Abstract
Molecular cytogenetic reveal details of fine chromosomal structure that are useful in studies of chromosomal diversification, genome organization, and evolution. However, these techniques have rarely been used to describe population-level variations in the tribe Meliponini. In the present study, we compared the karyotype, number, and localization of rDNA clusters and the distribution of a repetitive DNA sequence in the genome of T. spinipes from nine locations. Although karyotypes with 2n = 34 were found in all cases, five different karyotypic formulae were detected. The heterochromatin was preferentially associated with centromeric regions. The microsatellite probe (GA)15 signals were localized mainly in the euchromatic regions of all chromosomes in most of the analyzed populations. The 18S rDNA clusters were present in four chromosomal pairs, except for the population from Palotina, which showed only two rDNA clusters. Analyses revealed a karyotypic variation in T. spinipes along the geographic distribution, proving that cytogenetic variations were not detected when scattered colonies were analyzed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cytogenetic studies on the stingless bees of the tribe Meliponini demonstrated that chromosome number and heterochromatin distribution pattern are key features for the characterization of different species/genera and that their variation can be associated with interspecific diversification (Rocha et al. 2002). Recently, however, cytogenetic studies on these bees have advanced remarkably because of the application of molecular cytogenetic methods, which reveal details of the fine chromosomal structure (Andrade-Souza et al. 2018; Piccoli et al. 2018; Travenzoli et al. 2019a; Pereira et al. 2021).
Molecular studies employing fluorescent in situ hybridization (FISH) have provided a better understanding of chromosomal organization and evolution of Meliponini (Andrade-Souza et al. 2018; Piccoli et al. 2018; Travenzoli et al. 2019a, b). For example, studies using 45 or 18S rDNA probes demonstrated preferential mapping of ribosomal genes in pericentromeric regions, in a single chromosomal pair, in several Melipona species from the Eomelipona and Melipona subgenera that have low heterochromatin content. On the other hand, in species from the subgenera Michmelia and Melikerria, which have a high heterochromatin content, single labeling was localized in the terminal or interstitial regions of chromosomes (Andrade-Souza et al. 2018; Cunha et al. 2018; Piccoli et al. 2018; Travenzoli et al. 2019a, b). In the genera Friesella, Partamona, Tetragona, and Trigona, however, 18S rDNA mapping revealed the presence of multiple DNA sites (Ferreira 2015; Capoco 2016; Gonçalves et al. 2020; Elizeu et al. 2021). Together, these data indicate that the location of the ribosomal genes in the meliponines, as in several other organisms, may be related to differences in their genome organization, including the chromatin content, distribution, and composition, which play an important role in the chromosomal diversification of stingless bees (Rocha et al. 2002; Pereira et al. 2020, 2021).
In this context, molecular studies have demonstrated that different Meliponini species possess specific types of repetitive sequences in the heterochromatic regions of their chromosomes (Lopes et al. 2014; Cunha et al. 2020; Pereira et al. 2020, 2021). The repetitive sequence isolated from Melipona rufiventris, for example, did not hybridize with the chromosomes of Tetragonisca fiebrigi (Lopes et al. 2014). Similarly, the repetitive probe isolated from T. angustula did not hybridize with the heterochromatic regions of M. mondury, Partamona helleri, Nannotrigona testaceicornis, Frieseomelitta varia, and Austroplebeia australis (Pereira et al. 2020). The repetitive probe from T. angustula, however, hybridized with the chromosomes of T. fiebrigi (Pereira et al. 2020) while that from M. mondury hybridized with the chromosomes of M. flavolineata and M. rufiventris (Pereira et al. 2021), indicating that more closely related species may share repetitive DNA sequences and that heterochromatin is evolving divergently in this tribe.
Moreover, the distribution patterns of different repetitive sequences, using FISH with microsatellite probes, have already shown interspecific variations in the number and localization of repetitive clusters, contributing to ongoing evolutionary studies on this tribe (Piccoli et al. 2018; Travenzoli et al. 2019a; Barboza and Costa 2021; Elizeu et al. 2021). In contrast to most insects, clusters of repetitive DNA in the meliponines are usually localized in terminal euchromatic regions or scattered through most chromosomal pairs (Barbosa 2018; Piccoli et al. 2018; Travenzoli et al. 2019a). However, depending on the probe used, FISH has also identified variable quantities of repetitive DNA in heterochromatic regions in some species of Melipona (Barbosa 2018; Travenzoli et al. 2019a) and Frieseomelitta (Santos et al. 2018), showing that each group of species may accumulate specific microsatellite repeats in different portions of its chromosomes.
Together, these studies reveal important characteristics of chromosome organization and diversification in different Meliponini species. However, intraspecific studies assessing the chromosomal variation of different Meliponini species have rarely been performed (Duarte et al. 2009; Ferreira 2015; Barboza and Costa 2021; Elizeu et al. 2021). Among Meliponini, Trigona spinipes Jurine, 1807 is broadly distributed across South America and is found in 21 of the 26 Brazilian states (Camargo and Pedro 2013). With this wide geographical distribution, this supergeneralist species (Kleinert and Giannini 2012) inhabits several Brazilian biomes such as the savannah (Cerrado), Atlantic forest, Pantanal, Pampa, Amazon, and Caatinga.
Therefore, in this study, we compared the karyotype, number, and localization of rDNA clusters, as well as the distribution of repetitive DNA sequences in the chromosomes of nine populations of T. spinipes. This was done in order to verify whether the karyotype is conserved among these populations or if they present geographically distinct karyotypic variations. These data will contribute to our knowledge of the chromosome organization and evolution in this genus.
2 Materials and methods
-
a.
Biological material
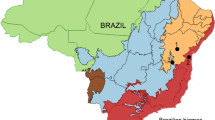
We analyzed T. spinipes samples from 23 nests collected from Minas Gerais, São Paulo, and Paraná States, Brazil (Figure 1; Table I). Voucher specimens were deposited in the Zoological Collections of the Federal University of Minas Gerais (UFMG) and the Regional Museum of Entomology of the Federal University of Viçosa (Central Apiary/UFV).
-
b.
Conventional cytogenetic analyses
Metaphase chromosomes were obtained from cerebral ganglia of larvae in the post-defecation stage. The cerebral ganglia from each larva (30 individuals/nest) were removed and processed following the technique developed by Imai et al. (1988) with certain modifications. Specifically, tissues were incubated in a hypotonic solution of colchicine (1% sodium citrate plus 0.005% colchicine) for 1 h. Conventional staining was carried out using 4% Giemsa in Sörensen’s phosphate buffer (0.06 M, pH 6.8) for 20 min at room temperature. To assess the location and distribution of heterochromatin, the C-banding technique for the BSG method (barium hydroxide/saline/Giemsa staining) was performed according to Rocha and Pompolo (1998) with modifications in the duration of the HCl treatment (7 min) and Ba(OH)2 incubation (14 min).
We analyzed an average of 15 metaphases per slide with an Olympus BX-60 microscope coupled to an image capturing system (Q-Color3 Olympus®). Chromosomes were classified according to Levan et al. (1964) after measuring five metaphases per colony using Image Pro Plus® (Online Resource 1).
-
c.
Fluorescence in situ hybridization
For physical mapping of the repetitive DNA sequences (microsatellite repeats), the microsatellite probe (GA)15 was used. This probe was directly labeled with Cy3 at the 5′-end during synthesis by VBC Biotech (Vienna, Austria). The hybridization procedures were performed following Kubat et al. (2008).
Ribosomal 18S genes were detected using FISH as reported by Pinkel et al. (1986) with the following modifications. The 18S rDNA probe was generated using PCR with primers F.1 (5′-GTCATATGCTTGTCTCAAAGA-3′) and R.1.1 (3′- TCTAATTTTTTCAAAGTAAACGC-5′) designed for Melipona quinquefasciata (Pereira 2006) and labeled by the indirect method with digoxigenin-11-dUTP (Roche, Mannheim, Germany). The probe was detected using anti-digoxigenin-rhodamine, and the slide preparations were counterstained with DAPI (4′,6-diamidino-2-phenylindole) and mounted in VECTASHIELD (Vector, Burlingame, CA, USA). The DAPI-stained slides were analyzed under an Olympus BX53 microscope with an Olympus DP73F camera. The images were processed using the CellSens Imaging software, with the filter WG (510–550 nm) for the probe detected with rhodamine/Cy3 and WU for the chromosomes (330–385 nm).
Collection sites of karyotyped Trigona spinipes in Brazil: (1) Januária/MG; (2) Janaúba/MG; (3) Bocaiúva/MG; (4) Mutum/MG; (5) Raul Soares/MG; (6) Florestal/MG; (7) Viçosa/MG; (8) Ribeirão Preto/SP; (9): Palotina/PR. MG, SP, and PR represents the Minas Gerais, São Paulo, and Paraná states, respectively (ArcGIS; Datum WGS 84)
3 Results
The nine analyzed populations of T. spinipes exhibited karyotypes with 2n = 34 chromosomes (females) or n = 17 (males). Despite this, based on morphology, five different karyotypic formulae were detected (Figure 2; Table I; Online Resource 1).
The heterochromatin was mostly located in centromeric regions, extending itself to the short arms of all chromosomes in populations from Bocaiúva, Janaúba, Januária, Mutum, Raul Soares, Viçosa, and Palotina. The only exception was the subtelocentric pair that was completely euchromatic. Contrarily, in the colony from Ribeirão Preto, the heterochromatic blocks were located in the long arms of the submetacentric pairs, and in the population from Florestal, the submetacentric pair 7 showed a large heterochromatic block, encompassing the small arm, the centromere, and part of the long arm (Figure 3).
The microsatellite probe (GA)15 signals were localized in the euchromatic regions of all chromosomes in the regionally different populations. In the colony from Ribeirão Preto, positive signals were seen in the eu- and/or heterochromatic arms of some chromosomes (Figure 4).
The 18S rDNA hybridization signals varied in number and location among populations. Most karyotypes exhibited 18S rDNA in three metacentric pairs (4th, 5th, and 8th pairs) and in the first submetacentric pair. In the population from Florestal, however, the submetacentric pair bearing the 18S rDNA markings was the 6th one, while in Raul Soares, two metacentric (4th and 5th pairs) and two submetacentric (1st and 6th pairs) pairs showed positive rDNA markings. Only two chromosome pairs (the 4th and the 8th metacentric pairs) carried rDNA genes in the Palotina population (Figure 5).
4 Discussion
Previously, the cytogenetic data obtained for Trigona have revealed that the chromosome number in this genus is conserved, with 11 of the 12 species studied presenting 34 chromosomes (Tavares et al. 2017). The only exception described for this genus was T. fulviventris (possibly, T. braueri), which has 32 chromosomes (Tavares et al. 2017). Therefore, the chromosome number detected in the present study corroborates the previous results. No differences were found between individuals from the same colony or between individuals from different colonies in the same location. The analyzed karyotypes also contained a pair of fully euchromatic subtelocentric chromosomes. We infer that this is a common characteristic of this and other Trigona species, such as T. chanchamayoensis, T. branneri, T. hyalinata, and T. recursa (Costa et al. 2004). Despite the same chromosomal number, our results identified five karyotypic formulae in the analyzed populations, due to a variation in the number of metacentric and submetacentric chromosomes in the different colonies (Figure 2; Table I; Online Resource 1).
In most populations, heterochromatin was evident in the short arm of the chromosomes and in the pericentromeric region. In the colony from Ribeirão Preto, however, some chromosomes had heterochromatic long arms. The detection of a heterochromatic chromosomal arm has also been revealed in other Trigona species (Costa et al. 2004; Ferreira 2015) and in different Meliponini genera, such as Frieseomelitta, Nannotrigona, Partamona, Schwarziana, Tetragona, Scaptotrigona, and Tetragonisca (Brito-Ribon et al. 1999; Duarte et al. 2009; Santos et al. 2018). In the Melipona genus, changes in the amount and distribution of heterochromatin represent the main source of karyotypic differentiation among species (Rocha et al. 2002). These data show that a variable amount of heterochromatin is present in the karyotype of most Meliponini. This variation may be the result of the amplification of the heterochromatic regions. Considering its diverse patterns, it appears that heterochromatin has important functions in the karyotypic evolution of this tribe (Rocha et al. 2002; see below).
The physical chromosome mapping of the (GA)15 sequence was identified along the euchromatic regions of all chromosomes in all populations, except in the case of Ribeirão Preto. In this colony, intense labeling was also detected in some heterochromatic regions. Considering that heterochromatin is enriched with repetitive DNA sequences, this pattern was expected. However, this situation rarely occurs in Meliponini (Travenzoli et al. 2019a). For example, in the 18 Melipona species, the above-mentioned dinucleotide DNA probe was detected in the euchromatic regions of most chromosomes, in the terminal regions, or widely scattered along the entire arms of chromosomes, depending on the species (Piccoli et al. 2018; Travenzoli et al. 2019a, b). However, in some insect species, as verified in this study for the colony of T. spinipes from Ribeirão Preto, some microsatellites have been also detected in heterochromatic regions (Milani and Cabral-de-Mello 2014; Ferreira 2015; Palacios-Gimenez and Cabral-de-Mello 2015; Barbosa 2018; Travenzoli et al. 2019a). Therefore, different DNA repeats can be distributed in specific regions of chromosomes depending on the species or group of organisms.
Although the presence of ribosomal genes in a single pair of chromosomes predominates in the Meliponini (Andrade-Souza et al. 2018; Cunha et al. 2018; Silva et al. 2018; Travenzoli et al. 2019a), our data showed an increase in the number of chromosomes that harbor the ribosomal genes, from 2 to 4 chromosome pairs. Considering that all populations of T. spinipes have the same chromosomal number, variations in the number of chromosomes bearing rDNA genes indicate the evolutionary dynamic of these sequences (Castro et al. 2001). Different mechanisms have already been used to explain the spreading of rDNA clusters throughout the genome of different organisms, and many times, more than one of them seems to operate when considering different groups of organisms or even species from the same genus. Among these mechanisms, we may cite ectopic recombination (Nguyen et al. 2010; Ferretti et al. 2019; Teixeira et al. 2021), Robertsonian chromosomal rearrangements (Nguyen et al. 2010; Arcanjo et al. 2013; Menezes et al. 2019), and transposition mediated by transposable elements adjacent to ribosomal genes or by a few rRNA genes to new chromosome locations (Cabrero and Camacho 2008; Panzera et al. 2012; Arcanjo et al. 2013; Pita et al. 2013; Ferretti et al. 2019).
More recently, Hirai (2020) demonstrated that the physical relationships among rDNA and other repeat arrays can interfere in the genomic dispersion of some repeat segments. He also showed that the affinity of the rDNA regions with the heterochromatic regions may facilitate dispersion. Considering this hypothesis, data obtained in the present and other studies (Ferreira 2015; Cunha et al. 2018; Travenzoli et al. 2019a) demonstrated that the 18S rDNA cluster in several Meliponini species is associated with heterochromatic sequences. Thus, heterochromatic sequences can play an important role in ribosomal cluster spreading in T. spinipes, which presented two, four, and even five chromosome pairs harboring 18S rDNA sequences (Barboza and Costa 2021). They could also explain the fact that in the different T. spinipes populations, the same chromosome pairs carried the 18S rDNA genes. Specifically, in the population from Palotina, which presented the 18S markings in only two chromosome pairs, they were positioned in homeologous pairs in the other populations.
Additionally, all 23 Melipona species analyzed (Andrade-Souza et al. 2018; Cunha et al. 2018; Picolli et al. 2018; Travenzoli et al. 2019b; Pereira et al. 2021) have a single chromosome pair harboring the rDNA sites, while in the other 21 Meliponini species studied (Gonçalves et al. 2020; Lopes et al. 2020; Barboza and Costa 2021; Elizeu et al. 2021), this number varies from one to five. When we compare these data with the phylogeny proposed by Rasmussen and Cameron (2010), it is clear that the Melipona sensu lato and the “other Meliponini” clade followed a different evolutionary path in relation to the 18S rDNA spreading mechanism. In this context, Lopes et al. (2014) and Pereira et al. (2021) have already demonstrated that the repetitive sequence present in the heterochromatin of different Meliponini species is not shared and can have arisen independently, in different genera. Thus, knowledge concerning the constitution of repetitive sequences, including the presence of mobile genetic elements, of different Meliponini species is necessary in order to better understand the spreading of the rDNA clusters in this group.
In summary, our analyses revealed karyotypic variation in T. spinipes along a geographic gradient. All analyzed populations possessed the same number of chromosomes. However, the morphology, number, and distribution of the analyzed markers varied between individuals from different locations. In this regard, populations from Ribeirão Preto and Palotina are the most divergent, displaying unique character combinations. The present research significantly expands the previously known cytogenetic characteristics of T. spinipes, revealing cytogenetic variations not detected in previous studies. Thus, this research improves our understanding of karyotypic diversification and the importance of using molecular cytogenetic techniques in population studies.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Andrade-Souza, V., Duarte, O.M.P., Martins, C.C.C., Santos, I.S., Costa, M.G.C., Costa, M.A. (2018) Comparative molecular cytogenetics in Melipona Illiger species (Hymenoptera, Apidae). Sociobiology 65, 696–705. https://doi.org/10.13102/sociobiology.v65i4.3480
Arcanjo, A., Cabral-de-Mello, D.C., Martins, C., Moura, R.C., Souza, M.J. (2013) Chromosomal diversification of diploid number, heterochromatin and rDNAs in two species of Phanaeus beetles (Scarabaeidae, Scarabaeinae). Gen. Mol. Biol. 36, 341-346. https://doi.org/10.1590/S1415-47572013005000031
Barbosa, I.C.O. (2018) Caracterização citogenética e análise do perfil de metilação em Melipona seminigra e Melipona interrupta (Hymenoptera, Apidae). Dissertação, Instituto Nacional de Pesquisas da Amazonia
Barboza, V.P., Costa, M.A. (2021) Cytogenetic analysis in Trigona spinipes Fabricius (Hymenoptera, Meliponina) reveals intraspecific variation. Neotrop. Entomol. https://doi.org/10.1007/s13744-021-00853-7
Brito-Ribon, R.M., Miyazawa, C.S., Pompolo, S.G. (1999) First karyotype characterization of four species of Partamona (Friese, 1980) (Hymenoptera, Apidae, Meliponinae) in Mato Grosso State, Brazil. Cytobios. 100, 19–26. https://www.researchgate.net/publication/282247312
Cabrero, J., Camacho, J.P.M. (2008) Location and expression of ribosomal RNA genes in grasshoppers: abundance of silent and cryptic loci. Chrom. Res. 16, 595-607. https://doi.org/10.1007/s10577-008-1214-x
Camargo, J.M. ., Pedro, S.R.M. (2013) Meliponini Lepeletier, 1836. In Moure, J. S., Urban, D. & Melo, G. A. R. (Orgs). Catalogue of Bees (Hymenoptera, Apoidea) in the Neotropical Region - online version. http://moure.cria.org.br/catalogue. Accessed 03 November 2020
Capoco, M.M. (2016) Mapeamento de DNA repetitivo na abelha sem ferrão Tetragonisca fiebrigi (Schwarz, 1938) com ênfase nos cromossomos Bs. Dissertação, Universidade Federal de Viçosa
Castro, J., Rodríguez, S., Pardo, B.G., Sánchez, L., Martínez, P. (2001) Population analysis of an unusual NOR-site polymorphism in brown trout (Salmo trutta L.). Heredity. 86, 291-302. https://doi.org/10.1046/j.1365-2540.2001.00834.x
Costa, K.F., Brito, R.M., Miyazawa, C.S. (2004) Karyotypic description of four species of Trigona (Jurine, 1807) (Hymenoptera, Apidae, Meliponini) from the State of Mato Grosso, Brazil. Gen. Mol. Biol. 27, 187-190. https://doi.org/10.1590/S1415-47572004000200010
Cunha, M.S.D., Travenzoli, N.M., Ferreira, R.D.P., Cassinela, E.K., Silva, H.B.D., Fernandes-Salomão, T.M.; Lopes, D.M. (2018) Comparative cytogenetics in three Melipona species (Hymenoptera: Apidae) with two divergent heterochromatic patterns. Gen. Mol. Biol. 41, 806-813. https://doi.org/10.1590/1678-4685-gmb-2017-0330
Cunha, M.S., Campos, L.A.O., Lopes, D.M. (2020) Insights into the heterochromatin evolution in the genus Melipona (Apidae: Meliponini). Insects Soc. 67, 391-398. https://doi.org/10.1007/s00040-020-00773-6
Duarte, O.M.P., Martins, C.C.C., Waldschmidt, A.M. (2009) Occurrence of multiple nucleolus organizer regions and intraspecific karyotype variation in Scaptotrigona xanthotricha Moure (Hymenoptera, Meliponini). Genet. Mol. Res. 8, 831-839. https://doi.org/10.4238/vol8-3gmr598
Elizeu, A.M., Travenzoli, N.M., Ferreira, R.P., Lopes, D.M., Tavares, M.G. (2021) Comparative study on the physical mapping of ribosomal genes and repetitive sequences in Friesella schrottkyi (Friese 1900) (Hymenoptera: Apidae, Meliponini). Zool. Anz. 292, 225-230. https://doi.org/10.1016/j.jcz.2021.04.006
Ferreira, R.P. (2015) Análise citogenética de abelhas do gênero Trigona Jurine, 1807 (Hymenoptera: Meliponini). Tese, Universidade Federal de Viçosa
Ferretti, A.B.S.M., Ruiz-Ruano, F.J., Milani, D., Loreto, V., Martí, D.A., Ramos, E., Martins, C., Cabral-de-Mello, D.C. (2019) How dynamic could be the 45S rDNA cistron? An intriguing variability in a grasshopper species revealed by integration of chromosomal and genomic data. Chromosoma 128, 165–175. https://doi.org/10.1007/s00412-019-00706-8
Gonçalves, G.C., Dalbosco, A.M., Barth, A., Miranda, E.A., Costa, M.A. (2020) Comparative cytogenetic analysis of three species of the genus Partamona (Apidae, Meliponini). Apidologie. https://doi.org/10.1007/s13592-020-00798-7
Hirai, H. (2020) Chromosome dynamics regulating genomic dispersion and alteration of nucleolus organizer regions (NORs). Cells. 9, 971. https://doi.org/10.3390/cells9040971
Imai, H.T., Taylor, R.W., Crosland, M.W.J., Crozier, R.H. (1988) Modes of spontaneous chromosome mutation and karyotype evolution in ants with reference to the minimum interaction hypothesis. Jpn. J. Gen. 63, 113-125
Kleinert, A.M.P., Giannini, T.C. (2012) Generalist Bee Species on Brazilian Bee-Plant Interaction Networks. Psyche. 291519. https://doi.org/10.1155/2012/291519
Kubat, Z., Hobza, R., Vyskot, B., Kejnovsky, E. (2008) Microsatellite accumulation in the Y chromosome of Silene latifolia. Genome 51, 350-356. https://doi.org/10.1139/G08-024
Levan, A., Fredga, K., Sandberg, A. (1964) Nomenclature for centromeric position on chromosomes. Hereditas 52, 201-220
Lopes, D.M., Fernandes, A., Diniz, D., Sobrinho-Scudeler, P.E., Foresti, F., Campos, L.A.O. (2014) Similarity of heterochromatic regions in the stingless bees (Hymenoptera: Meliponini) revealed by chromosome painting. Caryologia 67, 222-226. https://doi.org/10.1080/0144235X.2014.974349
Lopes, D.M., Travenzoli, N.M., Fernandes, A., Campos, L.A.O. (2020) Different levels of chromatin condensation in Partamona chapadicola and Partamona nhambiquara (Hymenoptera, Apidae). Cytogenet. Genome Res. 160, 206-213. https://doi.org/10.1159/000507835
Menezes, R.S.T., Gazoni, T., Costa, M.A. (2019) Cytogenetics of warrior wasps (Vespidae: Synoeca) reveals intense evolutionary dynamics of ribosomal DNA clusters and an unprecedented number of microchromosomes in Hymenoptera. Biol. J. Linnean Soc. 126, 925-935. https://doi.org/10.1093/biolinnean/bly210
Milani, D., Cabral-de-Mello, D.C. (2014) Microsatellite organization in the grasshopper Abracris flavolineata (Orthoptera: Acrididae) revealed by FISH mapping: remarkable spreading in the A and B chromosomes. PLoS One 9, e97956. https://doi.org/10.1371/journal.pone.0097956
Nguyen, P., Sahara, K., Yoshido, A., Marec, F. (2010) Evolutionary dynamics of rDNA clusters on chromosomes of moths and butterflies (Lepidoptera). Genetica. 138, 343-354. https://doi.org/10.1007/s10709-009-9424-5
Palacios‑Gimenez, O., Cabral‑de‑Mello, D.C. (2015) Repetitive DNA chromosomal organization in the cricket Cycloptiloides americanus: a case of the unusual X1X20 sex chromosome system in Orthoptera. Mol. Genet. Genomics. 290, 623-631. https://doi.org/10.1007/s00438-014-0947-9
Panzera, Y., Pita, S., Ferreiro, M.J., Ferrandis, I., Lages, C., Pérez, R., Silva, A.E., Guerra, M., Panzera, F. (2012) High dynamics of rDNA cluster location in kissing bug holocentric chromosomes (Triatominae, Heteroptera). Cytogenet. Genome Res. 138, 56-67. https://doi.org/10.1159/000341888
Pereira, J.O.P. (2006) Diversidade genética da abelha sem ferrão Melipona quinquefasciata baseada no sequenciamento das regiões ITS1 parcial e 18S do DNA ribossômico nuclear. Tese, Universidade Federal do Ceará
Pereira, J.A., Fernandes-Salomão, T.M., Lopes, D.M. (2020). Different repetitive DNA sequences make up heterochromatin in Meliponini. Apidologie 51, 855-860. https://doi.org/10.1007/s13592-020-00766-1
Pereira, J.A., Travenzoli, N.M., Oliveira, M.P., Werneck, H.A., Fernandes-Salomão, T.M., Lopes, D.M. (2021). Molecular cytogenetics in the study of repetitive sequences helping to understand the evolution of heterochromatin in Melipona (Hymenoptera, Meliponini). Genetica. https://doi.org/10.1007/s10709-020-00111-5
Piccoli, M.C.A., Bardella, V.B., Cabral-de-Mello, D.C. (2018) Repetitive DNAs in Melipona scutellaris (Hymenoptera: Apidae: Meliponidae): chromosomal distribution and test of multiple heterochromatin amplification in the genus. Apidologie 49, 497-504. https://doi.org/10.1007/s13592-018-0577-z
Pinkel, D., Straume, T., Gray, J.W. (1986) Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc. Nat. Ac. Sc. USA 83, 2934-2938
Pita, S., Panzera, F., Ferrandis, I., Galvão, C., Gomez-Palacio, A., Panzera, Y. (2013) Chromosomal divergence and evolutionary inferences in Rhodniini based on the chromosomal location of ribosomal genes. Mem. Inst. Oswaldo Cruz 108 (3), 376-382. https://doi.org/10.1590/S0074-02762013000300017
Rasmussen, C., Cameron, S.A. (2010) Global stingless bee phylogeny supports ancient divergence, vicariance, and long distance dispersal. Biol. J. Linn. Soc. Lond. 99, 206-232. https://doi.org/10.1111/j.1095-8312.2009.01341
Rocha, M.P., Pompolo, S.G. (1998) Karyotypes and heterochromatin variation (C-bands) in Melipona species (Hymenoptera, Apidae). Gen. Mol. Biol. 21, 41-45
Rocha, M.P., Pompolo, S.G., Dergam, J.A., Fernandes, A., Campos, L.A.O. (2002) DNA characterization and karyotypic evolution in the bee genus Melipona (Hymenoptera, Meliponini). Hereditas 136, 19-27. https://doi.org/10.1034/j.1601-5223.2002.1360104.x
Santos, J.M., Diniz, D., Rodrigues, T.A.S., Cioffi, M.B., Waldschmidt, A.M. (2018) Heterochromatin distribution and chromosomal mapping of microsatellite repeats in the genome of Frieseomelitta stingless bees (Hymenoptera: Apidae). Florida Entomologist 101, 33-39. https://doi.org/10.1653/024.101.0107
Silva, A.A., Rocha, M.P., Pompolo, S.G., Campos, L.A.O., Tavares, M.G. (2018) Karyotypic description of the stingless bee Melipona quinquefasciata Lepeletier, 1836 (Hymenoptera, Meliponini) with emphasis on the presence of B chromosomes. Comp. Cytogenet. 12, 471-482. https://doi.org/10.3897/CompCytogen.v12i4.29165
Tavares, M.G., Lopes, D.M., Campos, L.A.O. (2017) An overview of cytogenetics of the tribe Meliponini (Hymenoptera: Apidae). Genetica 145, 241-258. https://doi.org/10.1007/s10709-017-9961-2
Teixeira, G.A., Aguiar, H.J.A.C., Petitclerc, F., Orivel, J., Lopes, D.M., Barros, L.A.C. (2021) Evolutionary insights into the genomic organization of major ribosomal DNA in ant chromosomes. Ins. Mol. Biol. https://doi.org/10.1111/imb.12699
Travenzoli, N.M., Lima, B.A., Cardoso, D.C., Dergan, J.A., Fernandes-Salomão, T. M., Lopes, D.M. (2019a) Cytogenetic analysis and chromosomal mapping of repetitive DNA in Melipona species (Hymenoptera, Meliponini). Cytog. Gen. Res. 158, 213-224. https://doi.org/10.1159/000501754
Travenzoli, N.M., Barbosa, I.C.O., Carvalho-Zilse, G.A., Fernandes Salomao, T.M., Lopes, D.M. (2019b) Karyotypic description and repetitive DNA chromosome mapping of Melipona interrupta Latreille, 1811 (Hymenoptera: Meliponini). Caryologia 72, 91–95. https://doi.org/10.13128/cayologia-239
Acknowledgements
We are grateful to “Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG),” “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES),” and Universidade Federal de Viçosa (UFV) for financial support.
Funding
This research was supported by FAPEMIG, CAPES, and UFV.
Author information
Authors and Affiliations
Contributions
R.P.F. and D.M.L. conceived the study and designed the experiments. R.P.F., M.G.T., and N.T.M. performed the experiments and analyzed the data. M.G.T. wrote the manuscript draft, which was revised and had its final version approved by all co-authors.
Corresponding author
Ethics declarations
Ethics approval
The authors have no ethical conflicts to disclose.
Conflict of interest
The authors declare no competing interests.
Additional information
Manuscript Editor: Klaus Hartfelder
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tavares, M.G., Ferreira, R.d., Travenzoli, N.M. et al. Karyotypic variation in the stingless bee Trigona spinipes (Hymenoptera: Apidae: Meliponini) from different geographical regions of Brazil. Apidologie 52, 1358–1367 (2021). https://doi.org/10.1007/s13592-021-00906-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-021-00906-1