Abstract
Click reaction of alkynylthiacalix[4]arene with ethyl 2-azidoacetate, followed by ammonolysis with hydrazine hydrate and Schiff-base condensation with benzaldehyde or salicyic aldehyde, afforded two novel thiacalix[4]arene derivatives containing multiple aromatic groups in yields of 86% and 90%. Their complexation properties for four organic dyes were investigated by liquid-liquid extraction experiments, complexation UV-Vis spectra and mass spectrum. The highest extraction percentage was 97% for Neutral red. The UV-Vis spectra and ESI-MS spectrum indicated the 1:1 complexes in DMSO solution. The association constants were as high as 1 ∼ 8×104 M−1. These complexation experiments showed that thiacalix[4]arene receptors possess excellent complexation capabilities for dyes.

Two novel thiacalix[4]arene derivatives containing multiple aromatic groups were synthesized in yields of 86% and 90%. These complexation experiments showed that thiacalix[4]arene receptors possess excellent complexation capabilities for four tested dyes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Dyes are important chemicals for textiles, papers, leather, etc. However, the application of dyes also brought about serious pollution in these industries.[1,2] In order to remove the dyes from waste water, many conventional treatment processes have been developed, such as precipitation, extraction, adsorption, membrane filtration, photodegradation, etc.[3–7] Among them, liquid-liquid extraction is seen as an effective method due to its advantages of selective separation and recovery of dyes from the effluent mixtures. Normally, the extractants or carriers play the decisive roles in liquid-liquid extraction. Recently, supramolecular chemistry has been considered as a good way to search for molecular structures, which could be modified by introducing various functional groups to construct a suitable binding site for dyestuff.[8–10] Calixarenes are considered as the third generation supramolecules after crown ether and cyclodextrin. They possess tunable 3D-shaped cavities with versatile complexation abilities.[11,12] Lately, some calixarene derivatives exhibited good extraction abilities for dyes. A series of calixarene-based derivatives and polymers were reported as effective dye receptors by Yilmaz,[13–15] Memnon[16,17] and Diao.[18] Our group also described a series of calixarene derivatives with excellent dye complexation abilities.[19–22] These literature suggested that, although the small size of calix[4]arene cavity is a disadvantage for binding dyes, the calix[4]arene derivatives with aromatic conjugate groups could bind dyes effectively based on the new cavities constructed by calixarene skeleton and aromatic groups. Moreover, the π- π action between aromatic groups and dyes were usually the important factors in complexation. Lately, we prepared several thiacalix[4]arene derivatives with triazole rings by click chemistry and studied their excellent complexation abilities for dyes based on π- π interaction.[22] In this paper, this thiacalix [4]arene triazole derivatives were further modified by introducing new aromatic conjugate groups to construct novel π-cavity for binding dyes. The complexation results suggested that they possess better complexation abilities for dyes than the precursor compounds, as expected.
2 Experimental
2.1 Instruments and reagents
Melting points were tested on WRS-3 micro melting point instrument. 1H NMR spectra were recorded in CDCl3 on a Bruker-ARX 400 instrument, using TMS as reference. ESI-MS spectra were obtained from DECAX-30000 LCQ Deca XP mass spectrometer. Elemental analyses were performed at Vario EL III Elemental Analyzer. IR spectra were recorded on a Thermo Nicolet AVATAR 5700 FTIR spectrometer using KBr pellet. The UV-Vis measurements were performed on Varian UV-Vis spectrometer. Dyes were purchased from Acros Organics, China. All solvents were purified by standard procedures. Compounds 1, 2 and 3 were prepared according to the literature method.[23] Compound 4 was synthesized according to the procedure in reference.[22]
2.2 The procedure for syntheses of compound 5a
Under N2 atmosphere, the mixture of compound 4 (0.4 g, 0.3 mmol) and benzaldehyde (0.2 mL, 1.9 mmol) was stirred and refluxed in 15 mL of MeOH-CHCl3 (3:2, v/v). TLC analysis revealed that compound 4 disappeared in 6 h. After cooling, the solvent was removed under reduced pressure. The residue was treated with 10 mL of MeOH and white precipitation appeared. The precipitate was filtrated and dried under vacuum. Compound 5a was obtained as white solid in yield of 86%. 5a: M.p. 252 ∼254 ∘C. 1H NMR (400 MHz, CDCl3)δ: (ppm) 1.04 (s, 36H, CH3), 4.97 (s, 8H, NCH2), 5.66 (s, 8H, OCH2), 7.42 ∼8.31 (m, 36H, CH and ArH), 11.85 (s, 4H, NH). ESI-MS m/ z(%): 1684.9(M +, 100). Anal. Calcd. for C88 H 92 N 20 O 8 S 4: C 62.69, H 5.50, N 16.61; found C 62.61, H 5.45, N 16.56.
2.3 The procedure for syntheses of compound 5b
Under N2 atmosphere, the mixture of compound 4 (0.4 g, 0.3 mmol) and salicylaldehyde (0.2 mL, 2.0 mmol) was stirred and refluxed in 20 mL of MeOH-CHCl3 (1:1, v/v). TLC analysis revealed that compound 4 disappeared in 5 h. After cooling, the solvent was distilled under reduced pressure and 10 mL of MeOH was added to give white precipitation. Then the precipitate was filtered and dried under vacuum. Compound 5b was obtained as white solid in yield of 90%. 5b: M.p. 269–272 ∘C. 1H NMR (400 MHz, CDCl3)δ: (ppm) 1.02 (s, 36H, CH3), 5.00 (s, 8H, NCH2), 5.65 (s, 8H, OCH2), 6.89 ∼8.47 (m, 32H, CH and ArH), 10.06 (s, 4H, OH), 11.77 (s, 4H, NH). ESI-MS m/ z(%): 1749.4(M + 100). Anal. Calcd. for C88 H 92 N 20 O 12 S 4: C 60.39, H 5.30, N 16.01; found C 60.33, H 5.34, N 16.04.
2.4 Extraction of dyes
According to the reported method,[22], 10 mL of 2.0× 10 −5M aqueous solution of dye and 10 mL of 1.0×10−3M solution of receptor in CH2Cl2 were vigorously agitated in a stoppered glass tube with a mechanical shaker for 2 min. Then the mixture was stirred magnetically in a thermostated water-bath at 25 ∘C for 1 h, and was finally left standing for an additional 30 min. The concentration of dye remaining in the aqueous phase was subsequently determined by UV-Vis analyses. Blank experiments showed that dye extraction was less than 2% in the absence of extractant. The percent extraction (E%) was calculated as: E% = 100 (A o −A)/ A o.where A o and A are initial and final concentrations of the dye before and after the extraction, respectively. Each experiment was repeated thrice. The dye concentration in the receiving phase was reported as the mean of the determination and the relative standard deviation from the mean was less than 5%.
2.5 UV-Vis spectral studies of complexation experiments
All UV-Vis experiments were performed in DMSO solution by adding aliquots of stock solution of the respective dyes. The stoichiometry of the complexes was determined by the Job method of continuous variations. The association constants were calculated by Benesi-Hilderbrand formula with linear curve fitting procedure.[24,25]
3 Results and Discussion
3.1 Synthesis and characterization
The synthetic route was illustrated in scheme 1. As per our report[22] on click reaction, thiacalix[4]arene derivative 3 was prepared by treating alkynylthiacalix[4]arene 2 with compound 1 in yield of 70%. Then, by reacting compound 3 with hydrazine hydrate, the thiacalix[4] arene hydrazide derivative 4 was obtained in yield of 86%. Finally, by Schiff-base condensation of compound 4 with benzaldehyde or salicyic aldehyde, two novel thiacalix[4]arene derivatives 5a and 5b containing multiple aromatic groups were synthesized after simple recrystallization. The yields of compounds 5a and 5b were as high as 86% and 90%, respectively.
The structures of the new compounds 5a and 5b were confirmed by elemental analyses, IR, ESI-MS and 1H NMR spectra. Their IR spectra showed corresponding adsorption peaks of Schiff-base groups (C =N) at 1620 cm −1 approximately. The corresponding molecular peaks in their ESI-MS spectra were observed at 1684.9 (M +) and 1747.4(MH+), respectively. In the 1H NMR spectra, one singlet for the tert-butyl groups supported the cone conformations of compounds 5a and 5b.
3.2 Complexation studies for dyes
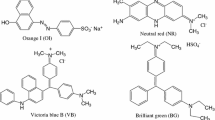
As can be seen that compounds 5a and 5b possess multiple aromatic triazole rings and phenyl groups, which could construct novel π-cavity with thiacalix[4]arene skeleton, resulting in excellent complexation abilities for dyes potentially. Thus, four common dyes with large π-conjugate system including Orange G (OG), Neutral red (NR), Methylene blue (MB) and Brilliant ponceau (BP), were used as representative dyes for the complexation studies of compounds 5a and 5b. Their structures are shown in Figure 1.
The two phase extraction experiments (H2O/CH2Cl2) were preliminarily applied to investigate the complexation abilities of compounds 5a and 5b towards four dyes. The extraction results are summarized in table 1. The control experiments without host showed that dye extractions were less than 2%. One can see that, as expected, compounds 5a and 5b exhibit excellent extraction abilities for the tested dyes. Their extraction percentages were higher than that of their precursor compound 4. The extraction percentage of compound 5b for Neutral red was as high as 97%. By comparing with the structures of compounds 5a, 5b and 4, the excellent extraction percentages of compounds 5a and 5b could be attributed to the introduction of Schiff-base aromatic groups, which enhance the π- π action between receptors and guests. On the other hand, the extraction percentages of compounds 5a and 5b were similar, although compound 5b showed a little higher extraction percentage than that of compound 5a.
Furthermore, the complexation behaviors of receptors 5a and 5b for dyes were studied by UV-Vis spectral titration. The respective UV-Vis spectra of compounds 5a and 5b, four dyes, and compounds 5a and 5b with four dyes at various concentrations (up to 2.0×10−5M) were studied. Figures 2 and 3 exhibit the representative UV-Vis spectra of complexation of compound 5a with MB. In Figure 2, absorption wavelength of MB at 660 nm shifted to 666 nm after complexation. Moreover, the absorbance at 294 nm increased but the absorbance at 665 nm decreased distinctly after complexation. These results certainly supported the existence of intermolecular complexation action between compound 5a and MB. In Figure 3, the maximal absorbance at 294 nm increased gradually in non-proportional manner with the increase of concentration of MB, also indicating the interaction between host and guest. Based on the absorbance at maximal absorption wavelength, the association constants and correlation coefficients were calculated by Benesi-Hildebrand equation, which was usually used to study the complexation behaviors of host-guest.[24,25] The calculation follow equations (1) and (2):
Where H is host, G is guest, n is the ratio of complexation. [H] and [G] are the concentrations of host and guest, respectively. Ks is association constant; Δε is difference in molar absorption coefficients. ΔA is the change of absorbance at maximal absorption wavelength.
The results of association constants (K s) and correlation coefficients (R 2) are summarized in table 2. It could be seen that the correlation coefficients (R 2) were near 1 when the values of the ratio of complexation (n) were set as 1. These results suggested the stoichiometric ratio of 1:1 for host-guest complexes of compounds 5a and 5b with dyes. On the other hand, the association constants of compounds 5a and 5b with dyes were as high as 1 ∼8×104 M −1. Moreover, compound 5b possessed higher association constants than that of compound 5a, indicating that the hydroxyl groups in compound 5b were favorable for dye complexation based on hydrogen bonding action. All these data implied the strong complexation action between hosts and guests, which were in agreement with the results of extraction experiments.
The ESI-MS spectrum of compound 5b with excess Neutral red (mol ratio = 1:4) was studied to investigate the complexation behavior in DMSO. The ESI-MS spectrum showed two strong peaks clearly (Figure S13 in SI). One peak at 1748.6 was for compound 5b and another one at 2001.7 was for the complex of compound 5b with Neutral red. It is interesting that, although excess Neutral red was added, only 1:1 complexation peak appeared and the relative abundance attained 100. This result suggested that strong intermolecular action between host and guest, which is also in accordance with the result of 1:1 complexation of UV-Vis spectra. In a word, all the complexation experiments supported that compounds 5a and 5b have excellent complexation abilities for dyes and prefer to 1:1 complexes in DMSO solution.
4 Conclusions
In summary, two novel thiacalix[4]arene derivatives 5a and 5b containing multiple aromatic groups were synthesized by click reaction subsequent ammonolysis with hydrazine hydrate and Schiff-base condensation with benzaldehyde and salicyic aldehyde. The yields were as high as 86% and 90%, respectively. Their complexation abilities for dyes were studied by liquid-liquid extraction experiments, UV-Vis spectra and mass spectrum. The extraction percentages were over 90%, which were higher than that of the precursor compound. The UV-Vis spectra indicated formation of 1:1 complexes between host and guest. The association constants are 1 ∼ 8×10 4 M −1. The ESI-MS spectrum of complex of 5b with Neutral red also implied the formation of 1:1 complex in DMSO. All these experiments supported that thiacalix[4]arene derivatives 5a and 5b containing multiple aromatic groups are excellent receptors for binding organic dyes.
References
Wong Y and Yu J 1999 Water Res. 33 3512
Crini G 2005 Prog. Polym. Sci. 30 38
Peternel I T, Koprivanac N and Bozic A M L 2007 J. Hazard. Mater. 148 477
Crini G and Badot P M 2008 Prog. Polym. Sci. 33 399
Purkait M K, DasGupta S and De S 2004 J. Colloid Interface Sci. 270 496
Chakraborty S, Purkait M K and DasGupta S 2003 Sep. Purif. Technol. 31 141
Ghosh D and Bhattacharyya K G 2002 Appl. Clay Sci. 20 295
Al-Degs Y, Khraisheh M A M and Allen S J 2001 Sep. Sci. Technol. 36 91
Jana P R, De S and Basu J K 2003 Chem. Eng. J. 95 143
Wu F C, Tseng R L and Juang R S 2000 J. Hazard. Mater. 73 63
Prakash C, Jayaraman D and Pulla C 2013 J. Chem. Sci. 125 1455
Gunupuru R, Maity D, Bhadu G, Chakraborty A, Srivastava D and Paul P 2014 J. Chem. Sci. 126 627
Akceylan E, Bahadir M and Yilmaz M 2009 J. Hazard. Mater. 162 960
Yilmaz E O, Sirit A and Yilmaz M 2007 J. Macromol. Sci. A: Pure Appl. Chem. 44 167
Yilmaz A, Yilmaz E and Yilmaz M 2007 Dyes Pigm. 74 54
Kamboh M A, Solangi I B and Sherazi S T H 2011 Desalination 268 83
Kamboh M A, Solangi I B and Memon S 2011 J. Hazard. Mater. 186 651
Chen M, Shang T, Fang W and Diao G 2011 J. Hazard. Mater. 185 914
Hong B Q, Yang F F, Xu B T, Xie J W and Guo H Y 2012 J. Macromol. Sci. Part A 49 648
Yang F F, Zhang Y M, Guo H Y and Wei X L 2013 Separation Sci. Tech. 48 1565
Bai X Y, Yang F F, Xie J W and Guo H Y 2013 J. Macromol. Sci. Part A. 50 334
Guo H Y, Yang F F, Jiao Z Y and Lin J R 2013 Chin. Chem. Lett. 24 450
Bew S P, Brimage R A and Hermite N L 2007 Org. Lett. 9 3713
Connors K A 1987 In Binding Constants (New York: Wiley)
Beresnev D G, Itsikson N A, Chupakhin Q N, Charushin V N, Kodess M I, Butakov A I, Rusinov G L, Morzherin Y Y, Konovalov A I and Antipin I S 2006 J. Org. Chem. 71 8272
Acknowledgements
Financial support from the National Natural Science Foundation of China (No: 21406036), Fujian Natural Science Foundation of China (No. 2014J01034), Project of Fujian provincial department of education(JA11044) and the Program for Innovative Research Team in Science and Technology in Fujian Province University and Program for Excellent young researchers in University of Fujian Province (JA10056) are greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
The electronic supporting information, including the characteristic data of new compounds and the complexation UV-Vis spectra for dyes are available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
YANG, C., WANG, Z., GUO, H. et al. Thiacalix[4]arene derivatives containing multiple aromatic groups: High efficient extractants for organic dyes. J Chem Sci 127, 1383–1388 (2015). https://doi.org/10.1007/s12039-015-0911-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-0911-1