, Kim KM, Leshnower BG, Maldonado T, Reece TB, Wang GJ. Society for vascular surgery (SVS) and Society of thoracic surgeons (STS) reporting standards for type B aortic dissections. J Vasc Surg 2020;71(3):723–747. https://doi.org/10.1016/j.jvs.2019.11.013
These simple, original classifications allowed for a better understanding of the contrasting natural history and difference in time-dependent clinical outcomes with medical and surgical management of type A and B aortic dissections. For example, International Registry of Aortic Dissections (IRAD) data showed that the 30-day mortality for patients with type A aortic dissections managed medically was significantly higher than those with type A aortic dissections managed surgically. On the other hand, type B aortic dissection patients showed lower 30-day mortality, compared to those who underwent surgery. This observation laid a foundation for the traditional paradigm of surgical management of type A aortic dissections and medical management of type B aortic dissections [4].
With continued research and emergence of clinical data, our understanding of type B aortic dissections became more sophisticated with increasing attention to clinical and radiographical signs of those who harbor higher risk for aortic complications. Furthermore, despite the wide adoption of these simple classification systems, neither addresses the full range of aortic dissection presentation, for example those with distal entry tears with retrograde extension through the aortic arch, those with entry tears focal to the transverse arch, or those with dissections extending into the great vessels, all of which can significantly impact our understanding of prognosis and outcomes.
Thus, in 2019, the Society for Vascular Surgery (SVS) and Society of Thoracic Surgeons (STS) published a new reporting standard, which contains a classification system more specifically identifying both the location of the entry tear as well as aortic extent involved by zone [5••]. By this classification, aortic dissections are distinguished as type A or B by entry tear location alone, with type A being those with entry tears in zone 0 only. A numeric subscript is then used to denote the zone of distal extent of the dissection. Similarly, type B dissections, which are those with the primary entry tear originating in zone 1 and beyond, utilizes two subscripts to denote the proximal and distal extent of involvement, respectively, regardless of false lumen patency. For example, a dissection with an entry tear at zone 2, retrograde extension to zone 0 and distal extension to zone 9 would be described as B0,9. Of note, this does not specify the zone of the entry tear, allowing for inclusion of all dissections from zone 1–9 as a type B dissection. For those with indeterminate entry tears with extension into zone 0, a designation “I” is used in place of “A”, and again described with a subscript to describe the distal extent of the dissection. (Fig. 2), Those with new acute on chronic presentations are described based on both the historic and new pathology, while those with prior repairs, for example open aortic reconstructions, can be described based on residual disease. Furthermore, intramural hematomas (IMH), a separation of the aortic wall layers without an identifiable entry tear, as well as penetrating aortic ulcers (PAU), ulcerations into the aortic wall secondary to atherosclerotic lesions, are two related aortic syndromes which can similarly be described using subscripts to denote zones involved (e.g., PAU2,5 and IMH3,7).
Society for vascular surgery and society of thoracic surgeons aortic dissection classification system. (Lombardi JV, Hughes GC, Appoo JJ, Bavaria JE, Beck AW, Cambria RP, Charlton-Ouw K, Eslami MH, Kim KM, Leshnower BG, Maldonado T, Reece TB, Wang GJ. Society for vascular surgery (SVS) and Society of thoracic surgeons (STS) reporting standards for type B aortic dissections. J Vasc Surg 2020;71(3):723–747. https://doi.org/10.1016/j.jvs.2019.11.013
Temporal
In addition to anatomic classification, the SVS/STS sought to incorporate a temporal classification. This was initially born out of the observation that mortality most rapidly increased in the first 14 days, supporting the idea of an “acute” phase of aortic dissections [4]. Subsequent studies then demonstrated the influence of chronicity on morbidity and mortality [6], as well as visible changes in flap morphology on computed tomography (CT) imaging depending on acuity of the aortic dissection [7]. Thus, the new reporting standards classify hyperacute as < 24 h, acute 1–14 days, subacute 15–90 days, and chronic > 90 days from symptom onset. With these new SVS/STS reporting standards of aortic dissections, further research can be framed with more granularity, allowing us to gain a deeper understanding of the factors influencing clinical outcomes, as well as a more standardized evaluation of novel treatment strategies.
Risk Factors, Presentation and Diagnosis
The classic risk factor for aortic dissection is hypertension, with other known risk factors being cocaine use, trauma, pregnancy, atherosclerosis, and genetic disorders such as inherited connective tissue disorders including Marfan syndrome, Loeys-Dietz syndrome, and Ehlers-Danlos syndrome.
Patients typically present with abrupt onset back, chest, or abdominal pain that is tearing in nature, often in the context of hypertension, or even cardiac tamponade or aortic valve complications for type A and retrograde type B dissections, and syncope or neurologic symptoms if there is involvement of the arch vessels. If the dissection extends into branch or peripheral vessels, patients may also present with symptoms of the corresponding end-organ such as renal failure or limb ischemia.
This heterogenous presentation prompted more descriptive terms of type B aortic dissections (TBAD) as complicated or uncomplicated. Complicated aortic dissections are those that present with rupture or clinical evidence of malperfusion, commonly affecting the renal, visceral, iliofemoral, and spinal vascular beds. Malperfusion can further be described as being caused by dynamic obstructions whereby the intimal flap is mobile with each cardiac cycle causing intermittent obstruction, or static obstructions which are caused by continuous false lumen pressurization creating constant obstruction (Fig. 3). Given the associated morbidity and mortality, complicated type B aortic dissections require prompt surgical (open or endovascular) management.
Static versus dynamic obstruction causing malperfusion in aortic dissection. (Lombardi JV, Hughes GC, Appoo JJ, Bavaria JE, Beck AW, Cambria RP, Charlton-Ouw K, Eslami MH, Kim KM, Leshnower BG, Maldonado T, Reece TB, Wang GJ. Society for vascular surgery (SVS) and Society of thoracic surgeons (STS) reporting standards for type B aortic dissections. J Vasc Surg 2020;71(3):723–747. https://doi.org/10.1016/j.jvs.2019.11.013
Uncomplicated aortic dissections are those without evidence of rupture or end-organ malperfusion and are typically managed medically. However, a number of studies have identified radiographic signs that are associated with increased risk for aortic events and late aneurysmal degeneration, and thus failure of medical management alone. For example, an entry tear located along the lesser curve was found to be more commonly associated with retrograde extension of the dissection [8, 9]. Furthermore, larger entry tears resulted in higher false lumen pressurization leading to increased false lumen degeneration [10]. Other studies evaluating the primary location for late aneurysmal degeneration demonstrated that the upper descending thoracic aorta was the major site for late (≥ 2 years) aneurysmal dilatation, and false lumen diameters ≥ 22 mm showed higher aneurysm and death rates, suggesting the need for earlier intervention [11]. Thus, based on these and other studies, the SVS/STS reporting standards suggest specifying “high-risk” features which include a maximum trans-aortic diameter > 40 mm on presentation, primary entry tear along the lesser curve, primary entry tear > 10 mm, and false lumen diameter > 22 mm (Table 1). This concept of “high-risk, uncomplicated” aortic dissections has since been adopted at many centers as expanded indications for endovascular aortic repair.
Given the described criteria above, diagnostic imaging, most commonly CT imaging, is crucial in the rapid diagnosis and appropriate management of aortic dissections. While less sensitive and specific, plain radiography can demonstrate a widened mediastinum or identify pleural effusions, and echocardiography can delineate involvement of the ascending aorta.
Management
Initial Management Strategy
Prompt initiation of medical treatment including anti-impulse therapy (systolic blood pressure 100–120 mmHg, mean arterial pressure 60–70 mmHg, heart rate < 60 beats per minute) and pain control should be applied for all patients upon the diagnosis of an aortic dissection. Medical treatment is aimed at reducing the hemodynamic stresses on the aortic wall and minimizing risk of aortic dissection propagation. The utility of medical management was described by Kodama et al. who reviewed 171 patients with acute aortic dissection, demonstrating a reduction in aortic events (organ or limb ischemia, rupture, recurrent dissection, pathologic aortic expansion, aortic surgery) when both blood pressure and tight heart rate control were achieved [12]. Furthermore, analysis of the IRAD global registry database found that the use of beta-blockers was associated with improved survival in all patients including those with type A dissections, and calcium channel blockers were associated with improved survival in type B aortic dissections, while ACE-Inhibitors had no association with mortality [13].
Subsequent management of aortic dissection is primarily determined by classification of the dissection. While open definitive surgical treatment has been well established for type A acute aortic dissections given the potentially lethal complications such as tamponade, rupture, myocardial infarction, and stroke, type B aortic dissections (TBAD) have historically been approached with more conservative measures given the high morbidity and mortality associated with open repair, prior to the wide adoption of thoracic endovascular aortic repair (TEVAR). The initial application of TEVAR was primarily focused on complicated dissections i.e., rupture or impending rupture, malperfusion, or failed medical therapy, with such application of TEVAR demonstrating favorable clinical and anatomical outcomes at 30-days, 1-year, and 2-year follow-up on prospective multicenter trials [14, 15], as well as more favorable perioperative outcomes when compared to open repair, now with emerging data on long-term outcomes of TEVAR in complicated dissections [16]. Furthermore, a number of high-risk features (as outlined above) have been identified as predictors of medical treatment failure, in which the indications for TEVAR has been expanded.
As endovascular technology continues to evolve, the conservative management strategy of uncomplicated aortic dissections has been challenged. Studies demonstrate lower long-term mortality after TEVAR for TBAD with a majority of patients failing medical therapy over time [17, 18]. Furthermore, while the first randomized control trials (INSTEAD) demonstrated comparable 2-year survival and adverse event rates between optimal medical therapy and TEVAR for uncomplicated aortic dissections, follow-up analysis demonstrated decreased mortality and disease progression beyond 2 years up to 5 years (INSTEAD-XL) [19]. This trial as well as the ADSORB trial [20] demonstrated notable disease progression and aneurysmal degeneration in almost half of the patients by one year, with many requiring aortic-related reintervention due to false lumen degeneration, at which point aortic involvement can be more extensive requiring more complex repairs. This is in contrast to favorable aortic remodeling with true lumen recovery and false lumen thrombosis in > 90% of patients who underwent TEVAR [21]. These results shifted the practice of using TEVAR beyond just complicated and high-risk aortic dissections, towards potentially all uncomplicated aortic dissections in efforts to mitigate future degeneration and need for aortic reintervention. This shift to achieve false lumen thrombosis and favorable aortic remodeling is echoed by Tsai et al., who demonstrated that partial thrombosis of the false lumen during the index hospitalization is associated with delayed post-discharge mortality as compared with those with no thrombosis of the false lumen [22].
Timing of Intervention
The optimal timing of TEVAR also continues to be controversial, as the only level 1 data regarding TEVAR in TBAD contained selection bias regarding the timing of TEVAR. The INSTEAD trial excluded acute (< 14 days) dissections with a median time to intervention of 57 days, whereas ADSORB only included acute dissections, with TEVARs performed within 48 h of randomization. Determining the optimal timing of intervention prompts questions regarding the risk for retrograde type A dissections and procedural complications when intervening acutely, against the benefits of long-term prevention of aortic degeneration and complications. It also questions the benefit of intervening on chronic dissections where aortic remodeling may not be easily achieved. In an effort to identify the potential complications associated with the timing of TEVAR for uncomplicated aortic dissections, an analysis of the vascular quality initiative (VQI) TEVAR registry was published in 2021, suggesting that TEVAR within 14 days versus 15–90 days of presentation, does not predict mortality or postoperative complications, although there was a strong association between repair within 14 days and a higher risk of reintervention, which may have been a reflection of the complexity of cases within that time period [23•]. Given the paucity of data and consensus on this topic, ongoing studies continue to evaluate optimal timing of TEVAR for uncomplicated dissections.
Regardless, the promising successes of endovascular management have since made open surgical repair for descending aortic dissections largely disfavored. Open repair is now reserved for patients with threatened or ruptured aortas with no identifiable proximal seal zone in those who are otherwise appropriate surgical candidates, or those with connective tissue disorders, although this too has been challenged with increasing use of TEVAR for connective tissue disorders demonstrating low perioperatively mortality, spinal cord ischemia, and stroke [24].
Technical Considerations
In addition to timing, appropriate technique has become increasingly of interest and is critical for successful outcomes after TEVAR. Special consideration must be given to the proximal and distal landing zones, as well as device selection and sizing, and branch incorporation.
Device Landing Zone
The traditional proximal landing zone length recommendation for degenerative aneurysms has been 2 cm of healthy aorta. However, on a review comparing 71 patients who underwent TEVAR for TBAD with proximal seal zones with and without intramural hematomas (IMH), achieving a full 2 cm of normal aortic seal zone would have required coverage of all three arch branch vessels in 31 (43.7%) patients [25•] (Fig. 4). Conversely, all patients [3] who developed retrograde type A dissections had seal zones entirely in IMH, while none occurred in those with proximal seal zones involving healthy aorta, suggesting that some proximal seal zone in normal aorta without IMH may avoid retrograde dissection and induce favorable aortic remodeling. In our opinion, the proximal extent of aortic dissection, not just the location of the entry tear alone, should be a determining factor in choosing the proximal landing zone, with an aim to maximize landing within healthy, disease-free aortic walls.
A Proximal seal zone in normal aorta. B Proximal seal zone in intramural hematoma. (Kuo EC, Veranyan N, Johnson CE, Weaver FA, Ham SW, Rowe VL, et al. Impact of proximal seal zone length and intramural hematoma on clinical outcomes and aortic remodeling after thoracic endovascular aortic repair for aortic dissections. J Vasc Surg 2019;69(4):987–995
Similarly, the distal landing zone is of particular importance, as suboptimal stent graft placement and sizing can result in stent-induced new entry tears (SINE) (Fig. 5) and is associated with substantial mortality and aortic reintervention [26, 27]. Furthermore, increased aortic coverage is associated with increased risk for spinal cord ischemia [28•]. Spinal drainage and careful intraoperative and postoperative blood pressure management are some ways to mitigate this risk of spinal cord ischemia, and emerging data now suggest that more extensive coverage through the celiac artery during TEVAR may not be associated with increased rates of perioperative mortality or spinal cord ischemia, but rather may improve thoracic aortic remodeling after TEVAR [29, 30]. Thus, clinician judgment with consideration of case-specific presentation, anatomy, and acuity should be considered when evaluating for distal landing zones.
Stent-induced new entry tear (SINE). (Lombardi JV, Hughes GC, Appoo JJ, Bavaria JE, Beck AW, Cambria RP, Charlton-Ouw K, Eslami MH, Kim KM, Leshnower BG, Maldonado T, Reece TB, Wang GJ. Society for vascular surgery (SVS) and society of thoracic surgeons (STS) reporting standards for type B aortic dissections. J Vasc Surg 2020;71(3):723–747. https://doi.org/10.1016/j.jvs.2019.11.013
Device Selection
With the proximal and distal landing zones determined, appropriate devices should be considered. Covered stents (stent grafts) should always be utilized for the proximal entry tear given the high risk for iatrogenic complications as well as likelihood for continued false lumen perfusion resulting in progressive degeneration and potential true lumen/stent collapse when using uncovered stents [31]. For complicated dissections, composite arrangements of covered stents proximally with distal uncovered dissection stents can be utilized to promote laminar flow and true lumen expansion without increasing aortic coverage [15].
Furthermore, devices should be oversized by no more than 10%, contrary to the traditional 20% oversizing used for degenerative aneurysms. While the axial images of CT angiography (CTA) is rapid and readily available, there can be significant inter- and intra-observer variability [32]. Instead, CTA with three-dimensional reconstruction and centerline measurements have become the standard for stent graft sizing.
The utility of intravascular ultrasound (IVUS) has also been assessed in TEVAR for TBAD. In addition to aiding in true lumen catheterization, IVUS can provide dynamic, orthogonal imaging of the aorta along the path of the stent graft, and assess variations secondary to changes with the cardiac cycle or the patient’s volume status to provide real-time estimations for stent graft sizing. IVUS demonstrated high correlation with CTA, especially at the base of the left subclavian. However, it should be noted that it may overestimate aortic diameters with the most discrepancy, when compared to CTA, at angulated portions of the aorta [33].
Branch Considerations
The subclavian artery provides collateral circulation for the spinal cord as well as contributes to posterior cerebral circulation. However, strategies to achieve adequate proximal landing zones often require coverage of the left subclavian artery. Although some studies suggest increased risk of stroke and spinal cord ischemia [34] with subclavian artery coverage and improved rates with revascularization [35], others demonstrate no significant difference in perioperative morbidity [36]. Thus, some practitioners routinely revascularize the left subclavian artery when coverage is needed to achieve adequate seal zones, while others have adopted a more selective approach with adjunct subclavian revascularization.
Traditional open techniques for subclavian artery revascularization include carotid-subclavian bypass or transposition. Total endovascular left subclavian artery incorporation can be achieved by use of parallel grafting, in-situ fenestration, and branched/fenestrated endografts [37,38,39,40,41,42,43,44,45,46,47,48,49,50,51], with promising early outcomes. Furthermore, custom and off-the-shelf branched/fenestrated stent grafts designed for subclavian artery incorporation are in clinical trials, and in the future may offer options for endovascular repair of arch pathology including aortic dissections.
Other Considerations
Other practical considerations include minimizing the use of balloon molding given the aortic fragility associated with dissections, as well as identifying adequate peripheral access whereby the pulseless or weaker femoral pulses often predicts easier access into the true lumen.
Technique for Complicated Dissections
Patients presenting with rupture should have the proximal tear covered first, with distal extension as needed. Malperfusion poses a particular challenge, as the often more extensive dissection with potentially multiple fenestrations and dynamic flap can make accurate identification of the true lumen especially difficult. In such cases, IVUS can be an extremely useful tool in identifying the dissection. The proximal tear should again be sealed first followed by an aortogram downstream to identify the need for further intervention. Branch intervention should be done on a case-by-case basis pending the severity of the clinical presentation of malperfusion, as there is currently no evidence to suggest a benefit for pre-emptive branch intervention [52]. If there is any concern for branch occlusion intraoperatively, it is crucial to maintain wire access and potentially place stents within the branch, as well as perform endofenestration of the intimal flap as needed to maintain perfusion.
Approach for Chronic Dissections
Aneurysmal degeneration due to persistent false lumen perfusion is a primary concern for aortic dissections in the chronic phase. Persistent false lumen perfusion can be addressed by embolization using amplatzer plugs, or specialized techniques such as the knickerbocker technique [53] whereby an oversized stent graft is deployed within the true lumen and used to create a controlled rupture of the dissection flap upon inflation of a large compliant balloon. The aim of this technique is to occlude the false lumen and promote proximal thrombosis while preventing continued false lumen perfusion. Other techniques, such as the candy-plug technique, utilizes implantation of a modified candy-shaped stent graft in the false lumen aneurysm, again to promote false lumen occlusion and subsequent aortic remodeling [54, 55]. The petticoat technique utilizes a bare metal stent for chronic type B dissections (with additional covered stents within the bare metal stent as parallel iliac stent grafts for the “extended” petticoat technique) to promote favorable remodeling and stabilize aortic diameters [56, 57].
In cases where the dissection significantly degenerates, strategies used to repair primary thoracoabdominal or complex abdominal aortic aneurysms can be applied. For example, complex endovascular aortic repair including custom-manufactured or physician-modified fenestrated-branched stent grafts can be utilized [58,59,60,61,62]. This approach incorporates tailor-made fenestrations, branch cuffs, and/or inner branches on aortic stent grafts in order to create an endovascular repair with adequate seal zones in anatomy otherwise unable to be addressed by standard infrarenal endovascular aortic stent grafts. This technology now continues to develop in hopes for off-the-shelf availability [63] for both primary aneurysmal and dissection-related aneurysmal disease.
Open repair remains an option and is traditionally considered the gold standard for durability. This approach utilizes synthetic or homografts for aortic replacement [64, 65]. Open repairs require adequate preoperative risk assessments and serious consideration of the risks and benefits given the increased morbidity and mortality often associated with such extensive repairs [66]. Hybrid repairs have also demonstrated success, which begins with open aortic debranching followed by concomitant or staged endovascular graft placement [67, 68]; however, again this approach can come with considerable morbidity [69] and is reserved for selective surgical candidates.
Postoperative Care and Surveillance
As we continue to understand the natural history of aortic dissections, it has become clear that regardless of initial management strategy, follow-up and surveillance are crucial for preventing late aortic complications.
As demonstrated in the INSTEAD and ADSORB trials, as well as IRAD registry data, medically managed aortic dissections often result in late aneurysmal degeneration at which point they may be more extensive, requiring more complex repairs. It should be noted, however, that patients who undergo TEVAR for acute aortic dissection are not protected from aneurysmal degeneration in unstented portions of the aorta [70]. This is in contrast to the prognosis of abdominal branches, which maintain stable branch perfusion with high intervention-free patency through the midterm period [52]. Thus, follow-up is primarily for aortic surveillance. Should a complication be identified during surveillance, patients can be evaluated for TEVAR, EVAR, hybrid, open, or fenestrated-branched endovascular aortic repairs as described above, depending on anatomic suitability and patient eligibility.
Conclusions
The literature on aortic dissections is continuously evolving as registries and endovascular technology develop. Although clear consensus on the management of aortic dissection can be difficult to obtain, it is becoming increasingly clear that this pathology and its related complications and natural history are significantly more complex than previously understood. The management of aortic dissections has shifted based on outcome-dependent nomenclature and classifications, and now with more focused efforts to standardize our definitions and outcomes, we can become increasingly deliberate about management strategies. Presently, the initial mainstay therapy for all dissections includes anti-impulse therapy. Type A dissections continue to depend largely on emergent open surgical intervention, while the management of type B dissections can range from medical management for uncomplicated dissections, to emergent TEVARs with or without adjunct procedures for complicated dissections and elective TEVARs for uncomplicated high-risk dissections. These approaches however continue to be challenged as newer endovascular devices develop such as arch fenestrated-branched stent grafts. Open surgical repair is largely reserved for patients without endovascular options who are otherwise acceptable surgical candidates, and those with connective tissue disorders. Continued patient compliance and surveillance are crucial for preventing long-term adverse outcomes.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Tsai TT, Trimarchi S, Nienaber CA. Acute aortic dissection: perspectives from the international registry of acute aortic dissection (IRAD). Eur J Asc Endovasc Surg. 2009;37(2):149–59.
Debakey ME, Henly WS, Cooley DA, Morris GC, Crawford ES, Beall AC. Surgical management of dissecting aneurysms of the aorta. J Thorac Cardiovasc Surg. 1965;49:130–49.
Daily PO, Trueblood HW, Stinson EB, Wuerflein RD, Shumway NE. Management of acute aortic dissections. Ann Thorac Surg. 1970;10(3):237–47.
Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, et al. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA. 2000;283(7):897–903.
•• Lombardi JV, Hughes GC, Appoo JJ, Bavaria JE, Beck AW, Cambria RP, et al. Society for vascular surgery (SVS) and society of thoracic surgeons (STS) reporting standards for type B aortic dissections. J Vasc Surg. 2020;71(3):723–47. Highlights most recent reporting standards and consensus international classifications.
Booher AM, Isselbacher EM, Nienaber CA, Trimarchi S, Evangelista A, Montgomery DG, et al. The IRAD classification system for characterizing survival after aortic dissection. Am J Med. 2013;126(8):730.e19-24.
Peterss S, Mansour AM, Ross JA, Vaitkeviciute I, Charilaou P, Dumfarth J, et al. Changing pathology of the thoracic aorta from acute to chronic dissection: literature review and insights. J Am Coll Cardiol. 2016;68(10):1054–65.
Loewe C, Czerny M, Sodeck GH, Ta J, Schoder M, Funovics M, et al. A new mechanism by which an acute type B aortic dissection is primarily complicated, becomes complicated, or remains uncomplicated. Ann Thorac Surg. 2012;93(4):1215–22.
Weiss G, Wolner I, Folkmann S, Sodeck G, Schmidli J, Grabenwöger M, et al. The location of the primary entry tear in acute type B aortic dissection affects early outcome. Eur J Cardiothorac Surg. 2012;42(3):571–6.
Evangelista A, Salas A, Ribera A, Ferreira-González I, Cuellar H, Pineda V, et al. Long-term outcome of aortic dissection with patent false lumen: predictive role of entry tear size and location. Circulation. 2012;125(25):3133–41.
Song JM, Kim SD, Kim JH, Kim MJ, Kang DH, Seo JB, et al. Long-term predictors of descending aorta aneurysmal change in patients with aortic dissection. J Am Coll Cardiol. 2007;50(8):799–804.
Kodama K, Nishigami K, Sakamoto T, Sawamura T, Hirayama T, Misumi H, et al. Tight heart rate control reduces secondary adverse events in patients with type B acute aortic dissection. Circulation. 2008;118(14):S167–70.
Suzuki T, Isselbacher EM, Nienaber CA, Pyeritz RE, Eagle KA, Tsai TT, et al. Type-selective benefits of medications in treatment of acute aortic dissection (from the international registry of acute aortic dissection [IRAD]). Am J Cardiol. 2012;109(1):122–7.
Lombardi JV, Gleason TG, Panneton JM, Starnes BW, Dake MD, Haulon S, et al. STABLE II clinical trial on endovascular treatment of acute, complicated type B aortic dissection with a composite device design. J Vasc Surg. 2020;71(4):1077-87.e2.
Lombardi JV, Cambria RP, Nienaber CA, Chiesa R, Teebken O, Lee A, et al. Prospective multicenter clinical trial (STABLE) on the endovascular treatment of complicated type B aortic dissection using a composite device design. J Vasc Surg. 2012;55(3):629-40.e2.
Hanna JM, Andersen ND, Ganapathi AM, McCann RL, Hughes GC. Five-year results for endovascular repair of acute complicated type B aortic dissection. J Vasc Surg. 2014;59(1):96–106.
Fattori R, Montgomery D, Lovato L, Kische S, Di Eusanio M, Ince H, et al. Survival after endovascular therapy in patients with type B aortic dissection: a report from the international registry of acute aortic dissection (IRAD). JACC Cardiovasc Interv. 2013;6(8):876–82.
Durham CA, Cambria RP, Wang LJ, Ergul EA, Aranson NJ, Patel VI, et al. The natural history of medically managed acute type B aortic dissection. J Vasc Surg. 2015;61(5):1192–8.
Nienaber CA, Kische S, Rousseau H, Eggebrecht H, Rehders TC, Kundt G, et al. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv. 2013;6(4):407–16.
Brunkwall J, Kasprzak P, Verhoeven E, Heijmen R, Taylor P, Alric P, et al. Endovascular repair of acute uncomplicated aortic type B dissection promotes aortic remodelling: 1 year results of the ADSORB trial. Eur J Vasc Endovasc Surg. 2014;48(3):285–91.
Nienaber CA, Zannetti S, Barbieri B, Kische S, Schareck W, Rehders TC, et al. Investigation of STEnt grafts in patients with type B aortic dissection: design of the INSTEAD trial–a prospective, multicenter European randomized trial. Am Heart J. 2005;149(4):592–9.
Tsai TT, Evangelista A, Nienaber CA, Myrmel T, Meinhardt G, Cooper JV, et al. Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med. 2007;357(4):349–59.
• Torrent DJ, McFarland GE, Wang G, Malas M, Pearce BJ, Aucoin V, et al. Timing of thoracic endovascular aortic repair for uncomplicated acute type B aortic dissection and the association with complications. J Vasc Surg. 2021;73(3):826–35. Evaluates an international vascular database to assess critical outcomes associated with aortic dissections.
Qato K, Conway A, Lu E, Tran NN, Giangola G, Carroccio A. Outcomes of thoracic endovascular aneurysm repair (TEVAR) in patients with connective tissue disorders. Vasc Endovascular Surg. 2020;54(8):676–80.
• Kuo EC, Veranyan N, Johnson CE, Weaver FA, Ham SW, Rowe VL, et al. Impact of proximal seal zone length and intramural hematoma on clinical outcomes and aortic remodeling after thoracic endovascular aortic repair for aortic dissections. J Vasc Surg. 2019;69(4):987–95. Challenges important technical concepts for endovascular repair of aortic dissections.
Dong Z, Fu W, Wang Y, Wang C, Yan Z, Guo D, et al. Stent graft-induced new entry after endovascular repair for Stanford type B aortic dissection. J Vasc Surg. 2010;52(6):1450–7.
Berkarda Z, Kondov S, Kreibich M, Czerny M, Beyersdorf F, Rylski B. Landing zone remodelling after endovascular repair of dissected descending aorta. Eur J Vasc Endovasc Surg. 2020;59(6):939–45.
• Piazza M, Squizzato F, Milan L, Miccoli T, Grego F, Antonello M, et al. Incidence and predictors of neurological complications following thoracic endovascular aneurysm repair in the global registry for endovascular aortic treatment. Eur J Vasc Endovasc Surg. 2019;58(4):512–9. Highlights predictors for a critical outcome of endovascular repair of aortic dissections.
Xue Y, Ge Y, Ge X, Miao J, Fan W, Rong D, et al. Association between extent of stent-graft coverage and thoracic aortic remodeling after endovascular repair of type B aortic dissection. J Endovasc Ther. 2020;27(2):211–20.
Lou X, Duwayri YM, Jordan WD, Chen EP, Veeraswamy RK, Leshnower BG. The safety and efficacy of extended TEVAR in acute type B aortic dissection. Ann Thorac Surg. 2020;110(3):799–806.
Kische S, D’Ancona G, Ortak J, Bermaoui B, Stoeckicht Y, Ince H. Complicated type B aortic dissection should not be treated with uncovered stents: a lesson not yet learned. Ann Vasc Surg. 2015;29(4):841.e13-7.
Han SM, Patel K, Rowe VL, Perese S, Bond A, Weaver FA. Ultrasound-determined diameter measurements are more accurate than axial computed tomography after endovascular aortic aneurysm repair. J Vasc Surg. 2010;51(6):1381–7.
Han SM, Elsayed RS, Ham SW, Mahajan A, Fleischman F, Rowe VL, et al. Comparison of intravascular ultrasound- and centerline computed tomography-determined aortic diameters during thoracic endovascular aortic repair. J Vasc Surg. 2017;66(4):1184–91.
Bradshaw RJ, Ahanchi SS, Powell O, Larion S, Brandt C, Soult MC, et al. Left subclavian artery revascularization in zone 2 thoracic endovascular aortic repair is associated with lower stroke risk across all aortic diseases. J Vasc Surg. 2017;65(5):1270–9.
Chen X, Wang J, Premaratne S, Zhao J, Zhang WW. Meta-analysis of the outcomes of revascularization after intentional coverage of the left subclavian artery for thoracic endovascular aortic repair. J Vasc Surg. 2019;70(4):1330–40.
Conway AM, Qato K, Nguyen Tran N, Giangola G, Carroccio A. Management of the left subclavian artery in TEVAR for chronic type B aortic dissection. Vasc Endovascular Surg. 2020;54(7):586–91.
Johnson CE, Zhang L, Magee GA, Ham SW, Ziegler KR, Weaver FA, et al. Periscope sandwich stenting as an alternative to open cervical revascularization of left subclavian artery during zone 2 thoracic endovascular aortic repair. J Vasc Surg. 2021;73(2):466-75
Magee GA, Veranyan N, Kuo EC, Ham SW, Ziegler KR, Weaver FA, et al. Anatomic suitability for off-the-shelf thoracic single side-branched endograft in patients with type B aortic dissection. J Vasc Surg. 2019;70(6):1776–81.
Wang ZG, Li C. Single-branch endograft for treating stanford type B aortic dissections with entry tears in proximity to the left subclavian artery. J Endovasc Ther. 2005;12(5):588–93.
Mougin J, Sobocinski J, Kratzberg J, Fabre D, Haulon S. Applicability of a standardized thoracic endograft with a single branch for the left subclavian artery to treat aortic disease involving the distal arch. J Vasc Surg. 2020;72(5):1516–23.
Tsilimparis N, Law Y, Rohlffs F, Spanos K, Debus ES, Kölbel T. Fenestrated endovascular repair for diseases involving the aortic arch. J Vasc Surg. 2020;71(5):1464–71.
Tsilimparis N, Detter C, Law Y, Rohlffs F, Heidemann F, Brickwedel J, et al. Single-center experience with an inner branched arch endograft. J Vasc Surg. 2019;69(4):977-985
Tsilimparis N, Debus ES, von Kodolitsch Y, Wipper S, Rohlffs F, Detter C, et al. Branched versus fenestrated endografts for endovascular repair of aortic arch lesions. J Vasc Surg. 2016;64(3):592–9.
Spear R, Clough RE, Fabre D, Roeder B, Hertault A, Martin Gonzalez T, et al. Total endovascular treatment of aortic arch disease using an arch endograft With 3 inner branches. J Endovasc Ther. 2017;24(4):534–8.
Spear R, Hertault A, Van Calster K, Settembre N, Delloye M, Azzaoui R, et al. Complex endovascular repair of postdissection arch and thoracoabdominal aneurysms. J Vasc Surg. 2018;67(3):685–93.
Gehringhoff B, Torsello G, Pitoulias GA, Austermann M, Donas KP. Use of chimney grafts in aortic arch pathologies involving the supra-aortic branches. J Endovasc Ther. 2011;18(5):650–5.
Huang C, Tang H, Qiao T, Liu C, Zhou M. Early results of chimney technique for type B aortic dissections extending to the aortic arch. Cardiovasc Intervent Radiol. 2016;39(1):28–35.
Ahanchi SS, Almaroof B, Stout CL, Panneton JM. In situ laser fenestration for revascularization of the left subclavian artery during emergent thoracic endovascular aortic repair. J Endovasc Ther. 2012;19(2):226–30.
Redlinger RE, Ahanchi SS, Panneton JM. In situ laser fenestration during emergent thoracic endovascular aortic repair is an effective method for left subclavian artery revascularization. J Vasc Surg. 2013;58(5):1171–7.
Li C, Xu P, Hua Z, Jiao Z, Cao H, Liu S, et al. Early and midterm outcomes of in situ laser fenestration during thoracic endovascular aortic repair for acute and subacute aortic arch diseases and analysis of its complications. J Vasc Surg. 2020;72(5):1524–33.
Zhao Z, Qin J, Yin M, Liu G, Liu X, Ye K, et al. In Situ Laser Stent Graft fenestration of the left subclavian artery during thoracic endovascular repair of type B aortic dissection with limited proximal landing zones: 5-year outcomes. J Vasc Interv Radiol. 2020;31(8):1321–7.
Han SM, Kuo EC, Woo K, Elsayed R, Nguyen BS, Ham SW, et al. Remodeling of abdominal aortic branch perfusion after thoracic endovascular aortic repair for aortic dissections. J Vasc Surg. 2016;64(4):902–11.
Kölbel T, Carpenter SW, Lohrenz C, Tsilimparis N, Larena-Avellaneda A, Debus ES. Addressing persistent false lumen flow in chronic aortic dissection: the knickerbocker technique. J Endovasc Ther. 2014;21(1):117–22.
Kotani S, Inoue Y, Kasai M, Suzuki S, Hachiya T. Modified candy-plug technique for chronic type B aortic dissection with aneurysmal dilatation: a case report. J Cardiothorac Surg. 2017;12(1):77.
Kölbel T, Lohrenz C, Kieback A, Diener H, Debus ES, Larena-Avellaneda A. Distal false lumen occlusion in aortic dissection with a homemade extra-large vascular plug: the candy-plug technique. J Endovasc Ther. 2013;20(4):484–9.
Kazimierczak A, Rynio P, Jędrzejczak T, Mokrzycki K, Samad R, Brykczyński M, et al. Expanded petticoat technique to promote the reduction of contrasted false lumen volume in patients with chronic type B aortic dissection. J Vasc Surg. 2019;70(6):1782–91.
Kazimierczak A, Rynio P, Jędrzejczak T, Samad R, Rybicka A, Gutowski P. Aortic remodeling after extended PETTICOAT technique in acute aortic dissection type III B. Ann Vasc Surg. 2020;66:183–92.
Han SM, Tenorio ER, Mirza AK, Zhang L, Weiss S, Oderich GS. Low-profile zenith alphaTM thoracic stent graft modification using preloaded wires for urgent repair of thoracoabdominal and pararenal abdominal aortic aneurysms. Ann Vasc Surg. 2020;67:14–25.
Oderich GS, Ribeiro M, de Reis SL, Hofer J, Wigham J, Cha S. Endovascular repair of thoracoabdominal aortic aneurysms using fenestrated and branched endografts. J Thorac Cardiovasc Surg. 2017;153(2):S32–41.
Starnes BW, Tatum B. Early report from an investigator-initiate investigational device exemption clinical trial on physician-modified endovascular grafts. J Vasc Surg. 2013;58(2):311–7.
Sweet MP, Starnes BW, Tatum B. Endovascular treatment of thoracoabdominal aortic aneurysm using physician-modified endografts. J Vasc Surg. 2015;62(5):1160–7.
Hu Z, Li Y, Peng R, Liu J, Jia X, Liu X, et al. Multibranched stent-grafts for the treatment of thoracoabdominal aortic aneurysms: a systematic review and meta-analysis. J Endovasc Ther. 2016;23(4):626–33.
Oderich GS, Farber MA, Silveira PG, Tadros R, Marin M, Fillinger M, et al. Technical aspects and 30-day outcomes of the prospective early feasibility study of the GORE EXCLUDER thoracoabdominal branched endoprosthesis (TAMBE) to treat pararenal and extent IV thoracoabdominal aortic aneurysms. J Vasc Surg. 2019;70(2):358-68.e6.
Coselli JS, Bozinovski J, LeMaire SA. Open surgical repair of 2286 thoracoabdominal aortic aneurysms. Ann Thorac Surg. 2007;83(2):S862–4.
Coselli JS, LeMaire SA, Preventza O, de la Cruz KI, Cooley DA, Price MD, et al. Outcomes of 3309 thoracoabdominal aortic aneurysm repairs. J Thorac Cardiovasc Surg. 2016;151(5):1323–37.
Rigberg DA, McGory ML, Zingmond DS, Maggard MA, Agustin M, Lawrence PF, et al. Thirty-day mortality statistics underestimate the risk of repair of thoracoabdominal aortic aneurysms: a statewide experience. J Vasc Surg. 2006;43(2):217–22.
Ham SW, Chong T, Moos J, Rowe VL, Cohen RG, Cunningham MJ, et al. Arch and visceral/renal debranching combined with endovascular repair for thoracic and thoracoabdominal aortic aneurysms. J Vasc Surg. 2011;54(1):30–40.
Fulton JJ, Farber MA, Marston WA, Mendes R, Mauro MA, Keagy BA. Endovascular stent-graft repair of pararenal and type IV thoracoabdominal aortic aneurysms with adjunctive visceral reconstruction. J Vasc Surg. 2005;41(2):191–8.
Moulakakis KG, Mylonas SN, Antonopoulos CN, Liapis CD. Combined open and endovascular treatment of thoracoabdominal aortic pathologies: a systematic review and meta-analysis. Ann Cardiothorac Surg. 2012;1(3):267–76.
Famularo M, Meyermann K, Lombardi JV. Aneurysmal degeneration of type B aortic dissections after thoracic endovascular aortic repair: a systematic review. J Vasc Surg. 2017;66(3):924–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Alyssa J Pyun, MD declares that she has no conflict of interest. Sukgu M Han, MD MS is a consultant for WL Gore and associates, Cook Medical, Terumo Aortic, Medtronic.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection on Vascular Surgery.
Rights and permissions
About this article
Cite this article
Pyun, A.J., Han, S.M. Modern Management of Type B Aortic Dissections. Curr Surg Rep 9, 22 (2021). https://doi.org/10.1007/s40137-021-00299-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s40137-021-00299-1