Abstract
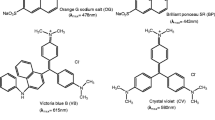
By introducing 1,2,3-triazole-ester groups on thiacalix[4]arene based on click chemistry and then ammonolysis with ethylenediamine, diethylenetriamine or triethylenetetramine, three novel 1,2,3-triazole-modified thiacalix[4]arene polymers were conveniently prepared in “4+2” condensation mode in ideal yields. The structures of polymers 4a–4c were confirmed by elemental analysis, FTIR and 1H NMR spectra. The surface morphologies of polymers 4a–4c were investigated by SEM micrographs. The M n of novel polymers 4a–4c indicated approximately 18–20 calixarene units in each polymer molecule. The dye adsorption abilities of polymers 4a–4c for series of organic dyes (alizarin green, orange I, neutral red (NR), Congo red (CR), orange G (OG), crystal violet, Victoria blue B and methylene blue) were studied by solid–liquid adsorption experiments. The adsorption experimental results implied that they had excellent adsorption abilities for tested organic dyes. The highest adsorption percentage reached 97 % for OG. The best saturation adsorption capacities for NR and CR were as high as 1.282 and 1.407 mmol/g, respectively. These novel polymers possessed stable adsorption abilities in the scopes of pH 5–9 and had good reused properties after desorption. The adsorption mechanism proposed that not only dipole interaction, electrostatic interaction and hydrogen bonding interaction, but also the π–π stacking interaction plays important role in binding dyes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dyes are widely used in various industries including textiles, leather, dyestuffs, papers, etc. However, serious water pollution is brought about by wastewaters in these industries [1, 2]. In order to remove the dyes from wastewaters, many conventional treatment methods, such as precipitation [3], extraction [4], adsorption [5, 6], membrane filtration [7] and photodegradation [8] have been developed. Among these wastewater treatments, much attention has been paid to adsorption due to its providing selective separation and recovery of dyes from the effluent mixtures. As a result, the design and synthesis of novel absorbent for dyes is the crucial task in this research field. Recently, some researches focused on the supramolecule-based hosts for binding dyestuff effectively. For example, the synthesis and dye complexation abilities of some calixarene-based derivatives and polymers were described by Yilmaz et al. [9–11], Memon et al. [12, 13] and Diao et al. [14], respectively. Our group also reported series of calixarene derivatives and polymers with high binding properties for aniline derivatives [15, 16], and organic dyes [17–19]. These literatures suggested that the structures of calixarene skeleton, the sizes of cavities and the influences of intermolecular acting forces produced by different functional groups played important roles in binding dyes.
Thiacalix[4]arene, of which the phenyl groups are bridged by four sulfur atom, possesses excellent structural flexibility and exhibits outstanding recognition ability for organic molecules, anions and cations [20–22]. Moreover, its flexible cavity might be favorable for adjusting its shape to match the structures of guests. On the other hand, the 1,2,3-triazole ring, which could be obtained conveniently by click chemistry [23–25] easily produce π–π stacking interaction with other aromatic systems, such as dyes. Lately, we synthesized several novel thiacalix[4]arene derivatives based on click chemistry and found for the first time that they possessed excellent binding capabilities for dyes in thiacalix[4]arene chemistry [26]. However, no thiacalix[4]arene polymers were reported to bind dyes up to now.
In this paper, we designed and synthesized series of novel thiacalix[4]arene polymers based on click chemistry and studied the dye adsorption abilities of thiacalix[4]arene polymers for the first time. The results indicated that they exhibited excellent adsorption capabilities for all kinds of dyes as expected.
Experimental
Materials
All solvents and reagents were purchased from Aladdin Chemical Reagents Factory, Shanghai, China. All solvents were purified by standard procedures before use. All other chemicals, except special instruction, were analytically pure and used without further purification. All reactions were carried out under nitrogen atmosphere. The azido compound 1 was easily obtained by the reaction of ethyl chloracetate with NaN3 in DMSO [27]. Thiacalix[4]arene derivative 2 was synthesized by literature method [26]. Polymers 4a–4c used in adsorption experiment were scrunched and sieved with diameter size of 500–1,000 μm.
Characterization
1H NMR spectra were recorded in CDCl3 on a Bruker-ARX 500 instrument (Switzerland) at room temperature, using TMS as an internal standard. Elemental analyses were performed at Carlo-Erba 1106 Elemental Analyzer (Italy). Osmometric molecular weight determinations were carried out a Knauer vapor pressure osmometer (USA) at concentrations of ca. 10−3 M (based on the polymeric units) in CHCl3 at 37 °C using sucrose octaacetate for calibration. The quotients of vapor pressure signal over concentration were plotted against C. The M n values were calculated by the inversely proportional to the intercept at [C] = 0. FTIR spectra were recorded on Perkin-Elmer 1605 FTIR spectrometer (USA) as KBr pellets. UV–Vis measurements were performed on a Varian UV–Vis instrument (Germany). Surface morphology of polymers, which were scrunched and sieved with diameter size of 500–1,000 μm, was studied by using SEM (JEOLJSM-6490LA, Japan) after the gold coating, operating at 10 kV.
Methods
Synthesis of tetra(1-ethylacetyl-1H-1,2,3-triazolyl-methyloxy)thiacalix[4]arene derivative 3
Compound 1 (1 mL), alkynylcalix[4]arene 2 (0.16 g, 0.2 mmol), CuSO4 (0.20 g, 0.8 mmol) and sodium ascorbate (0.79 g, 4 mmol) were stirred in dry DMF (40 mL). The mixture was heated at 90 °C for 15 h, and then diluted with CHCl3 (20 mL) and washed with water (3 × 20 mL). The organic phase was dried over MgSO4, filtered and the solvent removed under reduced pressure. The crude product was purified by recrystallization with CHCl3/MeOH, affording 3 as a white powder in yield of 80 %. 1H NMR δ H (500 MHz, CDCl3): 1.09 (36 H, s, But), 1.22–1.25 (12 H, t, J = 12.0 Hz, CH2CH3), 4.19–4.20 (8 H, m, COCH2), 5.02 (8 H, s, NCH2), 5.28 (8 H, s, OCH2), 6.95 (4 H, s, N–CH(=C)), 7.16 (8 H, s, ArH). ESI–MS m/z (%): 1,389.9 (M+, 100). Anal. calcd. for C68H84O12S4N12: C 58.77, H 6.09, N 12.09; Found: C 58.67, H 6.17, N 11.98.
Synthesis of 1,2,3-triazole-modified thiacalix[4]arene polymers 4a–4c
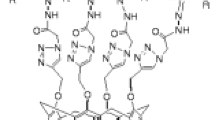
Under nitrogen atmosphere, the mixture of compound 3 (0.5 g, 0.36 mmol) and ethylenediamine (0.06 g, 1 mmol) in 12 mL of CHCl3/MeOH (v/v = 1:2) was stirred and refluxed for 6 h until the materials utterly vanished under the monitor of TLC. After reaction, the solvent was evaporated under reduced pressure. The residue was treated with distilled water (20 mL) and extracted with CHCl3 (20 mL). The organic layer was separated and dried by MgSO4. After evaporating off the solvent to dryness, the powder was washed by small amount of acetone to remove small molecules, and was dried in vacuum. 0.49 g of polymer 4a was obtained as light yellow powder. According to the same procedure with diethylenetriamine or triethylenetetramine instead of ethylenediamine, polymer 4b (0.48 g) and 4c (0.47 g) were obtained as light yellow powder, respectively (Fig. 1).
Polymer 4a: 1H NMR δ H (500 MHz, CDCl3): 1.13 (36 H, s, But), 3.91–5.12 (24 H, m, OCH2 and NCH2), 6.93 (4 H, bs, N–CH(=C)), 7.18 (8 H, bs, ArH), 8.92 (4H, bs, NH).
Polymer 4b: 1H NMR δ H (500 MHz, CDCl3): 1.14 (36 H, bs, But), 3.90–5.14 (34 H, m, OCH2, NCH2 and NH), 6.95 (4 H, bs, N–CH(=C)), 7.17 (8 H, bs, ArH), 8.89 (4H, bs, NH).
Polymer 4c: 1H NMR δ H (500 MHz, CDCl3): 1.16 (36 H, bs, But), 3.87–5.16 (44 H, m, OCH2, NCH2 and NH), 6.94 (4 H, bs, N–CH(=C)), 7.15 (8 H, bs, ArH), 8.81 (4H, bs, NH). The elemental analysis, FTIR spectrum, SEM, and osmometric M n of polymers 4a–4c are shown in Table 1 and Figs. 2 and 3, respectively.
Adsorption percentages of polymers 4a–4c
According to the published method [28], 10 mg of polymer was added to 10 mL of aqueous solution of corresponding dye (1.0 × 10−3 mol/L, pH 7). After stirring for 24 h at 25 °C, the mixture was filtered. The concentration of dye after adsorption in filtrate was examined by UV spectra. The percentage of dye adsorbed by polymer was calculated by the following equation:
where E is the adsorption percentage of polymer, C 0 and C are the concentrations of dye before and after the adsorption, respectively. Average of twice-independent experiments was carried out. Blank control experiment in the absence of the calixarene polymer showed that the change of concentration before and after stirring for 24 h at 25 °C was less than 2 %.
Saturated adsorption capacity of polymer 4a–4c
According to reported method [28], the approach of changing the volume of dye was employed to examine the saturated adsorption capacity. The polymer (10 mg) was added in sequences into solutions of dyes (1.0 × 10−3mol/L), of which the volumes were 5, 10, 15, 20, 25, 30, 35, 40, 45, 50 and 60 mL, respectively. Then the mixture was stirred for 24 h at 25 °C and was filtered. The concentration in the filtrate was determined by UV spectra. The adsorption capacity was calculated as follows:
where Q was the adsorption capacity, C was the initial concentration before adsorption, C* was the concentration after adsorption, V was the volume of solution and W was the mass of the polymer.
Adsorption percentages at different pH values
According to reported method [28], the polymer (10 mg) and solutions of dye (1 × 10−3 mol/L, 10 mL) at different pH values (the pH values were adjusted by HCl or NaOH) were mixed and stirred for 24 h at 25 °C. After filtration, the concentration of dye was examined by UV–Vis spectroscopy and the adsorption percentage was counted.
Results and discussion
Synthesis and characterization
The synthetic route of novel polymers 4a–4c is illustrated in Scheme 1. Compound 2 was synthesized by published method [26]. Then, the reaction of compound 2 with azide 1 was carried out in DMF at 90 °C with copper(II) sulfate and sodium ascorbate to afford 1,2,3-triazolyl-modified thiacalix[4]arene 3 in yield of 80 % via click chemistry. Further, by ammonolysis of compound 3 with corresponding polyamine, the 1,2,3-triazole-modified thiacalix[4]arene polymers 4a–4c were conveniently prepared in good yield. In order to convert the ester groups to amido groups completely, few excess amounts of polyamine were added in these ammonolysis reaction. Since compound 3 and polyamines possessed four and two reactive groups, respectively, and compound 3 possess the 1,3-alternate conformation, it could be deduced that polymers 4a–4c were a tridimensional netty “4+2” cross-linking polymers. It is worth noting that these polymers were the first examples of calixarene polymers based on click chemistry.
The structures and morphologies of polymers 4a–4c were studied by 1H NMR, elemental analysis, FTIR and SEM techniques. The signals of 1H NMR of polymers were well assigned for the corresponding protons (Fig. 1 exhibited the 1H NMR of polymer 4a as representative one). The elemental analysis data and molecular weight of polymers 4a–4c are shown in Table 1. The elemental analysis data were approximately in accordance with the calculated data obtained by the hypothesis that four ester groups in one molecule of compound 3 were ammonolyzed ideally by two times of diamines exactly. These results also indicated that few free primary amines existed in polymers 4a–4c. The molecular weight for polymers 4a–4c was determined by vapor pressure osmometric measurements. The M n of polymers 4a–4c after calculation was 23,537, 26,584 and 29,653, respectively, indicating average approximately 18–20 calixarene units in each polymer molecule.
The structures of polymers 4a–4c were also confirmed by FTIR spectra. Figure 2 exhibited the FTIR spectra of polymers 4a–4c and compound 3. Obviously, it can be seen that the strong absorption peaks at 1,755 cm−1 for C=O of ester groups in spectrum of compound 3 vanished completely in the FTIR spectra of polymers 4a–4c. On the other hand, the strong absorption peaks for amido groups appeared at 1,680 cm−1 approximately in the FTIR spectra of polymers 4a–4c. These FTIR spectra certainly supported that the esters’ groups of compound 3 were substituted utterly by amido groups. Also, the peak of free primary amines was not observed apparently, indicating that little primary amines existed in polymers 4a–4c. These results were in accordance with the results of elemental analysis. Moreover, it was reasonable to deduce that these polymers might prefer to adopt three-dimensional polymers than linear polymers because compound 3 possessed four ester groups in 1,3-alternate conformation [26]. The surface morphologies of polymers 4a–4c were also observed by scanning electron microscopy (SEM). The results are shown in Fig. 3. As expected, polymers 4a–4c exhibited loose, porous and netty morphologies, which were favorable for producing the excellent binding abilities for guests.
Dyes adsorption percentages of polymers 4a–4c
Lately, we had reported that the 1,2,3-triazole-modified thiacalix [4] arenes exhibited excellent extraction abilities for dyes due to the complexation action of flexible cavities and 1,2,3-triazole rings [26]. Because the novel polymers 4a–4c were the derived products of this 1,2,3-triazole-modified thiacalixarene and the new amido groups in polymers were favorable for complexation based on hydrogen bonding, polymers 4a–4c were expected to possess excellent dyes absorption capabilities. Thus, the adsorption abilities of polymers 4a–4c were investigated for a series of dyes including alizarin green (AG), orange I (OI), neutral red (NR), Congo red (CR), orange G (OG), crystal violet (CV), Victoria blue B (VB), methylene blue (MB) which are usual pollutants in water.
The adsorption percentages of polymers 4a–4c for dyes are shown in Table 2. It could be seen that all novel polymers had good adsorption capabilities for the eight tested dyes. The adsorption percentages for OI, CR, NR and OG were as high as over 90 %. The highest adsorption percentage reached 97 % for OG. These adsorption percentages were very outstanding when compared with the previous reports of calixarene resins [11–13] or polymers [16–18]. Also, the adsorption percentages of polymers 4a–4c were higher than the extraction abilities of their precursive compound 3, which could be attributed to the introduction of triazole-amide-amine units. Moreover, it could be observed that polymers 4a–4c exhibited similar adsorption abilities for both anionic dyes (AG, CR, OG and OI) and cationic dyes (MB, VB, CV and NR).
On the other hand, although the polymers 4a–4c possessed different bridging polyamines chains, no obvious differences were observed for their dye adsorption abilities. These adsorption results suggested that polymers 4a–4c have excellent dye adsorption capabilities. The similar adsorption abilities for anionic and cationic dyes, and no obvious differences for different bridging structures of polymers 4a–4c might support the speculation of the adsorption mechanism that the adsorption abilities of novel polymers were influenced not only by dipole, electrostatic and hydrogen bonding interactions which were usually observed in adsorption, but also by π–π stacking interaction of the phenyl groups and triazole ring with the aromatic conjugate system of dyes, and flexible cavities of thiacalix[4]arene skeleton for dyes complexation. Due to π–π stacking interaction, flexible cavities were influenced little by polyamines chains, thus, polymers 4a–4c with different bridging chains showed similar adsorption abilities for both anionic and cationic dyes.
Saturated adsorption capacities of polymers 4a–4c
In order to investigate the saturated adsorption capacities of polymers 4a–4c for dyes, NR and CR were chosen as the representative ones of cationic dyes and anionic dyes, respectively, to test the saturated adsorption capabilities. The results were shown in Fig. 4. It could be seen that the saturation adsorption capacities of polymers 4a–4c for NR and CR were as high as 1.2–1.4 mmol/g. The highest saturated adsorption capacities for NR and CR was 1.282 and 1.407 mmol/g, respectively. These saturated adsorption capacities were outstanding among all kinds of other absorbents for dyes [29–31]. On the other hand, polymers 4a–4c with different bridging polyamines chains showed similar saturated adsorption capabilities, which also could be explained by the previous speculated adsorption mechanism. These saturated adsorption results suggested that this kind of polymers possessed good applied prospective for the extraction of noxious dyes in polluted water.
The influence of pH values on adsorption percentages
The pH value is an important influencing factor for adsorption in practical application. Thus, the adsorption experiments under different pH values were done and the results are shown in Fig. 5. In order to avoid the protonation of amino groups at high acidity and the insolubilization of dyes at high acidity or high alkalinity, the scope of pH values was controlled at pH 5–9. The results showed that the adsorption percentages of polymers 4a–4c fluctuated in 77–96 %, which were not remarkable comparing with the other kinds of absorbents [29–31]. These results suggested that the adsorption abilities of polymers 4a–4c were fairly stable in the scope of pH 5–9. These adsorption results at different pH values exhibit good applicability in widely pH scopes.
Reuse of polymers 4a–4c after desorption
The recycling property is an important feature for an adsorbent. Thus, the reuse property of polymer 4c for CR was studied as the representative one. After adsorption for CR, the polymer 4c was desorbed by 10 % HCl and subsequently 10 % NaOH, washed adequately by distilled water and dried by vacuum. Then, the polymer was used for dye adsorption again. The five times’ adsorption percentages were measured and the results were 96.3, 88.8, 84.3, 82.7 and 80.4 %, respectively, which indicated that this kind of polymers have good recycling property.
Conclusion
This work reported the synthesis of the novel 1,2,3-triazole-modified thiacalix[4]arene polyamine polymers based on click chemistry. Their structures were characterized by 1H NMR, FTIR, and elemental analysis methods. The loose, porous and netty architectures were observed for novel polymers in their SEM analysis. The M n of novel polymers suggested average approximately 18–20 calixarene units in each polymer molecule. The adsorption experiments for dyes indicated that they have excellent adsorption abilities for both cationic and anionic dyes. The best saturation adsorption capacities for NR and CR were as high as 1.282 and 1.407 mmol/g, respectively. The adsorption abilities were stable at pH 5–9. The adsorption mechanism was speculated and the excellent adsorption capabilities were influenced not only by dipole, electrostatic and hydrogen bonding interactions, but also by the π–π stacking interaction of the phenyl groups and triazole ring with the aromatic conjugate system of dyes, and the flexible cavities of thiacalix[4]arene skeleton for dyes complexation.
References
Wong Y, Yu J (1999) Laccase-catalyzed decolorization of synthetic dyes. Water Res 33:3512–3519
Crini G (2005) Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog Polym Sci 30:38–70
Peternel IT, Koprivanac N, Bozic AML, Kusic HM (2007) Comparative study of UV/TiO2, UV/ZnO and photo-Fenton processes for the organic reactive dye degradation in aqueous solution. J Hazard Mater 148:477–484
Crini G, Badot PM (2008) Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using bath studies: a review of recent literature. Prog Polym Sci 33:399–447
Mahdavinia GR, Iravani S, Zoroufi S, Hosseinzadeh H (2014) Magnetic and K+-cross-linked kappa-carrageenan nanocomposite beads and adsorption of crystal violet. Iran Polym J 23:335–344
Barikani M, Oliaei E, Seddiqi H, Honarkar H (2014) Preparation and application of chitin and its derivatives: a review. Iran Polym J 23:307–326
Chakraborty S, Purkait MK, Das Gupta S, De S, Basu JK (2003) Nanofiltration of textile plant effluent for color removal and reduction in COD. Sep Purif Technol 31:141–151
Ghosh D, Bhattacharyya KG (2002) Adsorption of metheylene blue on kaolinite. Appl Clay Sci 20:295–300
Akceylan E, Bahadir M, Yilmaz M (2009) Removal efficiency of a calix[4]arene-based polymer for water-soluble carcinogenic direct azo dyes and aromatic amines. J Hazard Mater 162:960–966
Ozmen EY, Sirit A, Yilmaz M (2007) A calix[4]arene oligomer and two betacyclodextrin polymers: synthesis and sorption studies of azo dyes. J Macromol Sci Pure 44:167–173
Yilmaz A, Yilmaz E, Yilmaz M, Bartsch RA (2007) Removal of azo dyes from aqueous solutions using calix[4]arene and cyclodextrin. Dyes Pigment 74:54–59
Kamboh MA, Solangi IB, Sherazi STH, Memon S (2011) A highly efficient calix[4]arene based resin for the removal of azo dyes. Desalination 268:83–89
Kamboh MA, Solangi IB, Sherazi STH, Memon S (2011) Synthesis and application of p-tert-butylcalix[8]arene immobilized material for the removal of azo dyes. J Hazard Mater 186:651–656
Chen M, Shang T, Fang W, Diao G (2011) Study on adsorption and desorption properties of the starch grafted p-tert-butyl-calix[n]arene for butyl Rhodamine B solution. J Hazard Mater 185:914–920
Guo HY, Yang FF, Chai XF, Jiao ZY, Li CC (2012) Synthesis of novel calix[6]-1,4-crown-based netty polymer and its excellent adsorption capabilities for aniline derivatives. Iran Polym J 21:451–456
Yang FF, Huang ZS, Zhang XY, Guo HY (2010) Thiacalix[4]amido-based netty polymers: novel sorbents for heavy metal cations and derivatives of aniline. Iran Polym J 19:309–318
Yang FF, Liu WW, Xie JW, Bai XY, Guo HY (2013) Novel deep-cavity calix[4]arene derivatives with s-triazine conjugate systems: synthesis and complexation for dyes. J Incl Phenom Macrocycl Chem 76:311–316
Yang FF, Zhang YM, Guo HY, Wei XL (2013) Highly efficient liquid membrane transport of dyes using calix[4]arene-linked triphenylene dimers as carriers. Sep Sci Technol 48:1565–1571
Bai XY, Yang FF, Xie JW, Guo HY (2013) Novel 1,2-3,4-bridged and 1,3-bridged calix[4]arene based on large s-triazine conjugate systems: synthesis and complexation for dyes. J Macromol Sci Pure 50:334–339
Kumar M, Bhalla V, Dhir A, Babu JN (2010) A Ni2+ selective chemosensor based on partial cone conformation of calix[4]arene. Dalton Trans 39:10116–10121
Coquiere D, LeGac S, Darbost U, Seneque O, Jabin I, Reinaud O (2009) Biomimetic and self-assembled calix[6]arene-based receptors for neutral molecules. Org Biomol Chem 7:2485–2500
Kovalev VV, Khomich EV, Shokova EA, Babain AV, Alyapyshev MY (2008) Synerg istic extraction of cesium, strontium, and europium with adamanty lated thiacalix[4]arenes in the presence of chlorinated cobalt dicarbollide. Russ J Gen Chem 78:19–25
Feng NM, Zhao HY, Zhan JY, Tian D, Li H (2012) Switchable wettability sensor for ion pairs based on calix[4]azacrown clicking. Org Lett 14:1958–1961
Zhan JY, Fang F, Tian DM, Li HB (2012) Anthraquinone-modified calix[4]arene: click synthesis, selective calcium ion fluorescent chemosensor and INHIBIT logic gate. Supramol Chem 24:272–278
Hardman MJ, Thomas AM, Carroll LT, Williams LC, Parkin S, Fantini JL (2011) Synthesis and ‘click’ cycloaddition reactions of tetramethoxy- and tetrapropoxy-2-(omega-azidoalkyl)calix[4]arenes. Tetrahedron 67:7027–7034
Guo HY, Yang FF, Jiao ZY, Lin JR (2013) Click synthesis and dyes extraction capabilities of novel thiacalix[4]arene derivatives with triazole groups and hydrogen bond groups. Chin Chem Lett 24:450–452
Iki N, Kumakai H, Morohashi N, Ejima K, Hasegawa M, Miyanri S, Miyano S (1998) Selective oxidation of thiacalix[4]arenes to the sulfinyl- and sulfonylcalix[4]arenes and their coordination ability to metal ions. Tetrahedron Lett 39:7559–7562
Yang FF, Bai XY, Xu BT, Guo HY (2013) Triphenylene-modified chitosan: novel high efficient sorbent for cationic and anionic dyes. Cellulose 20:895–906
Xie K, Zhao W, He X (2011) Adsorption properties of nano-cellulose hybrid containing polyhedral oligomeric silsesquioxane and removal of reactive dyes from aqueous solution. Carbohydr Polym 83:1516–1520
Duan C, Zhao N, Yu X, Zhang X, Xu J (2013) Chemically modified kapok fiber for fast adsorption of Pb2+, Cd2+, Cu2+ from aqueous solution. Cellulose 20:849–860
Mao Y, Guan Y, Zheng Q, Feng X, Wang X (2011) Adsorption thermodynamic and kinetic of disperse dye on cotton fiber modified with tolylene diisocyanate derivative. Cellulose 18:271–279
Acknowledgments
Financial support from the National Natural Science Foundation of China (No: 21406036), Fujian Natural Science Foundation of China (No. 2014J01034), Project of Fujian Provincial Department of Education (JA11044, JA10056, JB13011) and the Program for Innovative Research Team in Science and Technology in Fujian Province University were greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lai, J., Yang, F., Guo, H. et al. Novel effective dye sorbents: synthesis and properties of 1,2,3-triazole-modified thiacalix[4]arene polymers based on click chemistry. Iran Polym J 23, 899–906 (2014). https://doi.org/10.1007/s13726-014-0289-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-014-0289-9