Abstract
Biopolymer-based magnetic beads, composed of kappa-carrageenan (κ-Car) and Fe3O4 nanoparticles, were synthesized. The magnetic beads were prepared through in situ precipitation of Fe2+/Fe3+ ions in the presence of carrageenan and subsequently treating with K+ solution. The structure of magnetic kappa-carrageenan beads (mκ-Carb) was characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), vibrating sample magnetometer, and thermal gravimetric analysis techniques. According to SEM micrographs, an undulant and coarse structure with cubic-shaped sections was obtained when the magnetic nanoparticles were incorporated in composition of beads. The TEM image confirmed the formation of magnetic nanoparticles with an average size of 3–7 nm. The synthesized beads were examined as adsorbent to remove crystal violet dye from aqueous solutions. It was found that due to coarse surface, the rate of dye adsorption on magnetic beads can be improved slightly. The experimental adsorption kinetics was analyzed according to pseudo-first-order and pseudo-second-order kinetic models and the adsorption kinetics followed well the pseudo-second-order model. Isotherm adsorption data of dye on beads were modeled according to Langmuir and Freundlich isotherm models. The results revealed that the experimental data have the best fit to Langmuir isotherm model, and maximum adsorption capacity of beads for dye obtained was 84.7 mg/g. The influence of pH on the variation of adsorption capacity of beads for crystal violet was not considerable. The thermodynamic parameters indicated that the adsorption of CV dye on beads is spontaneous.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carrageenan is a collective term for linear sulfated polysaccharides produced by alkaline extraction from red seaweed. The types of carrageenans differ only in the position and number of ester sulfate groups which determine their physicochemical properties, e.g., viscosity and gelation characteristics. Carrageenans comprise three main forms: lambda (non-gelling), kappa (strong gelling), and iota (weak gelling) [1]. Because of their exceptional properties, carrageenans are broadly used as ingredients in a variety of applications. They have several major characteristics that make them very useful in many food and non-food applications. They are used as cost-effective stabilizers, thermo-reversible gelling agents, binders, thickeners, texture modifiers, and moisture retainers. There are numerous reviews of their chemistry [2] and applications in foods [3] and drug delivery systems [4].
The gelling of kappa-carrageenan can occur physically and chemically. kappa-Carrageenan contains anion sulfate pendant on its backbones. Electrostatic interactions between sulfate on carrageenan with metal cations (especially K+) [5] and polycationic polyelectrolytes (especially chitosan) [6] are classes of physical cross-linking that have been used for synthesis of kappa-carrageenan hydrogels. Chitosan/carrageenan nanoparticles [6], alginate/carrageenan beads [7], and kappa-carrageenan/barium ferrite [8] have been synthesized and evaluated through physical cross-linking methods. Chemical cross-linking is originated from presence of reactive hydroxyl groups on kappa-carrageenan. Chemical cross-linking agents such as divinyl sulfone [9], genipin [10], and gamma irradiation [11] have been used for preparation of carrageenan-based hydrogel.
Industrial sources such as textile and paper printing produce large amount of wastewater containing dyes. Toxic dyes are the type of pollutants that can damage the environment and they must be removed from wastewater before being discharged into the environment. The removal techniques such as chemical precipitation, filtration, electrochemical treatment, reverse osmosis, and adsorption have been developed to remove dyes from water [12]. Because of its low cost and ease of operation, adsorption method has attracted many attentions to remove cationic dyes [12]. Adsorption of cationic dyes and other pollutants on biopolymer-based adsorbents such as alginate, chitin, and chitosan [13–15] has been developed widely. Despite the anionic sulfate groups on carrageenan, the adsorption of pollutants on carrageenan beads has not been extensively studied. Recently, biopolymer-based beads have been modified by the introduction of magnetic particles. It has been reported that unlike the traditional adsorbents, the recovery of magnetic adsorbents from solutions can be performed by external magnetic field [14]. Magnetic adsorbents are generally produced by modification of magnetic nanoparticles with polymers and organosilanes [16, 17].
In this study, we attempted to synthesize magnetic carrageenan beads through in situ precipitation of Fe3+/Fe2+ ions in the presence of kappa-carrageenan. The cross-linking of beads was performed by treatment of magnetic carrageenan with K+ solution. The cationic crystal violet dye is a triphenyl methane dye and has been shown to be as carcinogenic and in this project, the synthesized magnetic beads were evaluated for removal of cationic crystal violet dye from aqueous solutions. The influence of the contact time, initial concentration of CV, and pH of initial dye solution on the adsorption capacity of magnetic carrageenan beads for dye was investigated.
Experimental
Materials
kappa-Carrageenan was obtained from Condinson Co. (Denmark). FeCl2·4H2O and FeCl3·6H2O were purchased from Merck (Germany). All other chemicals were analytical grades and were used as received.
Preparation of beads
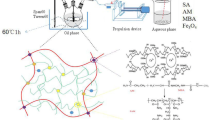
A simple method was applied to prepare in situ magnetic κ-Car beads. In brief, 2 g of κ-Car was poured into 50 mL of distilled water and the temperature was adjusted to 70 °C. After complete dissolution of κ-Car, the iron salt solutions (mκ-Carb5 0.4 g of FeCl3·6H2O and 0.2 g of FeCl2·4H2O; mκ-Crab12 0.8 g of FeCl3·6H2O and 0.4 g of FeCl2·4H2O) were added into κ-Car solution and allowed to stir at 70 °C for 10 min. The magnetic κ-Car solution was obtained through the precipitation of iron salts by addition of 2 M NH4OH solution. The dark solution confirmed the formation of magnetic particle. The pH of final solution was adjusted to 10. The obtained solution was stirred at the same temperature for 1 h. Then, the temperature of solution was decreased to 50 °C. The beads were obtained by dropwise addition of magnetic carrageenan solution into 3 wt% of KCl solution (stirring speed 60 rpm) using a 5-mL glass syringe. The beads were hardened by allowing to be stirred in KCl solution for 1 h. Then, the beads were collected and immersed in excess water to remove un-participated ingredients. After purification, the beads were dried at ambient temperature for constant weight. The magnetic-free beads (κ-Carb) were prepared according to above procedure in the absence of iron salts. The suffixes 5 and 12 in mκ-Carb5 and mκ-Carb12 show the magnetite weight percentage in beads. The approximate percentage of the magnetic nanoparticles was obtained from TGA thermograms of beads.
Swelling measurements
Dried carrageenan beads were used to determine the degree of swelling. The degree of swelling (DS) was determined by immersing the beads (~0.1 g) in distilled water and allowed to soak at room temperature for 24 h. Then, they were removed and blotted with filter paper to remove surface water, weighed and the DS was calculated using Eq. 1:
where W s and W d are the weights of the samples swollen in water and in dry state, respectively.
Dye adsorption
Dye adsorption was carried out by immersing 0.05 g of beads into 25 mL of dye solution with 25 mg L−1 of CV. All adsorption experiments were examined through a batch method on a shaker with a constant speed at 120 rpm. To study the adsorption kinetics, at specified time intervals, the amount of adsorbed CV was evaluated using a UV spectrometer at λ max = 590 nm. The content of adsorbed dye was calculated using Eq. 2:
where, C 0 is the initial CV concentration (mg L−1), C t is the remaining dye concentration in the solution at time t, V is the volume of dye solution used (L), and m is the weight of beads (g). Adsorption isotherm was carried out by immersing 0.05 g of the beads into 25 mL of dye solutions with 25, 50, 75, 100, 150, 200, and 250 mg L−1 of CV for 24 h. The equilibrium adsorption capacity of nanocomposites, q e (mg g−1), was determined using Eq. 2. In this equation, the C t and the q t are replaced with equilibrium concentration of dye in solution (C e) and equilibrium adsorption capacity (q e), respectively.
To evaluate the effect of pH on the adsorption capacity of beads, the pH of initial dye solutions was adjusted by adding dilute HCl and NaOH solutions.
Instruments
Dried beads were coated with a thin layer of gold and imaged in a SEM instrument (Vega/Tescan, Czech Republic). The magnetic properties of the beads were studied with a vibrating sample magnetometer (model 7400, Lakeshare Company, USA). TEM micrographs were recorded with a Philips CM10 (UK) operating at 60 kV tension. A thermal analyzer (Mettler Toledo, USA) was used for thermogravimetric analysis (TGA) under nitrogen. The heating rate was 20 °C/min.
Results and discussion
Synthesis and characterization
Biopolymeric magnetic beads, composed of kappa-carrageenan and Fe3O4 nanoparticles, were synthesized through in situ precipitation of Fe3+/Fe2+ ions in the presence of kappa-carrageenan. The cross-linking of beads was carried out by treating of magnetic kappa-carrageenan with K+ solutions [5]. Figure 1 indicates a simple scheme for synthesis of magnetic beads. The surface morphology of beads was investigated by SEM micrographs. It can be seen from Fig. 2 that the surface morphology of beads is significantly affected by the magnetic nanoparticles. Magnetic-free beads depict a relatively smooth surface (Fig. 2a). As the magnetic nanoparticles were introduced into composition of beads, the surface morphology of beads was changed and a coarse and undulant surface with cubic-shaped sections was appeared. According to Fig. 2a, this coarse surface cannot be due to combination of carrageenan and K+ ions. This observation may be attributed to the interaction of magnetic nanoparticles either with K+ ions or carrageenan component. Although the content of magnetic nanoparticles was not equal in mκ-Carb5 and mκ-Carb12 beads, the morphology of their surface was relatively similar (Fig. 2b mκ-Carb5 and Fig. 2c mκ-Carb12). The dispersion of nanoparticles was investigated by TEM and the TEM image of mκ-Carb5 is shown in Fig. 2d. As it may be observed from the figure, the magnetic nanoparticles in matrix of the bead have spherical structure and the size of nanoparticles is in the range of 3–7 nm.
The hysteresis loops of magnetic beads were investigated using VSM between ±9 kOe at 298 K. The results are depicted in Fig. 2e. According to the data, the saturation magnetization of mκ-Carb5 and mκ-Carb12 beads was obtained as 3.2 and 6.7 emu g−1, respectively. The saturation magnetization of mκ-Carb12 beads was obtained to be twice higher than that of mκ-Carb5 beads, in agreement with the amount of magnetic nanoparticles. Saturation magnetization of beads was sufficient to be removed from aqueous solution by a permanent magnet.
Thermal stability of beads was studied by thermogravimetric analysis (TGA) and the content of magnetic nanoparticles was also estimated from TGA data. The results are shown in Fig. 2f. As can be seen from Fig. 2f, the beads exhibited three decomposition stages. The first weight loss occurred at 50–195 °C due to the adsorbed water. Although the weight loss for κ-Carb was negligible, the weight losses for mκ-Car5 and mκ-Carb12 were obtained as 1.5 and 2.4 wt%, respectively. This observation may be attributed to the coarse surface of magnetic beads. The second stage of weight loss was observed at 200–260 °C. The temperatures of maximum weight loss rate for mκ-Carb5, mκ-Carb12, and κ-Carb were observed at 212, 203, and 200 °C, respectively. The increase in temperature of maximum weight loss for magnetic beads was higher than for non-magnetic bead indicating an improvement in thermal stability of magnetic beads. The weight loss at the third stage was observed around 400 °C and the rate of weight loss for magnetic beads was lower than that of the non-magnetic bead. At 800 °C, the residues for κ-Carb, mκ-Carb5, and mκ-Car12 were about 30.5, 40.6, and 47.6 wt%, respectively. The weight percentage of magnetic nanoparticles in composition of beads can be estimated from difference in residues of magnetic and non-magnetic beads. The amounts of magnetic nanoparticles were calculated as ~5 and 12 wt% for mκ-Carb5 and mκ-Carb12, respectively.
Swelling study
The swelling capacity of hydrogels is strongly influenced by their chemical composition [10, 16]. It was found that the degree of swelling of beads was decreased by incorporation of magnetic nanoparticles in composition of beads. The swelling capacity of κ-Carb, mκ-Carb5, and mκ-Carb12 were obtained 17.5, 14.7, and 10.2 g/g, respectively. Respective decrease in water absorbency of magnetic beads can be attributed to: (a) decrease in the ratio of hydrophilic carrageenan [18] and (b) interaction between magnetic nanoparticles and polymeric chains. These interactions can lead to physically cross-linked points and thus leading to reduced water absorbency [19].
Dye adsorption study
Adsorption kinetic
Adsorption kinetics as a useful information on the rate of dye adsorption can be considered as an important factor for proper design of adsorbent [20]. The effect of contact time on the adsorption of crystal violet on beads was investigated and it is shown in Fig. 3a. The equilibrium dye adsorption was achieved after 40, 80, and 100 min for mκ-Carb12, mκ-Carb5, and κ-Carb, respectively. The rate of adsorption of dye on magnetic beads was higher than on the non-magnetic beads. By considering the SEM micrographs, the surface of magnetic beads contained coarse structure. The coarse sections would lead to an enhanced surface area and thereby an increase in collision between dye molecules and adsorption centers on adsorbents. The removal efficiency of dye by the mκ-Carb12, mκ-Carb5, and κ-Carb was obtained as 96, 93.6, and 80 %, respectively. In fact, while the adsorption efficiency was decreased by combination of magnetic nanoparticles and carrageenan, an improvement in rate of dye adsorption on magnetic beads was observed. The decrease in dye adsorption capacity of beads with increasing amount of magnetic nanoparticles can be attributed to: (a) decrease in weight ratio of carrageenan and (b) decrease in swelling of magnetic beads that is originated from a decrease in surface area of beads.
The experimental kinetic data were analyzed by pseudo-first-order and pseudo-second-order models. The pseudo-first-order equation is described as below [21]:
where q e and q t (mg g−1) are the amounts of adsorbed dye on the beads at equilibrium and at time t, respectively. q e1 and k 1 (min−1) show the theoretical equilibrium adsorption and rate constant of pseudo-first-order kinetic, respectively. To obtain model calculations k 1, q e1, and R 2 (correlation coefficient for pseudo-first-order kinetic), we can plot Ln(q e−q t ) against t.
Also, kinetic data were analyzed using the pseudo-second-order model [21]:
where k 2 (g mg−1 min−1) is rate constant of pseudo-second-order kinetic and q e2 is the theoretical adsorbed dye (mg g−1). To obtain model calculations, k 2, and theoretically equilibrium adsorption (q e2) as well as R 2, we can plot \(\frac{t}{{q_{t} }}\) against t (Fig. 3b).
Model calculations for all beads are tabulated in Table 1. It was found that the plot of \(\frac{t}{{q_{t} }}\) against t gives a straight line with a high correlation coefficient (R2 > 0.98) and it can be concluded that adsorption kinetic of dye by all adsorbents has the best fit with the pseudo-second order. Also, according to pseudo-second-order kinetic, the theoretical equilibrium adsorption capacities (qe2) were consistent with the experimental data (qe.exp). Finally, introduction of magnetic particles led to an increase in k2 values and this is in agreement with adsorption kinetic profiles from Fig. 3a.
Adsorption isotherms
The effect of initial dye concentration on the adsorption capacity of beads for CV was investigated. The adsorption capacity of mκ-Carb5 as a function of equilibrium concentration of dye in the solution (Ce) is shown in Fig. 4a. As can be seen from figure, at low concentration of initial dye solution, the adsorption capacity of beads was sharply increased and then began to level off. In contrast, at high concentration of initial dye solution, the equilibrium concentration of dye (Ce) in solution was increased. In fact, at high concentration of dye, the adsorption sites on adsorbent reached a saturation state and while the equilibrium concentration of dye in the solution was increased, the adsorption remained constant. The types of interactions between adsorbate molecules and adsorbent surface were studied by adsorption isotherms. The experimental data were discussed according to Langmuir and Freundlich isotherm models. In the Langmuir adsorption model, adsorption of adsorbate took place at specific homogeneous sites within the adsorbent and valid for monolayer adsorption on adsorbents. The expression of the applied Langmuir model is given by Eq. 5 [22]:
where Ce is the equilibrium dye concentration in the solution (mg L−1), b is the Langmuir adsorption constant (L mg−1), and qm is the theoretical maximum adsorption capacity (mg g−1). The Langmuir constants qm and b can be calculated from the slope and intercept of plot between \(\frac{{C_{\text{e}} }}{{q_{\text{e}} }}\) and Ce (Fig. 4b).
In the Freundlich model, the adsorption of adsorbate occurs on a heterogeneous surface by multilayer sorption and the adsorption capacity can be increased with an increase in adsorbate concentration [22]. Freundlich model is expressed by Eq. 6:
where kf is the equilibrium adsorption coefficient (mg(1−n)Lng−1), and 1/n is the empirical constant. The kf and n values for beads can be calculated from the intercept and the slope of Lnqe against LnCe plots. The all expressions in Langmuir and Freundlich equations were calculated according to experimental data and are tabulated in Table 2. High correlation coefficient in Langmuir model (R2 > 0.98) indicated that this isotherm model has the best fit to the experimental data. In addition, as can be seen from Langmuir data, the theoretical maximum adsorption capacity (qm) is close to experimental data (qm.exp). So, it is concluded that the adsorption of CV onto beads occurred through monolayer adsorption. Although maximum adsorption capacity was experimentally obtained high for mκ-Carb (75 mg g−1), but according to Langmuir model, maximum adsorption capacity was achieved for mκ-Carb12 (84.7 mg g−1). The maximum adsorption capacities of some adsorbent including semi-IPN hydrogel [23], magnetic nanocomposite [24], raw kaolin [25], bagasse fly ash [26], CarAlg/MMt nanocomposite hydrogel [27], PAAm/laponite [28], and carrageenan/laponite RD nanocomposites [29] have been reported to be 35, 44.8, 26.2, 79, 88.8, 101.13, and 80.2 mg g−1, respectively. According to these data, the adsorption capacity of magnetic beads in this study is comparative with the given adsorbents.
The favorability of the adsorption (RL) was evaluated from parameters of Langmuir adsorption model. The RL can be calculated from the following equation [30]:
where b is the Langmuir constant (L mg−1) and C0 is the lowest initial concentration of dye. The RL is varied: for RL > 1 the adsorption is unfavorable; RL = 1 the adsorption is linear condition; the adsorption is favorable when 0 < RL < 1; and RL = 0 is for irreversible conditions [30]. According to Table 2, the RL values for beads were achieved between zero and 1 indicating favorable adsorption of CV onto the beads.
Effect of pH on the adsorption
The pH of initial dye solution can affect the adsorption capacity of adsorbents. This behavior originates from the nature of active centers on the adsorbents [31]. Thus, the effect of pH on the adsorption capacity of carrageenan beads for dye was studied at various pHs ranged from 2.0 to 8.0 (Fig. 5). No additional ions (through buffer solution) were added to medium for setting pH because absorbency of a hydrogel is strongly affected by ionic strength [32]. In addition, it has been reported that adsorption of dye is influenced by ionic strength [33]. Therefore, stock NaOH (pH 13.0) and HCl (pH 2.0) solutions were used to reach desired basic and acidic pHs, respectively. As it is clear from figure, by varying the pH of initial dye solution, the change in dye adsorption of both beads was not significant. This observation can be attributed to pH-independent behavior of κ-carrageenan component. The mκ-Carb beads comprise kappa-carrageenan and Fe3O4 particles. kappa-Carrageenan is an ionic polysaccharide carrying sulfate groups (-OSO3 −). These pendants are completely dissociated in the overall pH range and the hydrogels from this biopolymer show pH-independent swelling behavior [34]. In fact, in the overall pH range, these sulfate groups are in dissociated form. A small decrease in dye adsorption was observed at pH 2 and it may be attributed to charge screening effect of the H+ cations causing a restriction in electrostatic attraction between cationic dye and anionic sulfate [23]. Due to this behavior, the magnetic carrageenan beads may be assumed as good candidate for removal of cationic dyes from aqueous solution in the overall range of pHs.
Thermodynamic studies
The effect of temperature on the dye adsorption capacity of κ-Carb and mκ-Carb5 beads for crystal violet was studied. The changing in dye adsorption was explained according to thermodynamic parameters. Adsorption enthalpy (ΔH, kJ mol−1), adsorption free energy (ΔG, kJ mol−1), and adsorption entropy (ΔS, J K−1 mol−1) can be calculated according to the following equations [30]:
where KD is the equilibrium constant; Cd is the dye adsorbed onto nanocomposite (mg L−1); Cs is the equilibrium concentration (mg L−1); R is the universal gas constant (8.314 J mol−1 K−1); and T is the absolute temperature (K). According to Eq. 10, by plotting the LnKD versus 1/T, ΔH, and ΔS can be calculated from slope and intercept of curves, respectively (Fig. 6). The thermodynamic parameters are summarized in Table 3. By changing the temperature, the obtained thermodynamic parameters of two beads were not identical. While the magnetic-free beads indicated an enhancement in dye adsorption capacity by increasing the temperature of dye solution, a decrement in dye adsorption capacity was observed for magnetic mκ-Carb5 beads. The negative values of ΔG for both beads indicated that the adsorption process is spontaneous. According to values of ΔH, while the adsorption process for mκ-Car was endothermic, it was exothermic for magnetic-free beads. Also, the negative value of ΔS for mκ-Carb5 and positive value of ΔS for κ-Carb beads suggested a decrease and increase in degree of freedom of the adsorbed species on magnetic and non-magnetic beads, respectively.
Mechanism of adsorption of dye onto adsorbents can be estimated from values of ΔH parameter [35]. The values of ΔH lower than 20 kJ mol−1 depict that the physisorption interactions such as Van der Waals are dominated. The values of ΔH ranging from 20 to 80 kJ mol−1 indicate that the physisorption interaction such as electrostatic can be led to adsorption of adsorbate onto adsorbent. The chemisorption interaction occurs when the values of ΔH are between 80 and 450 kJ mol−1. The values of enthalpy for the adsorption of CV onto magnetic-free and magnetic carrageenan beads were obtained higher than 20 kJ mol−1. According to the values of ΔH, the positive crystal violet molecules can interact electrostatically by sulfate groups on carrageenan beads.
Desorption study
In addition to high adsorption capacity of adsorbents for dyes, due to economical problems, desorption of adsorbed dye can be considered as an important factor to reuse of adsorbents. To evaluate the regeneration of magnetic beads, desorption of dye from beads was examined with different solutions. First, we examined desorption of dye using 0.5 M NaCl and 0.5 KCl solutions. When the dye adsorbed beads were immersed in 0.5 M of NaCl, the beads were destroyed. So, desorption was evaluated using KCl solutions. 0.5 M of KCl in water and in water/ethanol (50/50 V/V) was placed in contact with the beads. While desorption efficiency was obtained 56 % in 0.5 of KCl solution in water, desorption efficiency was achieved 91 % by using 0.5 M KCl in water/ethanol solution. According to this observation, we endeavored to study the desorption–adsorption process for four times using the 0.5 M KCl in ethanol/water as regeneration agent. mκ-Carb5 was chosen for adsorption–desorption process. During four cycles, although desorption of dye was high, the adsorption efficiency showed a reduction of ~30 %.
Conclusion
The kappa-carrageenan-based magnetic nanocomposite beads were prepared by in situ precipitation of Fe2+/Fe3+ ions in the presence of kappa-carrageenan biopolymer and subsequently cross-linking with K+ ions. According to SEM images, a coarse surface was appeared for magnetic beads. TEM image confirmed the non-sized magnetic Fe3O4 nanoparticles. Magnetic beads were used for removal of cationic crystal violet from aqueous solutions. The incorporation of magnetic nanoparticles in composition of beads caused an improvement in the rate of dye adsorption. However, adsorption of dye on magnetic beads was decreased as magnetic Fe3O4 nanoparticles increased in beads composition. The adsorption kinetic data were analyzed by pseudo-first-order and pseudo-second-order kinetic models and the pseudo-second order was obtained as the best model to fit experimental data. Langmuir model was obtained as the best model for the adsorption of CV onto magnetic hydrogels and maximum adsorption capacity from Langmuir isotherm was obtained 84.7 mg g−1. The effect of pH revealed that the adsorption capacity of beads for CV was not significantly varied. The thermodynamic parameters indicated the spontaneously adsorption of CV dye on carrageenan beads.
References
Kirk RE, Othmer DF (1992) Encyclopedia of chemical technology. In: Kroschwitz I, Howe-Grant M (eds), 4th edn, vol. 4, Wiley, New York, pp 942–961
Usov AI (1998) Structural analysis of red seaweed galactans of agar and carrageenan groups. Food Hydrocoll 12:301–308
Theerkeelsen HG (1993) Industrial gums: carrageenan chapter. In: Whistler RL, BeMiller JN (eds), Academic Press, London
Li L, Ni R, Shao Y, Mao S (2014) Carrageenan and its application in drug delivery. Carbohydr Polym 103:1–11
Morris ER, Rees DA, Robinson G (1980) Cation-specific aggregation of carrageenan helices: domain model of polymer gel structure. J Mol Biol 138:349–362
Rodrigues S, Ros-da-Costa AM, Grenha A (2012) Chitosan/carrageenan nanoparticles: effect of cross-linking with tripolyphosphate and charge ratios. Carbohydr Polym 89:282–289
Mohamadnia Z, Zohuriaan-Mehr MJ, Kabiri K, Jamshidi A, Mobedi H (2007) pH-Sensitive IPN hydrogel beads of carrageenan-alginate for controlled drug delivery. J Bioact Compat Polym 22:342–356
Mitsumata T, Sakai K, Takimoto J (2006) Giant reduction in dynamic modulus of kappa-carrageenan magnetic gels. J Phys Chem B 110:20217–20223
Sagbas S, Butun S, Sahiner N (2012) Modifiable chemically crosslinked poli(κ-carrageenan) particles. Carbohydr Polym 87:2718–2724
Muhamad II, Fen LS, Hui NH, Mustapha NA (2011) Genipin-cross-linked kappa-carrageenan/carboxymethyl cellulose beads and effects on beta-carotene release. Carbohydr Polym 83:1207–1212
Tranquilan-Aranilla C, Nagasawa N, Bayquen A, Rosa AD (2012) Synthesis and characterization of carboxymethyl derivatives of kappa-carrageenan. Carbohydr Polym 87:1810–1816
Zu J, Shi F, Liu R, Ye M (2013) Amination of glycidyl methacrylate-grafted polystyrene particles and their adsorption capacity for Nd3+ and Cd2+. Iran Polym J 22:259–265
Sui K, Li Y, Liu R, Zhang Y, Zhao X, Liang H, Xia Y (2012) Biocomposite fiber of calcium alginate/multi-walled carbon nanotubes with enhanced adsorption properties for ionic dyes. Carbohydr Polym 90:399–406
Li G, Du Y, Tao Y, Deng H, Luo X, Yang J (2010) Iron(II) cross-linked chitin-based gel beads: preparation, magnetic property and adsorption of methyl orange. Carbohydr Polym 82:706–713
Wan Ngah WS, Teong LC, Hanafiah MAKM (2011) Adsorption of dyes and heavy metal ions by chitosan composites: a review. Carbohydr Polym 83:1446–1456
Sayar F, Guven G, Piskin E (2006) Magnetically loaded poly(methyl methacrylate-co-acrylic acid) nanoparticles. Colloid Polym Sci 284:965–978
Bruce IJ, Sen T (2005) Surface modification of magnetic nanoparticles with alkoxysilanes and their application in magnetic bioseparations. Langmuir 21:7029–7035
Starodubtsev SG, Saenko EV, Dokukin ME, Aksenov VL, Klechkovskaya VV, Zanaveskina IS (2005) Formation of magnetite nanoparticles in poly(acrylamide) gels. J Phys Condens Matter 17:1471–1480
Philippova O, Barabanova A, Molchanov V, Khokhlov A (2011) Magnetic polymer beads: recent trends and developments in synthetic design and applications. Eur Polym J 47:542–559
Liu P, Zhang L (2007) Adsorption of dyes from aqueous solutions or suspensions with clay nano-adsorbents. Sep Purif Technol 58:32–39
Dai J, Yang H, Yan H, Shangguan Y, Zheng Q, Cheng R (2011) Phosphate adsorption from aqueous solutions by disused adsorbents: chitosan hydrogel beads after the removal of copper (II). Chem Eng J 166:970–977
Shah A, Khuhawar MY, Shah SA (2012) Evaluation of sorption behavior of polymethylene-bis(2-hydroxybenzaldehyde) for Cu(II), Ni(II), Fe(III), Co(II) and Cd(II) ions. Iran Polym J 21:325–334
Li S (2010) Removal of crystal violet from aqueous solution by sorption into semi-interpenetrated networks hydrogels constituted of poly(acrylic acid-acrylamide-methacrylate) and amylose. Bioresour Technol 101:2197–2202
Singh KP, Gupta S, Singh AK, Sinha S (2011) Optimizing adsorption of crystal violet dye from water by magnetic nanocomposite using response surface modeling approach. J Hazard Mater 186:1462–1573
Nandi BK, Goswami A, Das AK, Mondal B, Purkait MK (2008) Kinetic and equilibrium studies on the adsorption of crystal violet dye using kaolin as an adsorbent. Sep Sci Technol 43:1382–1403
Mall ID, Srivastava VC, Agarwal NK (2006) Removal of orange-G and methyl violet dyes by adsorption onto bagasse fly ash—kinetic study and equilibrium isotherm analyses. Dyes Pigment 69:210–223
Mahdavinia GR, Aghaie H, Sheykhloie H, Vardini MT, Etemadi H (2013) Synthesis of CarAlg/MMt nanocomposite hydrogels and adsorption of cationic crystal violet. Carbohydr Polym 98:358–365
Li P, Siddaramaiah Kim NH, Heo SB, Lee JH (2008) Novel PAAm/laponite clay nanocomposite hydrogels with improved cationic dye adsorption behavior. Compos Part B 39:756–763
Mahdavinia GR, Massoudi A, Baghban A, Massoumi B (2012) Novel carrageenan-based hydrogel nanocomposites containing laponite RD and their application to remove cationic dye. Iran Polym J 21:609–619
Auta M, Hameed BH (2011) Preparation of waste tea activated carbon using potassium acetate as an activating agent for adsorption acid blue 25 dye. Chem Eng J 171:502–509
Nabid MR, Sedghi R, Sharifi R, Abdi-Oskooie H, Heravi MM (2013) Removal of toxic nitrate ions from drinking water using conducting polymer/MWCNTs nanocomposites. Iran Polym J 22:85–92
Peppas LB, Harland RS (1990) Absorbent polymer technology. Elsevier, Amsterdam, pp 45–66
Al-Degs YS, El-Barghouthi MI, El-Sheikh AH, Walker GM (2008) Effect of solution pH, ionic strength, and temperature on adsorption behavior of reactive dyes on activated carbon. Dyes Pigment 77:16–23
Durmaz S, Okay O (2000) Acrylamide/2-acrylamido-2-methylpropane sulfonic acid sodium salt-based hydrogels: synthesis and characterization. Polymer 41:3693–3704
Liu Y (2009) Is the free energy change of adsorption correctly calculated? J Chem Eng Data 54:1981–1985
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mahdavinia, G.R., Iravani, S., Zoroufi, S. et al. Magnetic and K+-cross-linked kappa-carrageenan nanocomposite beads and adsorption of crystal violet. Iran Polym J 23, 335–344 (2014). https://doi.org/10.1007/s13726-014-0229-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-014-0229-8